Abstract
Psychological stress has been shown to trigger systemic lupus erythematosus (SLE). However, objective evidence of symptom aggravation due to mental stress is difficult to identify. We aimed to investigate the relationship between SLE disease activity and mental stress, and the usefulness of saliva as an assessment index for stress in patients with SLE.
We prospectively assessed the salivary stress hormone and disease-related biomarkers, and questionnaire data regarding stress and depression in 100 patients with SLE and 49 sex- and age-matched normal controls (NCs).
Patients with SLE had higher mean salivary α-amylase levels (5.7 ± 4.6 U/mL vs 2.7 ± 2.5 U/mL, P < 0.001), anti-chromatin antibody levels (25.3 ± 22.9 U/mL vs 15.9 ± 10.9 U/mL, P < 0.001), and Beck Depression Index (BDI) scores (11.1 ± 9.2 vs 5.3 ± 5.1, P < 0.001) than NCs. However, salivary cortisol levels and Perceived Stress Scale (PSS) scores did not differ between the groups. The BDI scores correlated with the SLE disease activity index (SLEDAI) scores (r = 0.253, P = 0.011) and erythrocyte sedimentation rates (r = 0.234, P = 0.019). SLE patients with the highest-quartile PSS scores had significantly increased SLEDAI scores compared to those with the lowest-quartile PSS scores after 4 to 5 months’ follow-up. Moreover, SLE patients with elevated SLEDAI scores had higher baseline PSS scores.
Patients with SLE showed uncoupling of the sympathetic nervous system and hypothalamic–pituitary–adrenal axis; higher salivary α-amylase and no different cortisol levels compared with NCs. Also, patients with SLE were more depressed, which correlated with disease activity. Furthermore, perceived stress was not correlated with disease activity; however, disease activity worsened several months later with elevated perceived stress levels.
INTRODUCTION
Systemic lupus erythematosus (SLE) has undulating features that “wax and wane.” Aggravating factors hamper remission or develop disease flare-up. Factors such as viral infection, ultraviolet radiation, drugs, and hormones have been suggested to trigger the onset of SLE.1 Mental distress also has been known to provoke deterioration of disease course; this is a common thought among clinicians and patients suffering for a prolonged period.2 The theoretical background that supports such association is psychoneuroimmunology, which covers the key mechanistic evidence of the communications that connect immune, central nervous (CNS), and endocrine systems. The CNS, which is affected by mental distress, signals the immune system via hormonal and neuronal pathways, and the immune system affects the CNS through diverse cytokines. Immune cells are known to possess receptors for several hormones such as glucocorticoid, substance P, corticotrophin-releasing hormone (CRH), and sex hormone including estrogen and progesterone.3–5 SLE is characterized by diverse dysfunctional features of the immune system, including hyper-reactive immune cells and imbalanced cytokines production; therefore, signals from the neuroendocrine pathway might contribute to worsening of such dysregulation of the immune system in SLE.
The biomarkers used in the assessment of mental stress include cortisol, which reflects hypothalamic–pituitary–adrenal (HPA) axis activity; α-amylase, which represents the function of the autonomic nervous system (ANS); and pro-inflammatory cytokines, which are related to innate immunity.6 Increased psychological stress triggers activation of the HPA axis and related hormones such as CRH, adrenocorticotropic hormone, and cortisol. Especially salivary cortisol level was revealed to be directly proportional to mental stress and has been widely used in stress studies.7,8 In addition, the ANS responds to stress, and then, noradrenergic neurons synthesize and release catecholamines such as dopamine, norepinephrine, and epinephrine. While these biomarkers are measured in blood, α-amylase from acinar cells, which are innervated by sympathetic and parasympathetic branches of the ANS, can be detected in saliva. Therefore, in recent years, salivary α-amylase concentration has been used as a reliable stress marker.9 Pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α, which are involved in innate immunity have been suggested to respond to acute and chronic psychosocial stress.10 As their salivary concentrations are rapidly adjusted according to their blood concentrations, detecting their salivary concentrations is simple and stress-free, making them noninvasive biomarkers of psychological stress or anxiety.11
Anti-chromatin antibody is a well-known biomarker for diagnosis and disease activity of SLE. In our data from 100 SLE and 60 incomplete lupus patients, and 48 normal controls (NCs), significant correlation was found between the level of anti-chromatin antibodies and each of anti-double strand DNA (anti-dsDNA) antibody, leucopenia, complement, and SLE disease activity index (SLEDAI) score.12 The change of anti-chromatin antibody levels showed a positive correlation with the change in SLEDAI score in serial samples. Such reliability has been confirmed in many other studies.13,14 However, no data on salivary anti-chromatin antibody levels have been obtained from patients with SLE.
We investigated salivary cortisol, α-amylase, IL-1β, and anti-chromatin antibody levels as stress and disease activity biomarkers. We use 2 questionnaires to assess stress or depression among the patients with SLE and analyzed the results with disease-related markers for SLE.
