Abstract
This study sought to evaluate the predictive value of the DAP (diameter-axial-polar) nephrometry system on surgical outcomes following partial nephrectomy (PN).
This was a retrospective study of 237 patients who underwent open or minimally invasive PN for renal tumors at a single tertiary care center between 2009 and 2013. The primary outcomes included ischemia time >20 minutes and percentage of estimated glomerular filtration rate (eGFR) decline >10%. Statistical analysis was performed to study associations and predictions.
The DAP sum score exhibited a statistically significant correlation with ischemia time, operative time (OT), estimated blood loss (EBL), length of hospital stay (LOS), and percent change in eGFR. The DAP sum score (odds ratio [OR]: 1.749; 95% confidence interval [CI] 1.379–2.220; P < 0.001) and conventional laparoscopy and laparo-endoscopic single-site (CL&LESS) surgery versus the open surgical approach (OR: 5.736; 95% CI: 2.529–13.011; P < 0.001) independently predicted an ischemia time >20 minutes. Similarly, the DAP sum score (OR: 1.297; 95% CI 1.051–1.602; P = 0.016), age-weighted Charlson comorbidity index (CCI) (OR: 4.730; 95% CI 1.463–15.291; P = 0.009), EBL (OR 2.433; 95% CI 1.095–5.407; P = 0.029), and ischemia time (OR 3.332; 95% CI 1.777–6.249; P < 0.001) were identified as independent predictors of eGFR decline >10%. Furthermore, the DAP score × ischemia time interactions were statistically significant (P < 0.001).
We confirmed the predictive value of the DAP nephrometry score with respect to ischemia time and renal functional decline in an independent external cohort of patients undergoing PN. The effect of the DAP score on renal functional decline partially depends on that of ischemia time, and the individual component DAP scores may have different effects on clinical outcomes.
INTRODUCTION
Studies suggest that localized renal cell carcinoma (T1–2N0M0) is best treated by nephron-sparing surgery (NSS) rather than by radical nephrectomy irrespective of the surgical approach.1–5 Although the ideal outcome remains partial nephrectomy (PN), patient characteristics, tumor radiologic features, surgeon experience, and the availability of technology must be taken into consideration when determining the surgical approach. The morphometric characteristics of renal tumors are likely the most surgically relevant factors for the technical approach of PN. Customarily, surgeons have subjectively evaluated cross-sectional imaging to gain a sense for how to achieve an ideal PN. More recently, new measurements have been developed to systematically and quantitatively assess the most surgically relevant anatomical features of kidney tumors, namely the R.E.N.A.L. (Radius, Exophytic property, Nearness to the collecting system or sinus, Anterior/posterior location, Location relative to the polar lines), PADUA (Preoperative Aspects and Dimensions Used for Anatomical classification), and Centrality (C)-index systems.6–8 As a result, multiple studies have been published, and the results have extended the utility of these nephrometry systems to the prediction of surgical outcomes following PN.9–11 However, the measurement variability and complicated methodology of the above nephrometry systems make the translation of this information into practice difficult. In 2012, a study by Simmons et al described a novel system that integrates the optimized attributes of the R.E.N.A.L. and C-index systems, diameter-axial-polar (DAP) nephrometry. DAP nephrometry exhibits a simplified methodology, decreased measurement variability, and a much stronger correlation with clinical outcomes.12 However, the association of this novel nephrometry system with clinical outcomes requires external validation. Thus, we assessed the validity of the DAP scoring system to predict PN outcomes in an independent external cohort.
MATERIAL AND METHODS
Patient Population
On approval from the Institutional Review Board (IRB) of the Second Military Medical University, we identified 285 consecutive patients undergoing PN via open, conventional laparoscopic, laparoendoscopic single-site, or robotic approaches between 2009 and 2013 at a tertiary referral center (Kidney Cancer Center of Second Military Medical University). Patient information was anonymized and deidentified prior to analysis. The surgical technique and approach (open versus minimally invasive and transperitoneal versus retroperitoneal) were determined at the discretion of the primary urologist on a case-by-case basis. The electronic radiologic database was queried to identify those who had preoperative cross-sectional imaging (computerized tomography or magnetic resonance imaging) for DAP scoring assessment. Digital radiological data were available for 238 (83.5%) subjects, one of whom exhibited multiple ipsilateral tumors and was excluded from the final analysis. The assignment of a DAP score for all identified lesions was made by a radiologist (MML) and an urologist (ZJW) according to the established protocol described by Simmons et al.12
Outcomes
For all of the included patients, the clinical records were retrospectively reviewed, and the following data were extracted: patient age, sex, body mass index (BMI), American Society of Anesthesiologists classification, age-weighted Charlson comorbidity index (CCI) score, operative time (OT), estimated blood loss (EBL), ischemia time, length of hospital stay (LOS), pathological outcomes, intraoperative and postoperative complications, and the perioperative serum creatinine (SCr) levels. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease 2 equation.13 For baseline eGFR, the SCr level preceding surgery (generally within one week before surgery) was used. The follow-up eGFR was estimated based on the most recent available SCr level measured during the clinic oncological visits or the SCr value prior to discharge. The percentage decline in eGFR was calculated as follows:(postoperative eGFR- preoperative eGFR)/preoperative eGFR
Statistical Analysis
Patient demographics and surgical outcomes were presented as frequencies (percentages) for categorical variables and as the median and interquartile range (IQR) for continuous variables.
