Abstract
Swept-source optical coherence tomography (OCT) is the latest advancement in anterior segment imaging. There are limited data regarding its performance after laser in situ keratomileusis (LASIK). We compared the reliability of swept-source OCT and Scheimpflug imaging for evaluation of corneal parameters in refractive surgery candidates with myopia or myopic astigmatism. Three consecutive measurements were obtained preoperatively and 1 year postoperatively using swept-source OCT and Scheimpflug imaging. The study parameters included central corneal thickness (CCT), thinnest corneal thickness (TCT), keratometry at steep (Ks) and flat (Kf) axes, mean keratometry (Km), and, anterior and posterior best fit spheres (Ant and Post BFS). The main outcome measures included reliability of measurements before and after LASIK was evaluated using intraclass correlation coefficient (ICC) and reproducibility coefficients (RC). Association between the mean value of corneal parameters with age, spherical equivalent (SEQ), and residual bed thickness (RBT) and association of variance heterogeneity of corneal parameters and these covariates were analyzed. Twenty-six right eyes of 26 participants (mean age, 32.7 ± 6.9 yrs; mean SEQ, −6.27 ± 1.67 D) were included. Preoperatively, swept-source OCT demonstrated significantly higher ICC for Ks, CCT, TCT, and Post BFS (P ≤ 0.016), compared with Scheimpflug imaging. Swept-source OCT demonstrated significantly smaller RC values for CCT, TCT, and Post BFS (P ≤ 0.001). After LASIK, both devices had significant differences in measurements for all corneal parameters (P ≤ 0.015). Swept-source OCT demonstrated a significantly higher ICC and smaller RC for all measurements, compared with Scheimpflug imaging (P ≤ 0.001). Association of variance heterogeneity was only found in pre-LASIK Ant BFS and post-LASIK Post BFS for swept-source OCT, whereas significant association of variance heterogeneity was noted for all measurements except Ks and Km for Scheimpflug imaging.
This study reported higher reliability of swept-source OCT for post-LASIK corneal measurements, as compared with Scheimpflug imaging. The reliability of corneal parameters measured with Scheimpflug imaging after LASIK was not consistent across different age, SEQ, and RBT measurements. These factors need to be considered during follow-up and evaluation of post-LASIK patients for further surgical procedures.
INTRODUCTION
The precise measurement of corneal topography is essential for preoperative evaluation and monitoring of eyes undergoing refractive surgery. The accuracy of biometric parameters is equally important for future enhancement procedures, intraocular lens power calculation, and early detection of postoperative keratectasia.1,2 Several sophisticated instruments have been developed for the evaluation of anterior and posterior corneal surface including scanning-slit elevation topography, Scheimpflug imaging, and optical coherence tomography (OCT).3 Scanning-slit elevation topography utilizes a combination of a projective technique and a reflection technique based on Placido disc principle. Scheimpflug imaging provides a high depth of focus with minimal distortion by alternation of the lens and film planes during acquisition of anterior segment scans. OCT works on the principle of low-coherence interferometry, with time-domain and Fourier-domain methods of data acquisition and processing.
The reliability of modern tomography machines has been widely validated in normal corneas.4–10 Few cross-sectional studies also attempted to evaluate the precision of these devices in eyes that have undergone laser refractive surgery.11–15 Scanning-slit elevation topography has been demonstrated to be inferior to Scheimpflug imaging or time-domain OCT for measurement of corneal thickness after laser refractive surgery.12,16,17 Fourier-domain OCT, which has a higher resolution and acquisition speed, offers better reliability for corneal biometric measurements compared with its time-domain counterpart.18,19 It was also reported to have higher repeatability and reproducibility for pachymetric measurements compared with Scheimpflug imaging in normal,20–23 as well as postlaser in situ keratomileusis (LASIK) corneas,24 although both techniques offered highly precise measurement of corneal thickness.