MATERIALS AND METHODS
Study Participants
In this prospective study, all 100 patients with SLE and 49 sex- and age-matched healthy NCs from the outpatient rheumatology clinic of Ajou University Hospital were enrolled from August 2011 to August 2012. All of the patients with SLE met the revised American College of Rheumatology classification criteria.15 All of NCs had no history of autoimmune disease. Any patient with SLE and NC with previous history of mental disease or currently stressful condition was excluded. Full medical history and clinical manifestations with medication history were collected by conducting a chart review and interviews. Blood test results, including complete blood count, erythrocyte sedimentation rate (ESR), anti-nuclear antibody, complements (C3 and C4), and anti-dsDNA antibody levels, were collected. All subjects provided informed consents, which was regulated by the institutional review board of our hospital.
Saliva Sample Collection and Questionnaire Survey
Salivary sample collection from the patients with SLE and NCs was performed between 9:00 and 11:00 am because salivary proteins were known as having diurnal variations.16 To minimize the mixture of unwanted impurities in the samples, all the subjects were required not to eat, drink, smoke, or perform oral hygiene procedures for at least 1 h before the sample collection.17 They rinsed the mouth with water and collected their saliva for 5 min. In collection of saliva samples, no agent that stimulates saliva secretion was used, and subjects were asked to keep their mouth closed for a while and expectorate saliva into a tube once per minute.
At the same time of saliva sample collection, the subjects were asked to answer 2 questionnaires, namely the Perceived Stress Scale (PSS) and Beck Depression Index (BDI), which were translated into Korean by using easy-to-understand words and then validated.18,19 Among the 3 versions of PSS, the 10-item version, which has shown good internal consistency and maximum reliability, was used.18 Each question asks about current feelings or status during the last month such as anger, disappointment, and failure to control. Each answer has 1 to 5 points, with 1 point representing “never” and 5 points representing “very often.” The higher the total score, the higher the level of stress perceived by the individual.18,20,21 The BDI consists of 21 items regarding diverse emotions related with depression. It has been used widely as a self-questionnaire to assess early depression.19,22
Salivary Protein Analysis
After the saliva sample collection, the samples were immediately mixed with protease inhibitors to preserve the integrity of the proteins and centrifuged at 3000 rpm for 5 min, resulting in a clear supernatant. Then, the samples were stored at −20°C. Salivary cortisol, α-amylase, and IL-1β concentrations were measured by using an enzyme-linked immunosorbent assay kit (Salimetrics, State College, PA), according to the manufacturer's instructions. Salivary anti-chromatin antibody assay was conducted as previously described with modification.12 Chick chromatin (generous gift from Dr Philip L. Cohen, Temple University) at a concentration of 2.5 μg/mL (100 μL) was added at 96-well microplates (Corning, New York, NY) and then incubated at 4°C for 12 h. The plates were washed 5 times with borate-buffered saline (BBS; 0.5 M boric acid, 0.125 M sodium tetraborate decahydrate, 0.37 M sodium chloride). The plates were blocked with 200 μL of bovine serum albumin-buffered Triton (BBT; BBS 0.5% bovine serum albumin, 0.4% Tween 80) for 2 h at room temperature and were washed with BBS 5 times. Standard whole saliva samples (100 μL) that were twofold serially diluted from a concentration of 1:100 to that of 1:6400 by BBT and saliva samples (100 μL) from the patients or control subjects that were diluted at a concentration of 1:100 were incubated overnight at 4°C and washed 5 times with BBS. Biotinylated goat anti-human immunoglobulin G (1:2000, 100 μL), followed by avidin–alkaline phosphatase (1:4000, 100 μL; Sigma, St. Louis, MO), was added into each well; incubated for 2 h at room temperature; and washed 5 times with BBS. Then, 100 μL of 0.01 M diethanolamine (pH 9.8; Sigma, Steinheim, Germany) mixed with 1 mg/mL phosphatase substrate (Sigma, Steinheim, Germany) was added into each plate. After 4 h, absorbance values were read using an enzyme-linked immunosorbent assay reader at 405 nm. The mean optical density (OD) for each set of duplicate was determined. The reactivity in arbitrary units (AU) for each sample was then calculated by using a standard curve of the mean OD in the stepwise diluted standard serum (r2 = 0.99). The intra- and inter-assay variations were 2.9% and 4.6%, respectively.
Statistical Analysis
A 2-sample Wilcoxon rank-sum (Mann–Whitney) test was conducted to check the statistical significance of the difference between SLE and NCs. Differences in the salivary cortisol, α-amylase, IL-1β, and anti-chromatin antibody levels, and questionnaire scores were compared using the Kruskal–Wallis test. Correlations between each salivary molecules and manifestations, and disease activity markers in the patients with SLE were determined by using the Spearman's rank correlation technique. These were performed by using the Statistical Package for the Social Sciences version 22.0 (IBS Corp., Armonk, NY).