Associations between the DAP sum score as well as its individual categories and the main surgical outcomes were evaluated using Spearman correlation analysis. A logistic regression model was used for both univariable and multivariable analysis to identify predictors of ischemia time >20 minutes in cases where hilar clamping was used or the percent decline in eGFR >10%. Analyzed variables included patient age (≤50 years vs >50), sex, BMI (≤25 kg/m2 vs >25), American Society of Anesthesiologists score (0–2 vs >2), age-adjusted CCI (0–2 vs >2), clinical tumor size, surgical approach (open vs conventional laparoscopy and laparo-endoscopic single-site [CL&LESS] vs robotic), DAP score, OT (≤3 hours vs >3), and EBL (≤250 mL vs >250). The covariates tested were different according to the outcomes analyzed, and factors at P < 0.2 on univariable analysis were included in the multivariable models. For each reported outcome, we generated 2 models, including the DAP score as a continuous variable (model 1) or the DAP-size adjusted score as a continuous variable (model 2). The DAP-size adjusted score was calculated by adding axial and plane scores regardless of the tumor diameter score. A stepwise selection was used for multivariable analysis to control for the possible confounding effects and the collinearity between predictors. Moreover, interactions between predictors were estimated by multiplicative terms of the form x1 × x2. In both univariable and multivariable analysis, odds ratios (ORs) as well as their 95% confidence intervals (CIs) were presented.
All statistical analyses were performed using IBM SPSS Statistics v.20 (IBM Corp., Armonk, IL, USA). The null hypothesis was rejected for all analyses at P < 0.05, and all P values were 2-tailed.
RESULTS
Table 1 presents the clinical and pathological data of the 237 patients included in the present study. There were 64 (27%), 94 (39.6%), 8 (3.4%), and 71 (30%) patients who underwent open, CL, LESS, and robotic PN, respectively. The median patient age was 51 years (IQR: 43–60) with a median tumor size of 3 cm (IQR: 2.3–4). The median value of the DAP score was 6 (IQR: 5–7), and the median value of the DAP size-adjusted score was 4 (IQR: 3–5). The median ischemia time was 23 minutes (IQR: 16.5–28). The median follow-up time was 18 months (IQR: 6–24). Figure 1 presents the histogram analysis of the DAP score distribution. The DAP sum score as well as its component scores of diameter and axial scoring were normally distributed, although the polar scoring tended to exhibit an even distribution.
TABLE 1.
Baseline Characteristics and Surgical Outcomes of 237 Patients Analyzed in the Study
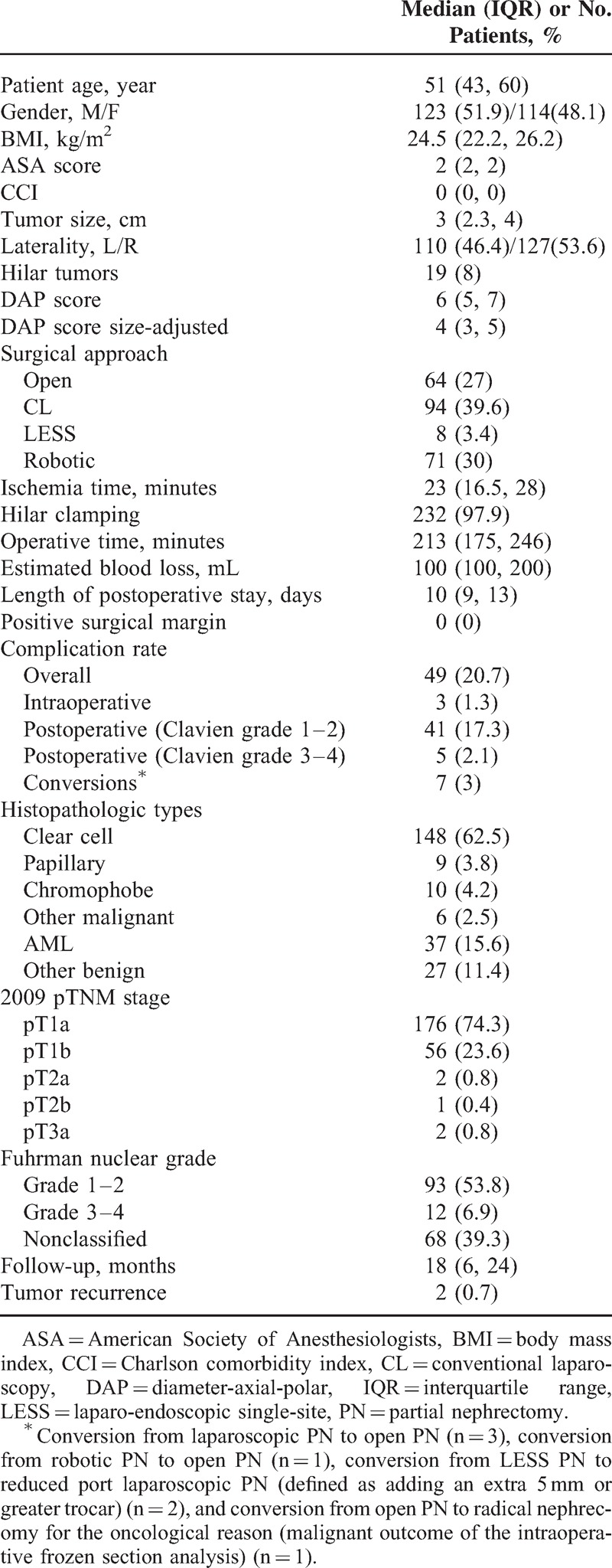
FIGURE 1.