The introduction of swept-source OCT for ocular imaging allows improved sensitivity and signal-to-noise ratio compared with the previous models of spectral-domain OCT.25,26 In a recently published study, we observed similar reliability between swept-source OCT and spectral-domain OCT with excellent interdevice agreement for measurement of LASIK flap thickness.27 The purpose of the present study was to compare the reliability of corneal topographic measurements between swept-source OCT and Scheimpflug imaging in eyes before, and 1 year after femtosecond-assisted laser-assisted keratomileusis (LASIK).
METHODS
This was a prospective, comparative study conducted at the Refractive Surgery Clinic of the Chinese University of Hong Kong Eye Centre between July 2013 and December 2014. An informed consent was obtained from all participants. An Institutional Review Board approved the conduct of the study. The study adhered to the tenets of the Declaration of Helsinki. All patients underwent a complete ophthalmic examination and had no ocular abnormality, except myopia or myopic astigmatism with a best-corrected distance visual acuity of 20/20 or better in both eyes. Patients with a stable refraction for more than 1 year, myopia of ≥3.0 D bilaterally, and anisometropia of ≤1 D were included. Patients with recent contact lens wear (rigid contact lens ≤4 weeks and soft contact lens ≤2 weeks), suspicion of keratoconus on corneal topography (displacement of the corneal apex, decrease in thinnest-point pachymetry, asymmetric topographic pattern), active ocular pathology, and any history of ophthalmic surgery were excluded. All eyes underwent a complete ophthalmic assessment, including measurement of visual acuity and refraction, slit-lamp and fundus examination, and corneal topography before recruitment into the study.
Instruments
Swept-Source Optical Coherence Tomography
Casia (Casia SS-1000, Tomey, Nagoya, Japan) is a swept-source anterior segment-OCT that uses a wavelength of 1310 nm and measures with a speed of 30,000 axial scans per second. The axial and transverse resolution of the device is <10 and 30 μm, respectively. It can perform large depth scans with 6.0 mm tissue penetration and 16.0 mm × 16.0 mm horizontal and vertical scan ranges. In the anterior-segment mode, each 3D image consists of 128 B-scans (cross-sectional images) and 512 A-scans. In the corneal-map mode, each 3D image contains 16 B-scans and 512 A-lines. The Topo-Pachy-Map scan protocol was used in the present study. It comprises evenly spaced 16 radial B-scans. The total scan duration is 0.3 seconds for measurement of corneal thickness and corneal topography. The topographic data of both anterior and posterior corneal surfaces, as well as cornea thickness, were obtained from the map.
During image acquisition, the patient's chin was positioned with forehead touching the headrest while the patient was instructed to look at the internal fixation target. The scan was initiated when a cross-sectional image of the cornea was visualized on a computer screen. Patients were asked to blink in between consecutive scans. Collected data are processed by the system to achieve cross-sectional images. The topography maps of both corneal surfaces and cornea thickness are calculated. Subsequently, the sphere is fitted (best-fit sphere) to anterior and posterior cornea surfaces, and differences between the fitted surface and real data are plotted on elevation maps. A fit zone diameter of 8 mm was applied.
Scheimpflug Imaging
Pentacam (Pentacam HR, Oculus, Wetzlar, Germany) captures 100 slit images with a slit depth of 14.0 mm in 2 seconds by rotating along the optical axis from 0 to 360°. It evaluates more than 138,000 true elevation points. The participants were instructed to fixate upon the red central fixation target and keep their eyes wide open just before image capture. Once aligned correctly, Pentacam's digital camera (1.45-megapixel) and slit illumination system (475-nm monochromatic slit of light) automatically rotate around the corneal apex to capture cross-sectional Scheimpflug images of the anterior eye, each separated by 3.6°. Patients were asked to blink in between consecutive scans. Any measurements that are unreliable because of poor alignment, excessive eye movements, or any missing or invalid data are flagged. Scans that were registered as “OK” on the instrument's Examination Quality Specification were included for analysis. A fit zone diameter of 8 mm was applied.
Measurement Technique
Three consecutive measurements were obtained for all eyes during a single sitting for each device. All measurements were obtained between 10 am and 4 pm under dim room illumination by a single examiner. The study parameters included central corneal thickness (CCT), thinnest corneal thickness (TCT), keratometry at steep (Ks) and flat (Kf) axes, mean keratometry (Km), and anterior and posterior best fit spheres (Ant and Post BFS). Measurements were performed preoperatively and 1 year after LASIK.