RESULTS
Clinical Characteristics of the Patients With SLE and NCs
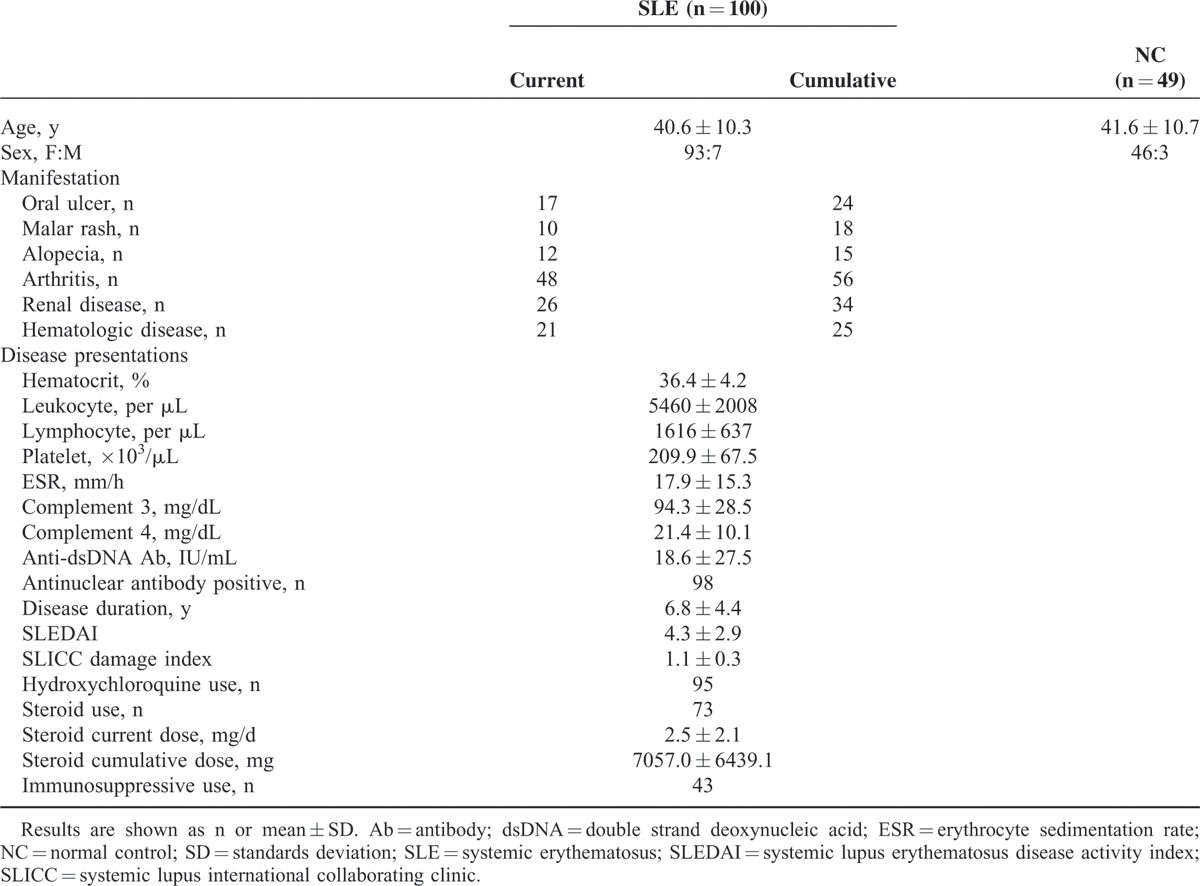
The characteristics of the patients with SLE and NCs are shown in Table 1. The mean age of the patients with SLE was 40.6 ± 10.3 years, which was not different from that of the NCs (41.6 ± 10.7 years, P = 0.42). Seven male patients were included, and the male-to-female ratio did not differ between the groups (P = 0.84). The most common manifestation was arthritis (48/100 cases), followed by renal and hematologic diseases (26/100 and 21/100 cases, respectively). The mean SLEDAI score was 4.3 ± 2.9, and the mean Systemic Lupus International Collaborating Clinics damage index was 1.1 ± 0.3. Ninety-five patients received hydroxychloroquine, and 73 patients received steroids with a mean dosage of 2.5 ± 2.1 mg/d prednisolone equivalent. Moreover, 43 patients received immunosuppressive drugs.
TABLE 1.