Histogram demonstrating the distribution of the DAP score and its categories. DAP = diameter-axial-polar.
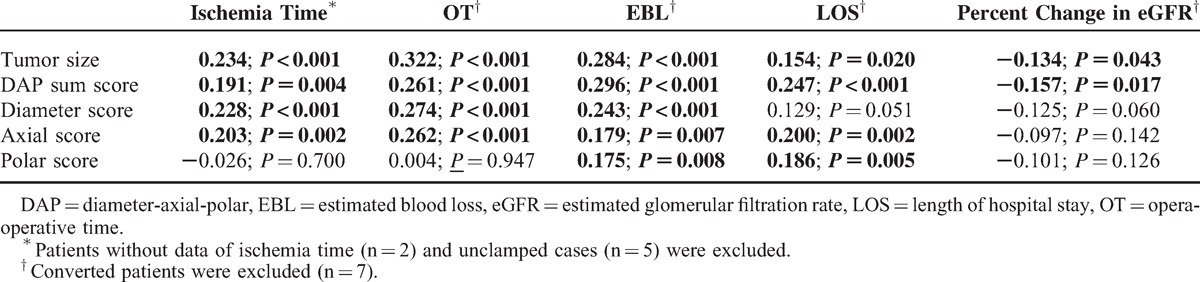
Table 2 presents the correlations between tumor size and the DAP score as well as its individual item scores with ischemia time, OT, EBL, LOS, and percent change in eGFR. Both the DAP sum score and tumor size exhibited a significant correlation with all tested parameters. However, the component scores of DAP nephrometry otherwise did not generally exhibit a statistically significant association with the examined variables. The diameter scoring and axial scoring performed better than the polar scoring, which was significantly correlated with only EBL and LOS. None of the DAP component scores exhibited a significant correlation with the percent change in eGFR.
TABLE 2.
Correlation Between Morphometric Characteristics of Kidney Tumors and Surgical Outcomes (Coefficient, P Value) (n = 230)
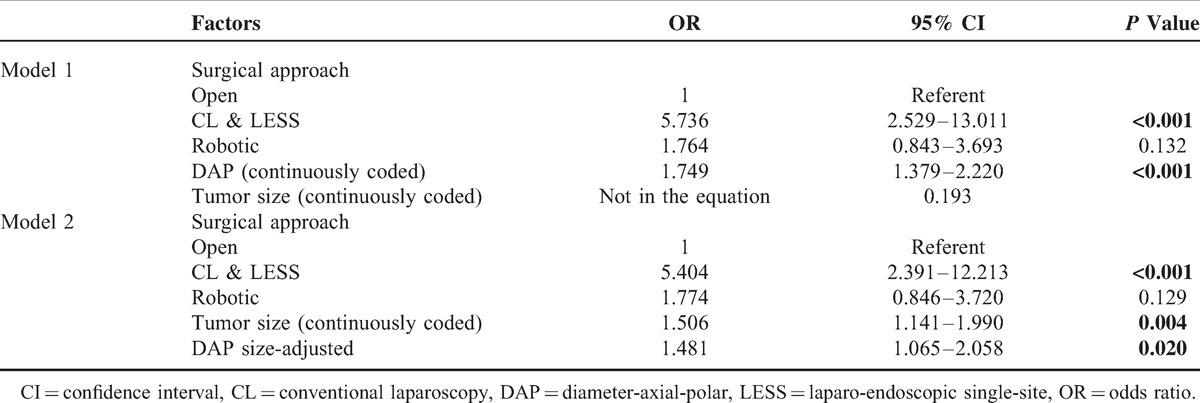
Ischemia time longer than 20 minutes was observed in 137 patients (59.6%). In univariable analysis, tumor size (P = 0.002), surgical approach (P = 0.015), DAP score (P = 0.002), and DAP size-adjusted score were predictive of ischemia time >20 minutes, whereas patient age, sex, BMI, tumor side, and robotic versus open surgical approach were not statistically significant (Table 3). On multivariable logistic regression, only the DAP sum score (OR: 1.749; 95%CI: 1.379–2.220; P < 0.001) and CL&LESS versus open surgical approach (OR: 5.736; 95%CI: 2.529–13.011; P < 0.001) independently predicted an ischemia time >20 minutes (Table 4, Model 1). Moreover, the DAP size-adjusted score (OR: 1.481; 95%CI: 1.065–2.058; P = 0.020) was able to predict ischemia time >20 minutes irrespective of clinical tumor size (OR: 1.506; 95%CI: 1.141–1.990; P = 0.004) and surgical approach (CL&LESS vs open, OR: 5.404; 95%CI: 2.391–12.213; P < 0.001; robotic vs open, OR: 1.774; 95%CI: 0.846–3.720; P = 0.129) (Table 4, Model 2).