Surgical Procedure
The corneal flaps were created using a 150-kHz IntraLase femtosecond laser platform (Abott Medical Optics, Chicago, IL). All flaps had a superior hinge. The intended thickness and flap diameter were 110 μm and 9.0 mm, respectively. Other settings included hinge angle, 55°; bed energy, 0.75 μJ; spot separation, 6 μm; line separation, 6 μm; side-cut energy, 1.1 μJ; pocket width, 200 μm; pocket start depth, 210 μm; and both pocket tangent and radial spot separation, 4 μm. Stromal ablation was performed with Allegretto Wave & Eye-Q 400 Hz laser (WaveLight Laser Technologie AG, Germany) using a 6.5-mm optical zone. Postoperatively, all patients received topical levofloxacin 0.5% eye drops four times a day for 1 week. Topical prednisolone acetate 1% eye drops were used four times daily for the first postoperative week and then tapered over 1 month. Preservative free artificial tear were used for 6 months postoperatively.
Statistical Analysis
Statistical analysis was performed using R 2.15.2 (R Foundation, Vienna, Austria). Reliability of Ks, Kf, Km, CCT, TCT, Ant BFS and post BFS measurements obtained with swept-source OCT and Scheimpflug imaging before and after LASIK were evaluated using intraclass correlation coefficient (ICC) and reproducibility coefficients (RC). ICC is an index of reliability between 0 and 1 that measures the proportion of variation attributed to variation among individuals. An ICC above 0.90 represents adequate reliability. RC is defined as the 95% confidence limit of the difference of measurements between examinations that is equal to 1.96 × √ (2 × pooled test–retest variance). It measures variability in measurements taken from the same eye that can be ascribed to errors due to the measurement process itself. Comparison of ICC and RC between pre- and post-LASIK, and between swept-source OCT and Scheimpflug imaging, was performed by bootstrap resampling with 5000 replications. Mean values of all 7 corneal parameters obtained by both devices before and after LASIK were estimated by one-way analysis of variance (ANOVA) with repeated measures. Mean comparison between pre- and post-LASIK, and between swept-source OCT and Scheimpflug photography, was performed by two-way ANOVA with repeated measures.
Association of the corneal parameters with age, spherical equivalent, and residual bed thickness was modeled using linear mixed effect model formulate:
yi,j = μ + γxi + μ i + ei,j
where yi,j represents the jth observation of a corneal parameter from subject i; xi represents the observation of a covariate from a subject i; μ is a fixed intercept that represents the mean value of a corneal parameter; μi is a random intercept that represents the subject-by-subject variation of a parameter; γ represents a fixed association effect between a parameter and a covariate; and ei,j represents the random measurement error. Association of the corneal parameters with the 3 covariates were evaluated by testing γ = 0 through Wald test. Since the standard deviation of the random measurement error, ei,j, is directly proportional to the RC of the parameter, association of RC of the corneal parameters with the 3 covariates was modeled using the above linear mixed effect model with variance of the random error following an exponential distribution model:
var(ei,j) = αeβxi
where α represents a fixed variance component; and β represents an exponentially proportional component of the variance with the covariate, xi. Association of RC of corneal parameters with the 3 covariates was evaluated using likelihood ratio test. For a few linear mixed effect models with exponential variance that cannot converge numerically, Breusch–Pagan variance tests were applied on the estimated residuals from linear mixed effect models without exponential variance with 1000 bootstrap resampling replications. P-value <0.050 was considered statistically significant.