Clinical Characteristics of the Patients With SLE and Normal Controls
Salivary Biomarkers and Questionnaire Survey Results
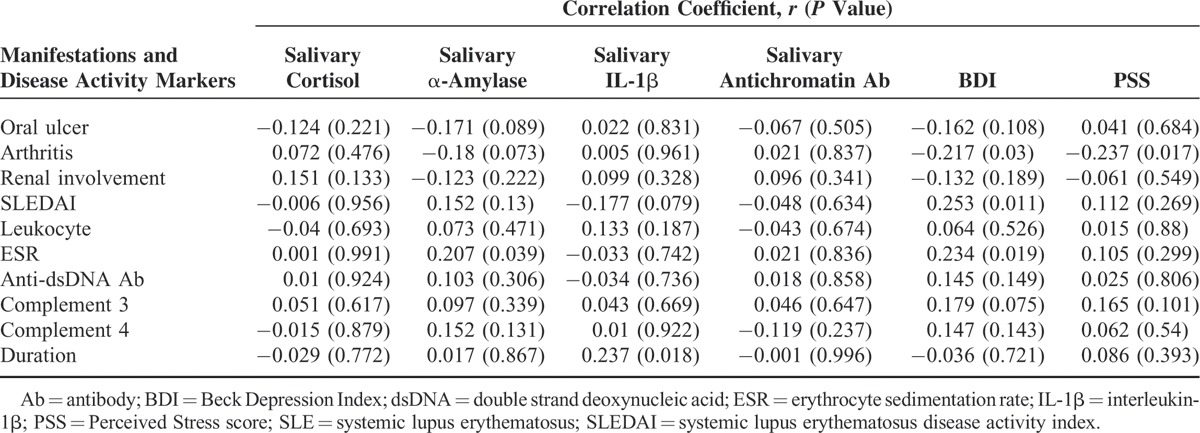
The mean salivary α-amylase level was higher in the patients with SLE (5.7 ± 4.6 U/mL) than in the NCs (2.7 ± 2.5 U/mL, P < 0.001). However, the mean salivary cortisol and IL-1β levels did not significantly differ. The mean anti-chromatin antibody level was higher in the SLE group (25.3 ± 22.9 AU) than in the NC group (15.18 ± 10.9 AU, P < 0.001). The results of the questionnaire survey indicate that the mean BDI score was higher (11.1 ± 9.2) in the SLE group than in the NC group (5.3 ± 5.1, P < 0.001). However, the PSS scores did not significantly differ between the groups (Table 2). When salivary markers were analyzed with the manifestations and disease activity markers of SLE, only salivary IL-1β level correlated with disease duration. BDI score correlated with SLEDAI score and ESR, but PSS score did not correlate with any disease activity marker (Table 3).
TABLE 2.
Questionnaire Scores and Salivary Hormones in Patients with SLE and NC
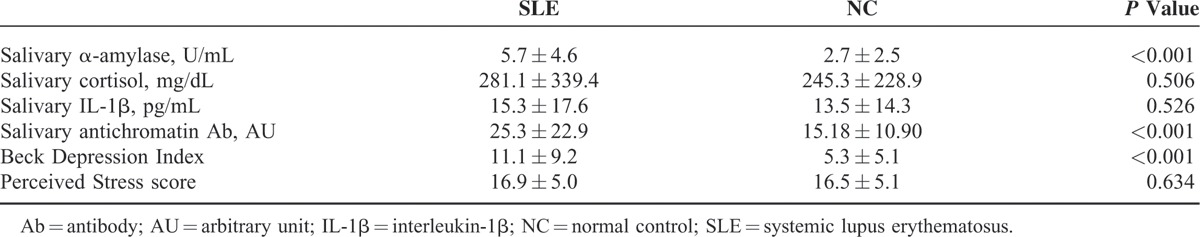
TABLE 3.