TABLE 3.
Univariate Analysis to Predict Ischemia Time >20 min (n = 230)
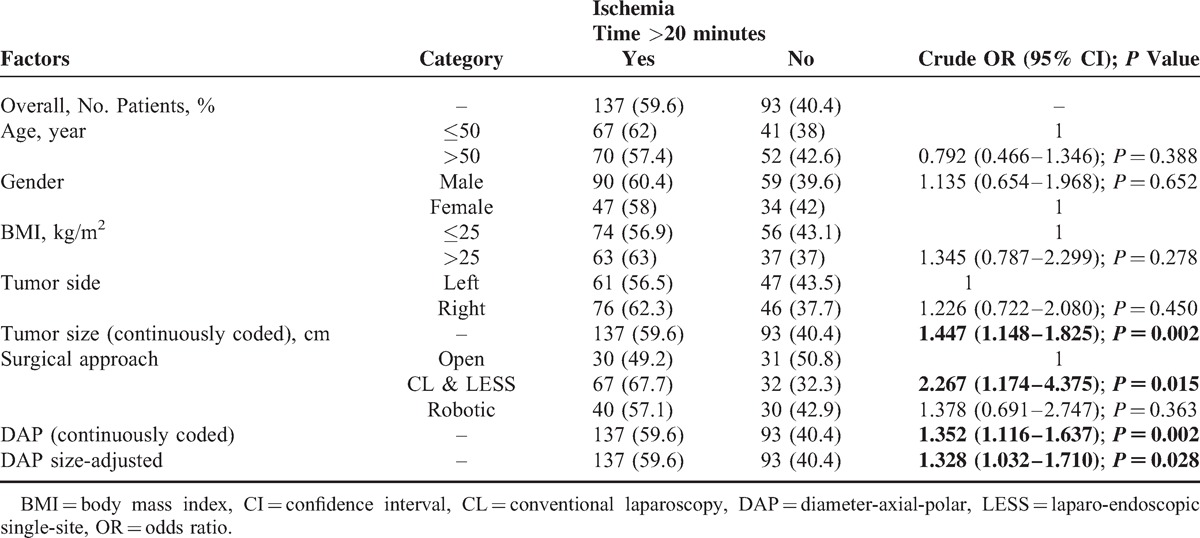
TABLE 4.
Multivariate Models Predicting Ischemia Time >20 minutes (n = 230)
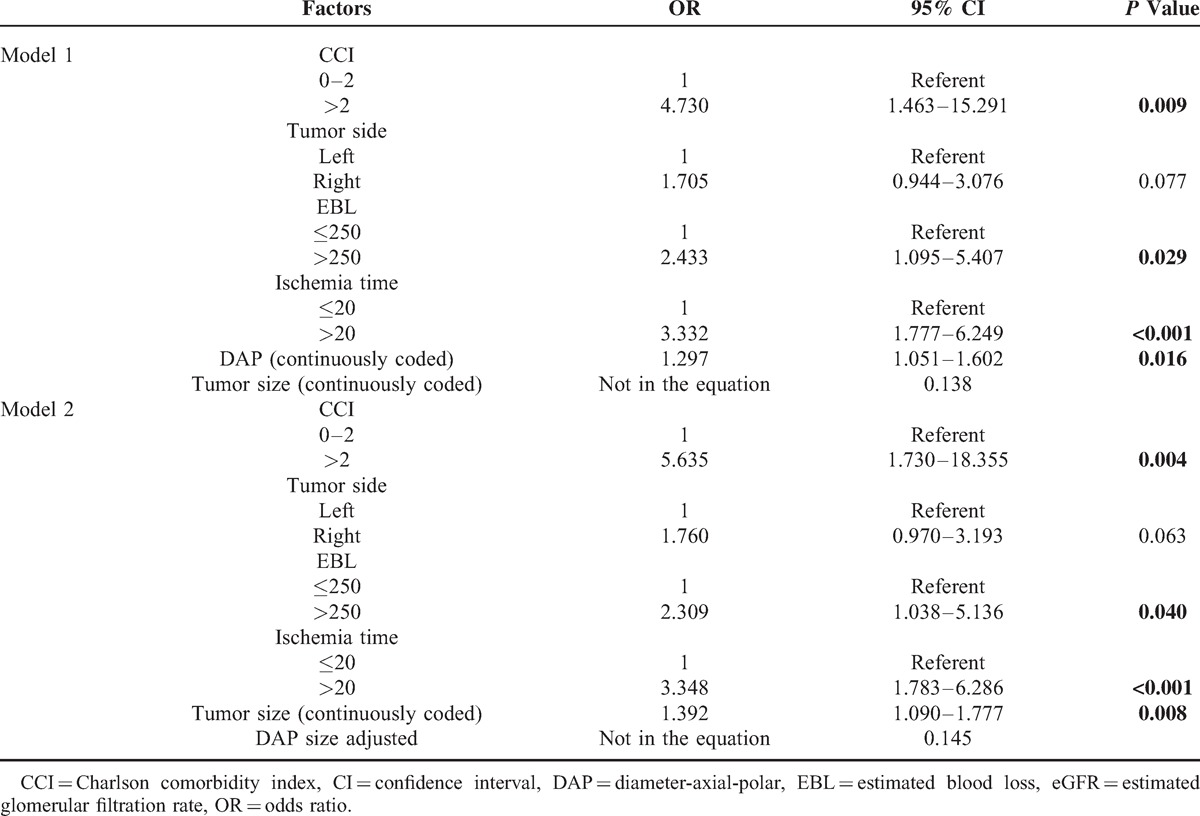
With respect to postoperative renal functional impairment, a decrease in eGFR greater than 10% was observed in 116 (50.7%) patients. Univariable analysis revealed that tumor size (P = 0.002), the DAP sum score (P = 0.001), the DAP size-adjusted score (P = 0.002), ischemia time (P < 0.001), and EBL (P = 0.002) were potential risk factors for eGFR decline >10% (Table 5). In multivariable analysis, the DAP sum score (OR: 1.297; 95%CI: 1.051–1.602; P = 0.016) was an independent predictor of eGFR decline >10% as well as CCI (OR: 4.730; 95%CI: 1.463–15.291; P = 0.009), EBL (OR: 2.433; 95%CI: 1.095–5.407; P = 0.029), and ischemia time (OR: 3.332; 95%CI: 1.777–6.249; P < 0.001) (Table 6, Model 1). However, the DAP size-adjusted score (P = 0.145) was not significantly associated with an eGFR decline >10% after adjustment for the effects of CCI (OR: 5.635; 95%CI: 1.730–3.193; P = 0.004), EBL (OR: 2.309; 95%CI: 1.038–5.136; P = 0.040), and ischemia time (OR: 3.348; 95%CI: 1.783–6.286; P < 0.001) (Table 6, Model 2). Nevertheless, the interactions of both DAP score × ischemia time (OR: 1.221; 95%CI: 1.104–1.350; P < 0.001) and tumor size × ischemia time (OR: 1.448; 95%CI: 1.237–1.693; P < 0.001) were statistically significant.
TABLE 5.
Univariate Analysis to Predict eGFR Decline >10% in Percentage (n = 229)∗
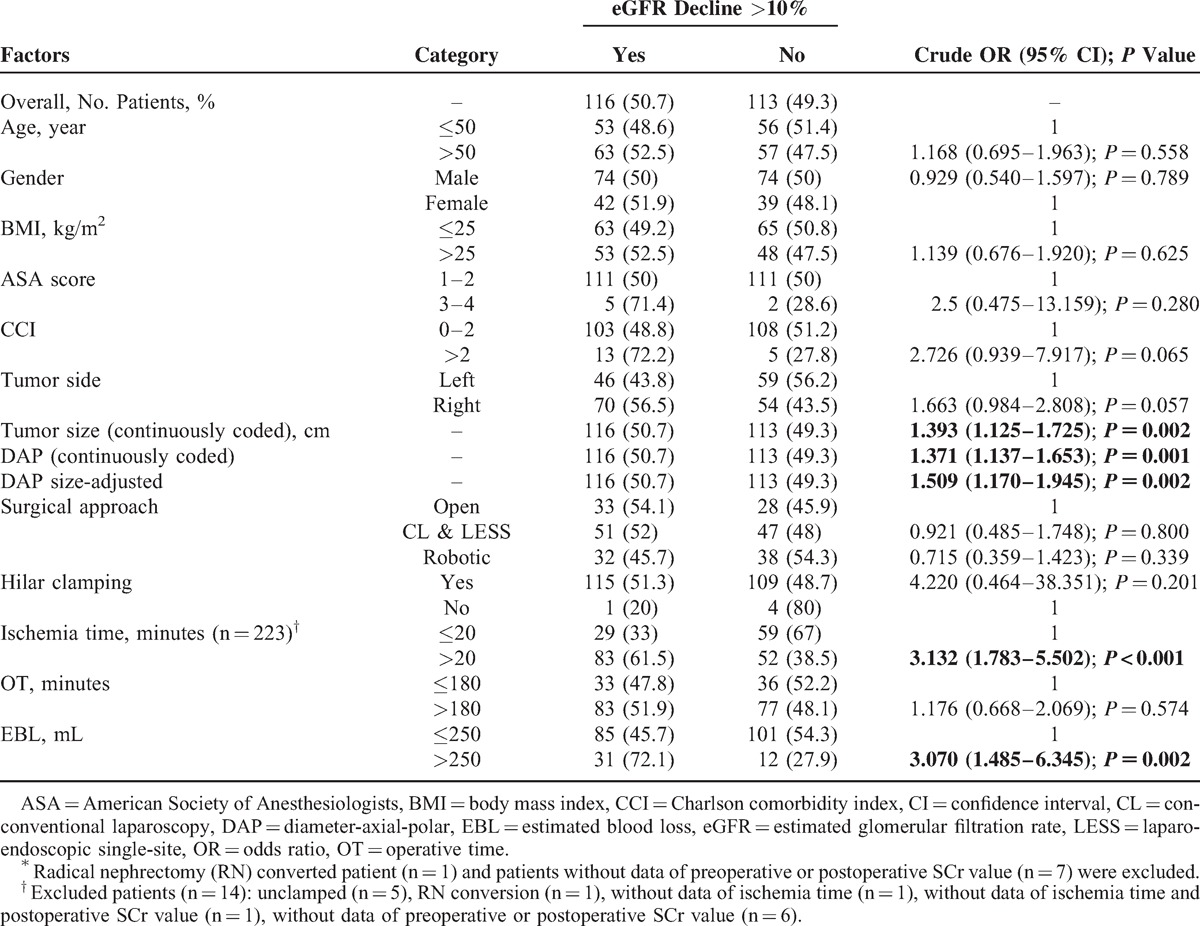
TABLE 6.
Multivariate Models Predicting eGFR Decline >10% in Percentage (n = 223)
DISCUSSION