RESULTS
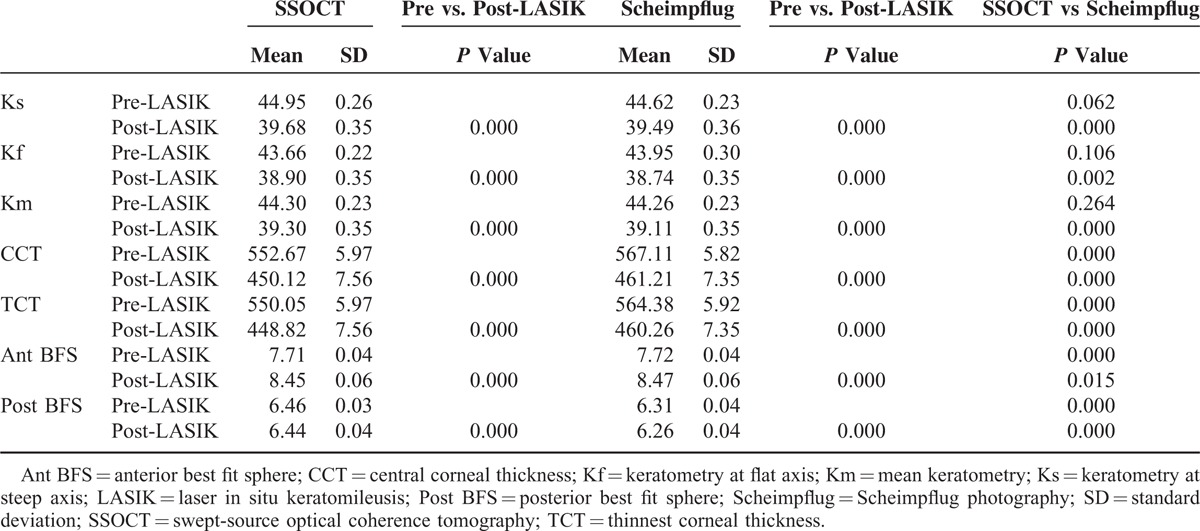
A total of 52 eyes of 26 patients were imaged over the study period. Twenty-six right eyes of 26 participants with mean age of 32.7 ± 6.9 years, mean spherical equivalent −6.27 ± 1.67 D, and mean residual bed thickness 358.1 ± 33.8 μm were included in the analysis. Preoperatively, the mean Ks, Kf, Km, CCT, TCT, Ant BFS, and Post BFS measured with swept-source OCT were 44.95 D, 43.66 D, 44.30 D, 552.7 μm, 550.1 μm, 7.71 mm, and 6.46 mm, respectively. The mean Ks, Kf, Km, CCT, TCT, Ant BFS, and Post BFS measured with Scheimpflug imaging were 44.62 D, 43.95 D, 44.26 D, 567.1 μm, 564.4 μm, 7.72 mm, and 6.31 mm, respectively (Table 1). Significant differences were found in preoperative CCT, TCT, Ant BFS, and Post BFS readings (P ≤ 0.001) between both devices.
TABLE 1.
Comparison of Mean vValues of Corneal Parameters Preoperatively and 1 Year After Laser In Situ Keratomileusis Using Swept-Source Optical Coherence Tomography and Scheimpflug Photography