Correlations With Manifestations and Disease Activity Markers of SLE and PSS, BDI, and Salivary Hormones
Changes in Disease Activity According to the Salivary Biomarkers and Questionnaire Survey Results
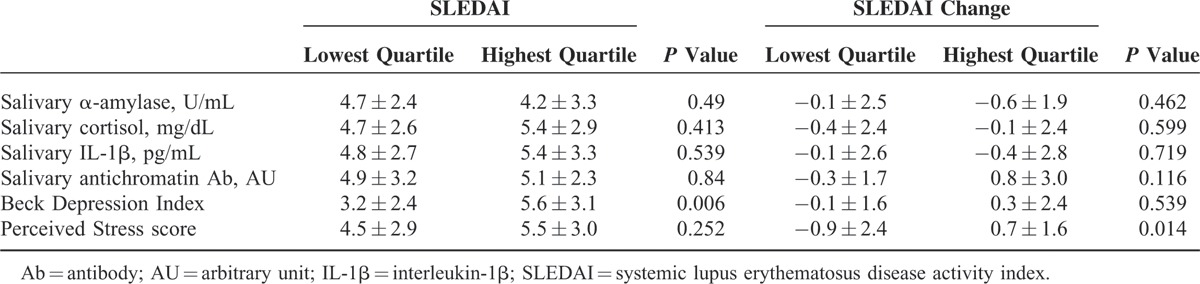
In order to find out changes in disease activity according to mental distress or depression status, patients were divided into groups according to SLEDAI changes during 4 or 5 months’ follow-up. One group consisted of patients with increased SLEDAI score, and the other group consisted of patients with decreased or unchanged SLEDAI score. The SLE patients with increased SLEDAI score had higher initial PSS scores (18.9 ± 5.4) than those with decreased or unchanged SLEDAI score (16.3 ± 4.7, P = 0.029; Table 4). The higher PSS scores at baseline were associated with the increased SLEDAI score at 4 or 5 months’ follow-up (r = 0.212, P = 0.034; Figure 1). Moreover, SLEDAI scores and SLEDAI changes were analyzed between the patient groups that were divided according to quartile of salivary markers and questionnaire scores. Although disease activity and disease activity changes did not differ between the lowest- and highest-quartile groups of salivary markers, SLEDAI scores were significantly higher in the patients with the highest BDI scores than in the patients with the lowest BDI scores (5.6 ± 3.1 vs 3.2 ± 2.4, P = 0.006). SLEDAI changes increased significantly in the patients with the highest PSS scores than in the patients with the lowest PSS scores (0.7 ± 1.6 vs −0.9 ± 2.4, P = 0.014; Table 5).
TABLE 4.
Comparison With Groups Divided by SLEDAI Changes
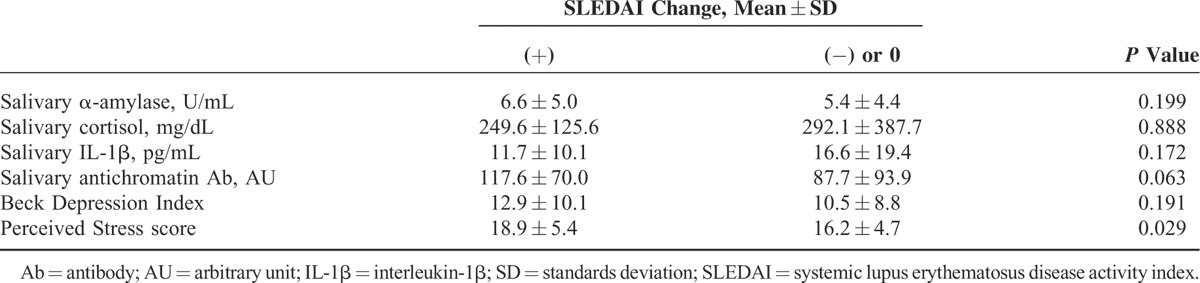
FIGURE 1.
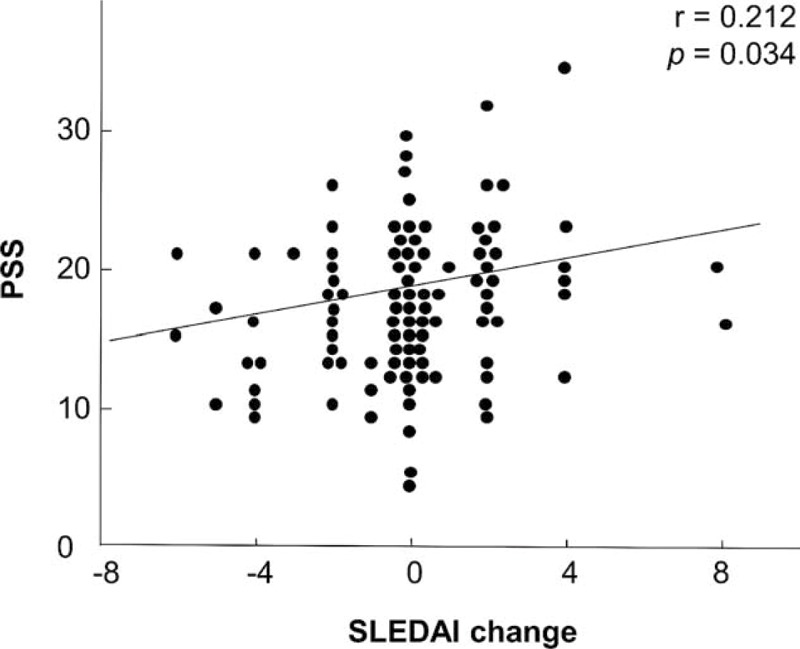
Correlation between changes of disease activity and the PSS scores in patients with SLE. It showed that changes of SLEDAI levels were positively correlated with the PSS scores (r = 0.212, P = 0.034). They were determined using the Spearman's rank correlation technique. PSS = Perceived Stress Scale; SLE = systemic lupus erythematosus; SLEDAI = SLE disease activity index.
TABLE 5.