Our data confirmed the correlations of the DAP nephrometry score with clinical outcomes and demonstrated that the DAP nephrometry score is an independent predictor of both ischemia time and renal function decrease in patients who undergo PN with 2 models. Furthermore, our results provide additional insights into the different effects of individual DAP nephrometry component scores on PN patient outcomes.
Using linear regression analysis, Simmons et al12 reported strong associations between all DAP scoring parameters and clinical outcomes, including warm ischemia time, EBL, and percent functional volume preservation (each P < 0.001). Nevertheless, the coefficients and 95% CIs were not reported in their study, and tumor size as a continuous variable was not included in the multivariable analysis, which precludes the in-depth comparison of data generated by different cohorts. Moreover, we believe that such results would be more convincing using a conservative nonparameter statistical test due to the nonnormal distribution and heterogeneity of variance of most surgical data in a size-limited sample. The relevance of scoring variables in the current study was determined by Spearman correlation analysis. Our results suggest that both the DAP sum score and tumor size were significantly associated with ischemia time, OT, EBL, LOS, and percent change in eGFR, which indicates the key role of tumor size as a component score in the DAP nephrometry system.
However, we failed to confirm the consistent correlations of 3 DAP component scores with the outcomes examined. Specifically, the diameter score and axial score performed better than polar score. The histogram analysis confirmed higher discriminatory power for the diameter score and axial score, where 27% versus 16% and 21% versus 31% of scores were in the low and high score categories, respectively, whereas the polar