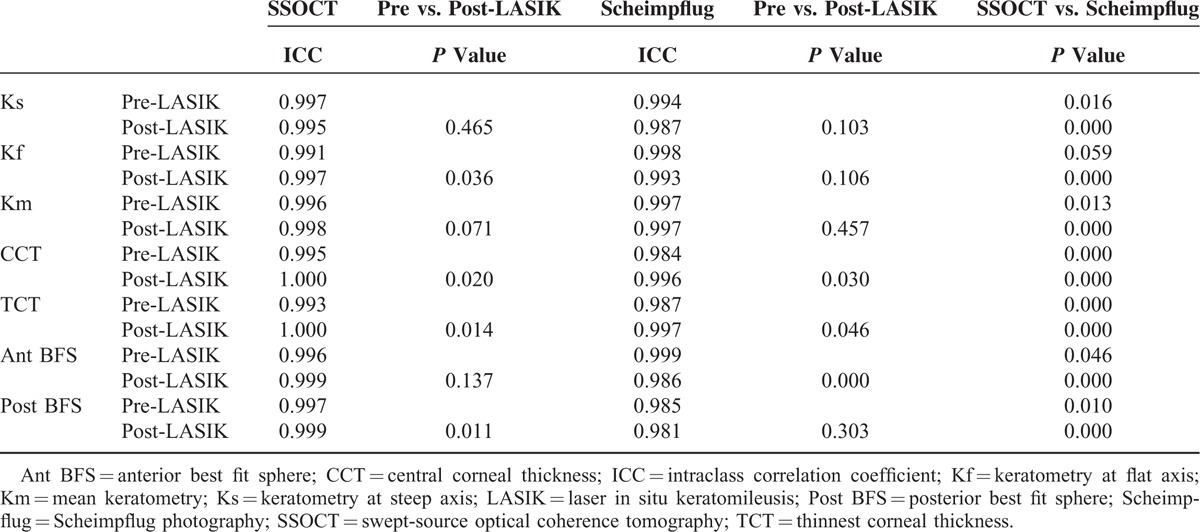
Preoperatively, both swept source OCT and Scheimpflug imaging showed adequate intraclass correlation coefficient values for all measurements with ICC ranging between 0.98 and 1.00. Swept-source OCT demonstrated significantly higher ICC for Ks, CCT, TCT, and Post BFS (P ≤ 0.016), but significantly lower ICC for Km, and Ant BFS (P ≤ 0.046) compared with Scheimpflug imaging (Table 2).
TABLE 2.
Comparison of Intraclass Correlation Coefficient Preoperatively and 1 Year After Laser In Situ Keratomileusis Using Swept-Source Optical Coherence Tomography and Scheimpflug Photography
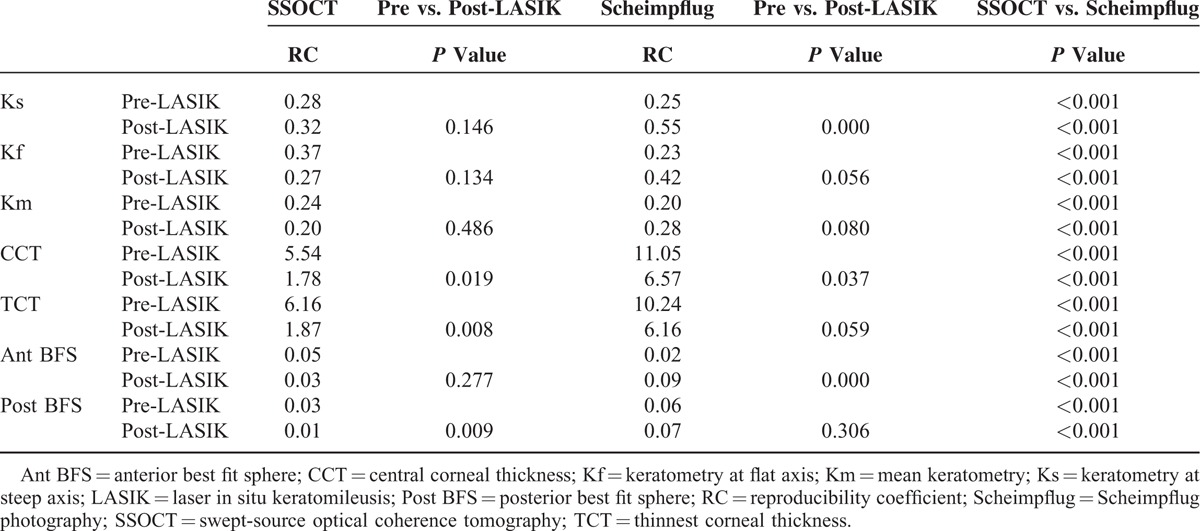
For RC, swept-source OCT demonstrated significantly smaller values for CCT, TCT, and Post BFS, whereas the RC values were significantly better for Ks, Kf, Km, and Ant BFS measurements with Scheimpflug imaging (P ≤ 0.001) (Table 3).
TABLE 3.