Disease Activity and Its Change Between the Lowest and Highest Group of Salivary Hormones and Questionnaire Scores
DISCUSSION
In this study, salivary α-amylase and anti-chromatin antibody levels were elevated in the patients with SLE than in the NCs. However, salivary cortisol and IL-1β levels did not differ. The mean BDI score was higher in the SLE group than in the NC group. The SLE patients with high disease activity had high BDI scores. The PSS scores were similar in both groups. In the comparison between the 2 groups divided according to SLEDAI changes during several months, the PSS scores were higher in the SLE patients with increased disease activity than in those with unchanged or decreased disease activity. In addition, the SLE patients with the highest-quartile PSS scores had increased SLEDAI scores at 4 or 5 months’ follow-up, but those with the lowest-quartile PSS scores had decreased SLEDAI scores.
This is the first time that salivary proteins were evaluated in patients with SLE. Most molecules in the blood can transfer to the saliva; thus, the salivary levels of specific molecules represent their blood levels.17 Saliva has been increasingly used as a good diagnostic or disease activity marker in many diseases.23 Without any invasive and painful procedures, saliva can be obtained easily without incurring adverse effects. For this reason, saliva has been used widely in stress research. Moreover, salivary disease-specific markers have been investigated in the field of oral cancer, Sjogren syndrome, asthma, and diabetes.24–27
The elevated salivary α-amylase levels in the patients with SLE compared with the NCs mean that the patients with SLE might had a more-activated stress response than the NCs. Although a large portion of the patients with SLE continuously received small amounts of corticosteroids as treatment of SLE, salivary cortisol levels did not differ significantly between the patients with SLE and the NCs. Continued corticosteroid administration might interrupt increases in cortisol levels due to psychological stimuli, and the results could not represent an intact HPA axis. We found no correlation when we analyzed the relationship between current or cumulative doses of steroids and salivary cortisol levels in the patients with SLE. Pool et al28 reported reduced serum cortisol levels in patients with SLE after exercise and van der Goes et al29 reported significantly different salivary cortisol levels between high and low ESRs in patients with SLE. The serum or salivary cortisol levels in the patients with SLE had some confounding factors such as length of administration, dose, and impairment of the HPA axis.
In addition, our finding of paradoxical salivary α-amylase and cortisol levels in the patients with SLE could represent the abnormal coupling of 2 major endogenous response axes. The sympathetic nervous system (SNS) and HPA axis have been known to cooperatively function in normal conditions, having parallel up-regulations. In rheumatoid arthritis (RA) or lupus mice, the uncoupling of the SNS and the HPA axis, in which SNS tone increases with a defective HPA axis, was demonstrated, and similar results were obtained from patients with RA and SLE.30–32 This uncoupling of the SNS tone and HPA axis supports the continuing inflammatory process.
Psychological effects on SLE disease activity have been studied.33–35 Twenty-two patients with SLE were evaluated for depression and anxiety status, and their effects on SLE disease activity during 2 weeks. It was concluded that psychological distress may change the assessments of disease activity in patients, but have no effect on SLE disease activity itself.36 In a study of 92 patients with SLE underwent a stress reduction program, the intervention showed improvement in pain, psychological function, and perceived physical function at a 9-month follow-up assessment, but no change in SLEDAI score and Systemic Lupus Activity Measure, Revised score.37 Similar to our study in term of BDI scores obtained, an observation study showed that patients with SLE had poorer in psychological function, which is closely related with coping styles influenced by stress, social support, and cognition than patients with RA and NCs.33
Although other factors have shown to contribute to disease deterioration, high stress scores in patients with SLE tended to be associated with worse symptoms. In underlying mechanism, stressors induce hormonal changes through brain stimulation, and the hormones affect diverse immune cells. In detail, mental stressors can stimulate the hypothalamus or pituitary gland, and activate the sympathetic–adrenal–medullary axis and ANS. Increased glucocorticoid, noradrenaline and adrenaline, prolactin, and growth hormone levels affect T and B lymphocytes, NK cells, and monocytes, resulting in the dysregulation of the production of cytokines such as interferon-γ (IFN-γ), IL-1, IL-2, IL-6, and TNF-α.38,39 SLE occurs in genetically susceptible individuals and can be caused by environmental factors, shows diverse immune dysregulation, and results in tissue damage.40 Patients with SLE have been shown to have defective B-cell tolerance, auto-antigen-responsive T-helper cells, functional and biochemical changes in intrinsic T cells with overproduction of auto-antibody, and abnormal cytokine concentrations.41,42 Therefore, hormonal changes may occur because of the effect of mental stress on the already vulnerable immune system of patients with SLE and may exacerbate immune system dysregulation.4 Several cytokines that are known to have imbalanced concentrations in stress conditions, such as IFN-γ, IL-1, and TNF-α, are also major players in the pathogenesis of SLE. Similarly, immunologic deteriorations by stress have been observed in multiple sclerosis (MS), a demyelinating disease with autoimmune inflammation of the CNS.43 It has been found that diverse types of stress are correlated closely with relapse of MS from mice and human studies and activation of HPA axis, microglia, and mast cells by stress are involved in the pathogenesis of MS.