score exhibited an even distribution. However, the discrepancy in the associations of component scores with clinical outcomes may also reflect surgeon habits. For instance, surgeons are used to obtaining surgically relevant information from cross-sectional images, including maximum diameter, endophytic/exophytic property, and other morphologic information, which could help explain the greater contributions of diameter score and axial score to surgical complexity. It is equally interesting to note that only the DAP sum score was significantly associated with the percentage of eGFR decline and that the DAP sum score performed marginally better than tumor size. These results were confirmed in multivariable analysis (Tables 5 and 6). Thus, these findings may be explained by the approximately linear association of the DAP sum score with the percent functional volume preservation following PN.12 All 3 items of the DAP nephrometry system are required to appropriately estimate 3-dimensional morphologic changes in the operated kidney. In our previous study, the DAP sum score was found to be significantly associated with the unilateral GFR decline in the operated kidney using 99TcmDTPA scintigraphy evaluation.14
To achieve standardized outcomes reporting of NSS, investigators recently proposed the “trifecta” and MIC (margin, ischemia, and complications) systems to determine the ideal outcome.15,16 Both systems take into account 3 variables for evaluating the safety and efficacy of NSS: surgical margin status, ischemia/renal functional damage, and complications. For the no occurrence of positive margin and few events of high-grade complications in our cohort, we assessed only the relevance of the DAP nephrometry system to ischemia time and renal functional impairment.