Comparison of Reproducibility Coefficient Preoperatively and 1 Year After Laser In Situ Keratomileusis Using Swept-Source Optical Coherence Tomography and Scheimpflug Photography
After LASIK, the mean Ks, Kf, Km, CCT, TCT, Ant BFS, and Post BFS values measured with swept-source OCT were 39.68 D, 38.90 D, 39.30 D, 450.1 μm, 448.8 μm, 8.45 mm, and 6.44 mm, respectively. The mean Ks, Kf, Km, CCT, TCT, Ant BFS, and Post BFS measured with Scheimpflug imaging were 39.49 D, 38.74 D, 39.11 D, 461.2 μm, 460.2 μm, 8.47 mm, and 6.26 mm, respectively (Table 1). Significant differences were found between both devices for all parameters (P ≤ 0.015). The ICC values ranged between 0.98 and 1.00. Swept-source OCT demonstrated significantly higher ICC for all parameters as compared with Scheimpflug imaging (P ≤ 0.001) (Table 2). Likewise, RCs were significantly smaller with swept-source OCT for all postoperative parameters as compared with Scheimpflug imaging (P ≤ 0.001) (Table 3).
A significant change in the mean values was observed in all parameters after LASIK (P ≤ 0.001) (Table 1). Statistically significant improvement in ICC was observed for Kf, CCT, TCT, and Post BFS measured with swept-source OCT after LASIK (P ≤ 0.036). An improvement in ICC was observed in post-LASIK CCT and TCT measurements with Scheimpflug imaging (P ≤ 0.030). However, significantly lower ICC was observed for Ant BFS measurements with Scheimpflug imaging after LASIK (P ≤ 0.001) (Table 2).
Postoperatively, smaller RC was observed for CCT, TCT, and Post BFS measured with swept-source OCT (P ≤ 0.019). Using Scheimpflug imaging, smaller RC for CCT (P = 0.037) and larger RC for Ks and Ant BFS measurements (P ≤ 0.001) were observed after LASIK (Table 3).
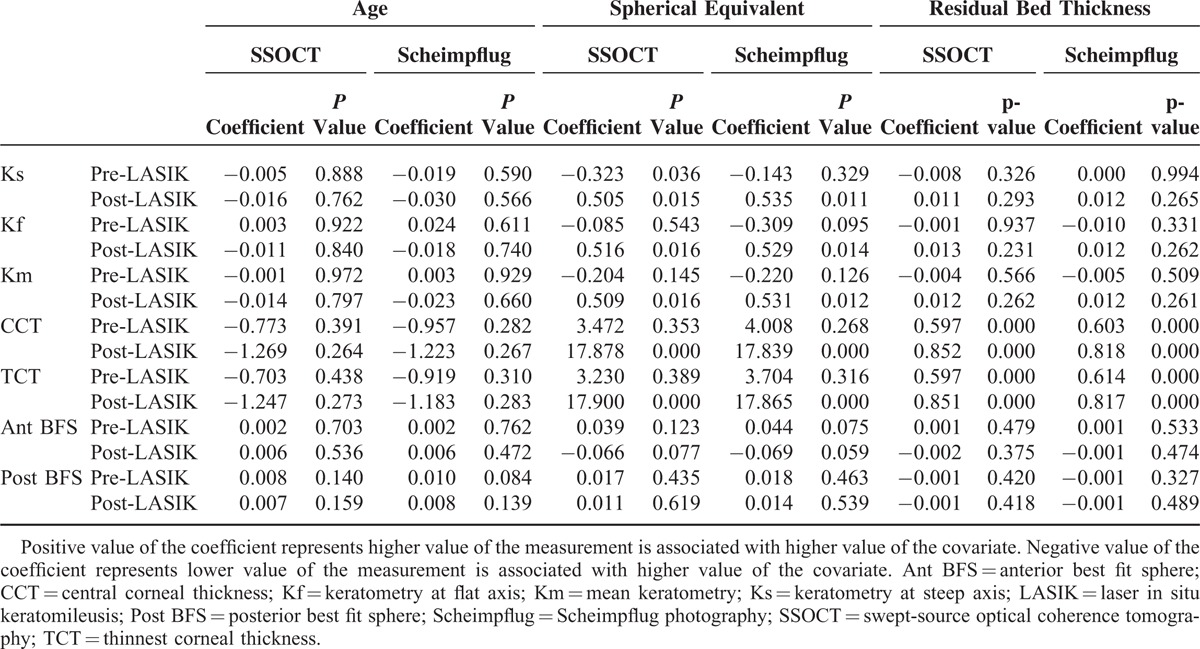
Association between the mean value of corneal parameters with age, spherical equivalent, and residual bed thickness is shown in Table 4. A positive association was noted between spherical equivalent and post-LASIK keratometry, as well as corneal thickness measurements for both swept-source OCT and Scheimpflug imaging (P ≤ 0.016). A positive association was also noted between residual stromal bed thickness and post-LASIK corneal thickness measurements for both devices (P ≤ 0.001) (Table 4).