Salivary anti-chromatin antibody levels were elevated in the patients with SLE compared with the NCs. However, they did not correlate with several disease activity markers, including SLEDAI score. The amount of anti-chromatin antibody secreted from blood might differ depending on age, sex, and personal differences. Such confounding factors should be clarified in order to improve the usefulness of salivary molecules as biomarkers.
The limitation of the study is relatively small patients in 1 center. However, SLE is a rare disease and the patient number is enough to reveal the difference. Also, we included age- and sex-matched NCs. There is a need of multicenter study with more patents to generalize our results.
In conclusion, patients with SLE showed uncoupling response of the SNS and HPA axis with higher salivary α-amylase and no different cortisol levels compared with NCs. Also, patients with SLE had more depressive symptoms which correlated with disease activity. Furthermore, stress perceived by patients could contribute to the aggravation of SLE several months later.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr Philip L. Cohen of the Temple University for his generous gift of chick chromatin.
Footnotes
Abbreviations: ANS = autonomic nervous system, Anti-dsDNA = anti-double strand DNA, BDI = Beck Depression Index, CNS = central nervous system, CRH = corticotrophin-releasing hormone, ESR = erythrocyte sedimentation rate, HPA = hypothalamic–pituitary–adrenal, IL = interleukin, MS = multiple sclerosis, NCs = normal controls, PSS = Perceived Stress Scale, SLE = systemic lupus erythematosus, SLEDAI = SLE disease activity index, TNF = tumor necrosis factor.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet 2007; 369:587–596. [DOI] [PubMed] [Google Scholar]
- 2.Roussou E, Iacovou C, Weerakoon A, et al. Stress as a trigger of disease flares in SLE. Rheumatol Int 2013; 33:1367–1370. [DOI] [PubMed] [Google Scholar]
- 3.Wick G, Sgonc R, Lechner O. Neuroendocrine-immune disturbances in animal models with spontaneous autoimmune diseases. Ann N Y Acad Sci 1998; 840:591–598. [DOI] [PubMed] [Google Scholar]
- 4.Szyper-Kravitz M, Zandman-Goddard G, Lahita RG, et al. The neuroendocrine-immune interactions in systemic lupus erythematosus: a basis for understanding disease pathogenesis and complexity. Rheum Dis Clin North Am 2005; 31:161–175. [DOI] [PubMed] [Google Scholar]
- 5.Tan IJ, Peeva E, Zandman-Goddard G. Hormonal modulation of the immune system—a spotlight on the role of progestogens. Autoimmun Rev 2015; 14:536–542. [DOI] [PubMed] [Google Scholar]
- 6.Nater UM, Skoluda N, Strahler J. Biomarkers of stress in behavioural medicine. Curr Opin Psychiatry 2013; 26:440–445. [DOI] [PubMed] [Google Scholar]
- 7.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 1989; 22:150–169. [DOI] [PubMed] [Google Scholar]
- 8.Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009; 34:163–171. [DOI] [PubMed] [Google Scholar]
- 9.van Stegeren A, Rohleder N, Everaerd W, et al. Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology 2006; 31:137–141. [DOI] [PubMed] [Google Scholar]
- 10.Berndt C, Strahler J, Kirschbaum C, et al. Lower stress system activity and higher peripheral inflammation in competitive ballroom dancers. Biol Psychol 2012; 91:357–364. [DOI] [PubMed] [Google Scholar]
- 11.Vining RF, McGinley RA, Maksvytis JJ, et al. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem 1983; 20:329–335. [DOI] [PubMed] [Google Scholar]
- 12.Kim HA, Jeon JY, Choi GS, et al. The antichromatin antibodies can be useful as a diagnostic tool and disease activity marker of systemic lupus erythematosus in Koreans. Clin Immunol 2008; 128:277–283. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Puerta JA, Burlingame RW, Cervera R. Anti-chromatin (anti-nucleosome) antibodies: diagnostic and clinical value. Autoimmun Rev 2008; 7:606–611. [DOI] [PubMed] [Google Scholar]
- 14.Bizzaro N, Villalta D, Giavarina D, et al. Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of meta-analysis. Autoimmun Rev 2012; 12:97–106. [DOI] [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 16.Schubert C, Lampe A, Geser W, et al. Daily psychosocial stressors and cyclic response patterns in urine cortisol and neopterin in a patient with systemic lupus erythematosus. Psychoneuroendocrinology 2003; 28:459–473. [DOI] [PubMed] [Google Scholar]
- 17.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci 1993; 694:72–77. [DOI] [PubMed] [Google Scholar]