We used 20 minutes as the cut-off value for ischemia time according to the recommendations proposed by a panel of experts and the definition of the MIC concept.15,17 After adjusting for the surgical approach, each 1-unit score increase in the DAP score was associated with a 1.749-fold higher risk of ischemia time >20 minutes. For example, patients with a score of 9 would have a 28.6-fold higher risk than those with a score of 3. In this way, the DAP nephrometry system can be used as a user-friendly algorithm, and its association with ischemia time becomes more intuitive. Notably, our analysis also demonstrated that the anatomic features of axial and polar location included in the DAP size-adjusted model were able to predict ischemia time >20 minutes regardless of the tumor size and surgical approach. This result implies that the anatomical characteristics of kidney tumors, apart from size, can be incorporated into the DAP nephrometry system to impart information that could be translated into patient benefit. In a multicenter, international series of robot-assisted PN conducted by Ficarra et al,18 the PADUA size-adjusted score was found to be an independent predictor of ischemia time longer than 20 min.
The median decrease in renal function in PN patients with a normal contralateral kidney ranges from 0% to 19% in literature reports.11,12,14,19–22 Some authors have reported that the preoperative anatomic characteristics of treated tumors are predictive of the postprocedure renal function damage. Specifically, Cha et al22 reported a median 12% decrease in eGFR at a median follow-up of 38 months, and higher R.E.N.A.L. nephrometry scores were correlated with greater renal functional decline. Similarly, Simmons et al12 reported that R.E.N.A.L., C-index, and the optimized combination form of DAP were consistently and strongly correlated with functional preservation.23 Conversely, Bylund et al11 failed to confirm a relationship between any of the R.E.N.A.L., C-index or PADUA scoring systems, and postoperative functional outcomes.11 In the current study, we confirmed that the DAP sum score and ischemia time were able to predict renal function decline. However, multiple studies have demonstrated, in addition to the present analysis, that the anatomic complexity of renal tumors has a strong correlation with ischemia time. Thus, we further studied the interactions between the DAP score and tumor size and ischemia time. With respect to the functional outcome, the effect of the DAP score at least partially depends on that of ischemia time. However, we do not deny the possibility that the effect of the DAP score can be interpreted alone because its association with the percent functional volume preservation is nearly linear (r2 = 0.97).12 Indeed, the collinearity and interactions between strongly correlated predictors should be evaluated, which may help explain the discrepancy in the effectiveness of nephrometry systems and ischemia time on renal function impairment after PN in the literature.11,19,23–25
The main limitations of this study are that it represents a retrospective analysis collected from a single surgeon's series with a limited number of patients and potential effects of measurement variability. Moreover, patients with a solitary kidney were enrolled (n = 6, 2.5%). Finally, the percent eGFR decrease was not adjusted according to the preserved kidney volume, and the percent functional volume preservation was not captured.
CONCLUSION
The predictive value of the DAP nephrometry score on ischemia time and renal function decline was confirmed in an independent external cohort of patients undergoing PN, and the individual component DAP scores may have different effects on these outcomes. However, our findings need to be further confirmed in a well-designed prospective global multi-institutional study.
Acknowledgments
The authors thank Scientific and Technological Talents Project of Shanghai (13XD1400100), the Scientific and Technological Medical Guiding Program of Shanghai 2015 (Clinical Study of LESS surgery in Urology), and The “Leading Talent” Project of Shanghai (2013046) for the support.
Footnotes
Abbreviations: DAP = diameter-axial-polar, PN = partial nephrectomy, CL = conventional laparoscopy, LESS = laparo-endoscopic single-site, eGFR = estimated glomerular filtration rate, SCr = serum creatinine, BMI = body mass index, CCI = charlson comorbidity index, ASA = American Society of Anesthesiologists, OT = operative time, EBL = estimated blood loss, LOS = length of hospital stay, OR = odds ratio, CI = confidence interval, IQR = interquartile range.
Mingmin Li, Yi Gao, Jiwen Cheng, and Le Qu share the first authorship of this article.
The abstract of this article has been posted at 2015 Conference of American Urological Association.