TABLE 4.
Association of Corneal Parameters with Age, Spherical Qquivalent, and Residual Bed Thickness Preoperatively and 1 Year After Laser In Situ Keratomileusis Based on Linear Mixed Effect Models Using Swept Source-Optical Coherence Tomography and Scheimpflug Photography
Association of variance heterogeneity of corneal parameters with age, spherical equivalent, and residual bed thickness is shown in Table 5, where variance is a quantity proportional to the square of RC. For swept-source OCT, association of variance heterogeneity was found in pre-LASIK Ant BFS and post-LASIK Post BFS. Variance of pre-LASIK Ant BFS was negatively associated with spherical equivalent and residual bed thickness (P ≤ 0.001), suggesting a larger RC was associated with lower spherical equivalent and lower residual bed thickness. Variance of post-LASIK Post BFS was negatively associated with age (P = 0.028), suggesting a smaller RC was associated with older age.
TABLE 5.
Association of Variance Heterogeneity of Corneal Parameters with Age, Spherical Equivalent, and Residual Bed Thickness Preoperatively and 1 Year After Laser In Situ Keratomileusis Based on Linear Mixed Effect Models with Exponential Variance Using Swept-Source Optical Coherence Tomography and Scheimpflug Imaging
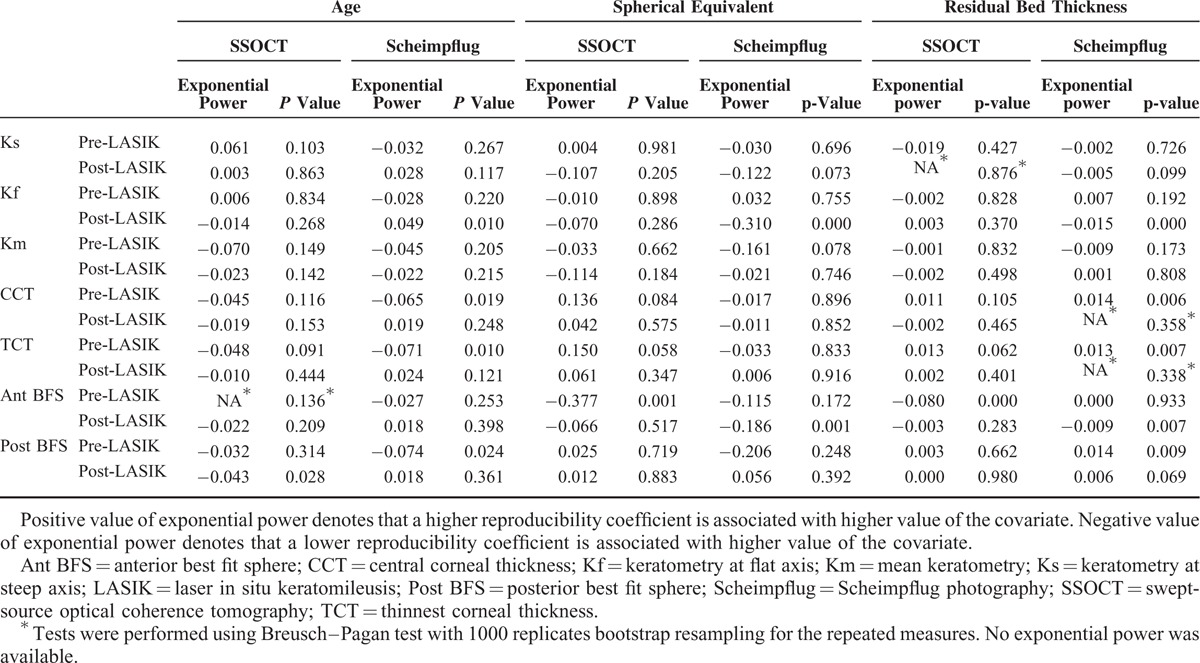
For Scheimpflug imaging, association of variance heterogeneity was found in all parameters except Ks and Km. Variances of pre-LASIK CCT, TCT, and Post BFS were negatively associated with age and positively associated with residual bed thickness (P ≤ 0.024). Variances of post-LASIK Kf and Ant BFS were negatively associated with spherical equivalent and residual bed thickness (P ≤ 0.007), and variance of post-LASIK Kf was positively associated with age (P = 0.010) (Table 5).