- 18.Lee EH, Chung BY, Suh CH, et al. Korean versions of the Perceived Stress Scale (PSS-14, 10 and 4): psychometric evaluation in patients with chronic disease. Scand J Caring Sci 2015; 29:183–192. [DOI] [PubMed] [Google Scholar]
- 19.Hahn H, Yum T, Shin Y, et al. A standardization study of beck depression inventory in Korea. J Korean Neuropsychiatr Assoc 1986; 25:487–502. [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24:385–396. [PubMed] [Google Scholar]
- 21.Hewitt PL, Flett GL, Mosher SW. The Perceived Stress Scale: factor structure and relation to depression symptoms in a psychiatric sample. J Psychopathol Behav Assess 1992; 14:247–257. [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571. [DOI] [PubMed] [Google Scholar]
- 23.Malamud D. Saliva as a diagnostic fluid. Dent Clin N Am 2011; 55:159–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang EH, Lee YJ, Hyon JY, et al. Salivary cytokine profiles in primary Sjogren's syndrome differ from those in non-Sjogren sicca in terms of TNF-alpha levels and Th-1/Th-2 ratios. Clin Exp Rheumatol 2011; 29:970–976. [PubMed] [Google Scholar]
- 25.Little FF, Delgado DM, Wexler PJ, et al. Salivary inflammatory mediator profiling and correlation to clinical disease markers in asthma. PLoS ONE 2014; 9:e84449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zalewska A, Knas M, Niczyporuk M, et al. Salivary lysosomal exoglycosidases profiles in patients with insulin-dependent and noninsulin-dependent diabetes mellitus. Adv Clin Exp Med 2013; 22:659–666. [PubMed] [Google Scholar]
- 27.Lisa Cheng YS, Jordan L, Gorugantula LM, et al. Salivary interleukins 6 and 8 in oral cancer patients and in patients with chronic oral inflammatory diseases. J Periodontol 2014; 85:956–965. [DOI] [PubMed] [Google Scholar]
- 28.Pool AJ, Whipp BJ, Skasick AJ, et al. Serum cortisol reduction and abnormal prolactin and CD4+/CD8+ T-cell response as a result of controlled exercise in patients with rheumatoid arthritis and systemic lupus erythematosus despite unaltered muscle energetics. Rheumatology (Oxford) 2004; 43:43–48. [DOI] [PubMed] [Google Scholar]
- 29.van der Goes MC, Bossema ER, Hartkamp A, et al. Cortisol during the day in patients with systemic lupus erythematosus or primary Sjogren's syndrome. J Rheumatol 2011; 38:285–288. [DOI] [PubMed] [Google Scholar]
- 30.Gudbjornsson B, Skogseid B, Oberg K, et al. Intact adrenocorticotropic hormone secretion but impaired cortisol response in patients with active rheumatoid arthritis: effect of glucocorticoids. J Rheumatol 1996; 23:596–602. [PubMed] [Google Scholar]
- 31.Harle P, Straub RH, Wiest R, et al. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann Rheum Dis 2006; 65:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zietz B, Reber T, Oertel M, et al. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. II. Dissociation between androstenedione, cortisol, or dehydroepiandrosterone and interleukin 6 or tumor necrosis factor. J Rheumatol 2000; 27:911–918. [PubMed] [Google Scholar]
- 33.Kozora E, Ellison MC, Waxmonsky JA, et al. Major life stress, coping styles, and social support in relation to psychological distress in patients with systemic lupus erythematosus. Lupus 2005; 14:363–372. [DOI] [PubMed] [Google Scholar]
- 34.McCracken LM, Semenchuk EM, Goetsch VL. Cross-sectional and longitudinal analyses of coping responses and health status in persons with systemic lupus erythematosus. Behav Med 1995; 20:179–187. [DOI] [PubMed] [Google Scholar]
- 35.Braden CJ, McGlone K, Pennington F. Specific psychosocial and behavioral outcomes from the systemic lupus erythematosus self-help course. Health Educ Q 1993; 20:29–41. [DOI] [PubMed] [Google Scholar]
- 36.Ward MM, Marx AS, Barry NN. Psychological distress and changes in the activity of systemic lupus erythematosus. Rheumatology (Oxford) 2002; 41:184–188. [DOI] [PubMed] [Google Scholar]
- 37.Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis Rheum 2004; 51:625–634. [DOI] [PubMed] [Google Scholar]
- 38.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005; 5:243–251. [DOI] [PubMed] [Google Scholar]
- 39.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol 2002; 20:125–163. [DOI] [PubMed] [Google Scholar]
- 40.Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011; 365:2110–2121. [DOI] [PubMed] [Google Scholar]
- 41.Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther 2011; 13:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 2006; 25:383–392. [DOI] [PubMed] [Google Scholar]
- 43.Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev 2013; 12:947–953. [DOI] [PubMed] [Google Scholar]