This work has been supported by the Scientific and Technological Talents Project of Shanghai (13XD1400100), the Scientific and Technological Medical Guiding Program of Shanghai 2015 (Clinical Study of LESS surgery in Urology), The “Leading Talent” Project of Shanghai (2013046).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.B, Bensalah K, Bex A, et al. Guidelines on Renal Cell Carcinoma. Uroweb 2013. Available at: http://www.uroweb.org/gls/pdf/10_Renal_Cell_Carcinoma_LRV2.pdf [Accessed January 28, 2014]. [Google Scholar]
- 2.MacLennan S, Imamura M, Lapitan MC, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol 2012; 61:972–993. [DOI] [PubMed] [Google Scholar]
- 3.MacLennan S, Imamura M, Lapitan MC, et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol 2012; 62:1097–1117. [DOI] [PubMed] [Google Scholar]
- 4.Weight CJ, Larson BT, Gao T, et al. Elective partial nephrectomy in patients with clinical T1b renal tumors is associated with improved overall survival. Urology 2010; 76:631–637. [DOI] [PubMed] [Google Scholar]
- 5.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA 2012; 307:1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method. J Urol 2010; 183:1708–1713. [DOI] [PubMed] [Google Scholar]
- 7.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009; 182:844–853. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 2009; 56:786–793. [DOI] [PubMed] [Google Scholar]
- 9.Weight CJ, Atwell TD, Fazzio RT, et al. A multidisciplinary evaluation of inter-reviewer agreement of the nephrometry score and the prediction of long-term outcomes. J Urol 2011; 186:1223–1228. [DOI] [PubMed] [Google Scholar]
- 10.Okhunov Z, Shapiro EY, Moreira DM, et al. R.E.N.A. L. nephrometry score accurately predicts complications following laparoscopic renal cryoablation. J Urol 2012; 188:1796–1800. [DOI] [PubMed] [Google Scholar]
- 11.Bylund JR, Gayheart D, Fleming T, et al. Association of Tumor Size, Location, R.E.N.A.L., PADUA and Centrality Index Score with Perioperative Outcomes and Postoperative Renal Function. J Urol 2012; 188:1684–1689. [DOI] [PubMed] [Google Scholar]
- 12.Simmons MN, Hillyer SP, Lee BH, et al. Diameter-axial-polar nephrometry: integration and optimization of R.E.N.A.L. and centrality index scoring systems. J Urol 2012; 188:384–390. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Li M, Chen W, et al. Is diameter-axial-polar scoring predictive of renal functional damage in patients undergoing partial nephrectomy? An evaluation using technetium Tc 99m ((9)(9)Tcm) diethylene-triamine-penta-acetic acid (DTPA) glomerular filtration rate. BJU Int 2013; 111:1191–1198. [DOI] [PubMed] [Google Scholar]
- 15.Buffi N, Lista G, Larcher A, et al. Margin, ischemia, and complications (MIC) score in partial nephrectomy: a new system for evaluating achievement of optimal outcomes in nephron-sparing surgery. Eur Urol 2012; 62:617–618. [DOI] [PubMed] [Google Scholar]
- 16.Hung AJ, Cai J, Simmons MN, et al. “Trifecta” in partial nephrectomy. J Urol 2013; 189:36–42. [DOI] [PubMed] [Google Scholar]
- 17.Becker F, Van Poppel H, Hakenberg OW, et al. Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 2009; 56:625–634. [DOI] [PubMed] [Google Scholar]
- 18.Ficarra V, Bhayani S, Porter J, et al. Predictors of warm ischemia time and perioperative complications in a multicenter, international series of robot-assisted partial nephrectomy. Eur Urol 2012; 61:395–402. [DOI] [PubMed] [Google Scholar]
- 19.Khalifeh A, Autorino R, Eyraud R, et al. Three-year oncologic and renal functional outcomes after robot-assisted partial nephrectomy. Eur Urol 2013; 64:744–750. [DOI] [PubMed] [Google Scholar]
- 20.Choi JD, Park JW, Choi JY, et al. Renal damage caused by warm ischaemia during laparoscopic and robot-assisted partial nephrectomy: an assessment using Tc 99m-DTPA glomerular filtration rate. Eur Urol 2010; 58:900–905. [DOI] [PubMed] [Google Scholar]
- 21.Mufarrij PW, Krane LS, Rajamahanty S, et al. Does nephrometry scoring of renal tumors predict outcomes in patients selected for robot-assisted partial nephrectomy? J Endourol 2011; 25:1649–1653. [DOI] [PubMed] [Google Scholar]
- 22.Cha EK, Ng CK, Jeun B, et al. Preoperative radiographic parameters predict long-term renal impairment following partial nephrectomy. World J Urol 2013; 31:817–822. [DOI] [PubMed] [Google Scholar]
- 23.Simmons MN, Hillyer SP, Lee BH, et al. Nephrometry score is associated with volume loss and functional recovery after partial nephrectomy. J Urol 2012; 188:39–44. [DOI] [PubMed] [Google Scholar]
- 24.Buethe DD, Moussly S, Lin HY, et al. Is the R.e.N.a.L. Nephrometry scoring system predictive of the functional efficacy of nephron sparing surgery in the solitary kidney? J Urol 2012; 188:729–735. [DOI] [PubMed] [Google Scholar]
- 25.Png KS, Bahler CD, Milgrom DP, et al. The role of R.E.N. A. L. nephrometry score in the era of robot-assisted partial nephrectomy. J Endourol 2013; 27:304–308. [DOI] [PubMed] [Google Scholar]