DISCUSSION
LASIK is the most commonly performed laser refractive surgery worldwide. It has been shown to be a safe and effective procedure with predictable results over a long-term follow-up.28 It is important to accurately measure corneal parameters after LASIK particularly for patients who require future enhancement procedure or cataract surgery. Accurate measurement of corneal thickness and elevation in post-LASIK eyes is mandatory for detection of postrefractive keratectasia.1,2 To the best of our knowledge, the present study is the only study comparing swept-source OCT and Scheimpflug imaging for measurement of corneal topography longitudinally before and after LASIK.
OCT is an optical analog of ultrasound that enables noninvasive cross-sectional, in vivo, imaging of tissue microstructure.29 In Fourier-domain OCT, light beams reflected by the sample arm and stationary reference arm are combined, and the interference spectrum is detected and extracted using the Fourier transformation. Swept-source OCT employs a fast wavelength scanning light source. It has higher sensitivity at greater scanning depths compared with other types of Fourier-domain OCT.25 Scheimpflug imaging, on the other hand, is based on the Schiempflug principle that involves an optical imaging scenario, whereby the plane of an object is not parallel to the film of the camera. Scheimpflug imaging employs a rotating camera, which determines light scattering profiles and biometry of the anterior segment of the eye. Compared with the traditional imaging techniques, it offers images with minimal distortion and wider depth of focus.30
High repeatability for measurement of corneal keratometry has been demonstrated in post-LASIK eyes (RC of 0.25) using spectral-domain OCT (RTVue, Optovue, Fremont, CA) with an even lower RC (0.19) in normal patients.8 It was suggested that the difference in preoperative and postoperative measurements might be attributed to a change in the corneal shape, as well as the anterior–posterior curvature ratio after LASIK. Although we observed a similar RC for keratometry measurements using swept-source OCT in post-LASIK corneas, no significant change between the preoperative and postoperative ICC and RC was found in our cohort. Scheimpflug imaging has also been reported to have a high repeatability for corneal power measurements after LASIK.31 In a recent study comparing a custom-made, motion-corrected spectral-domain OCT and Scheimpflug imaging, OCT demonstrated a better accuracy in measuring corneal power change after LASIK, although both devices were found to be highly reliable.32 Likewise, swept-source OCT was found to be more reliable compared with Scheimpflug photography in measuring corneal power postoperatively in the present study. However, the reliability in preoperative keratometry measurement was similar between both devices in our study. The variability in results can be attributed to the difference in scanning speeds of the custom-made spectral-domain OCT (10,000 axial scans per second), commercially available spectral-domain OCT (26,000 axial scans per second), and swept source OCT (30,000 axial scans per second).
The reliability for post-LASIK corneal thickness measurement has also been evaluated using OCT and Scheimpflug imaging.12–15,24,33 Ciolino et al showed that post-LASIK corneal thickness measured with Scheimpflug imaging (Pentacam) is highly agreeable to that measured with ultrasound pachymetry.14 Park et al reported a similar observation.15 Ho et al reported a high correlation between time-domain OCT (Visante, Carl Zeiss Meditec, Jena, Germany) and Scheimpflug imaging (Pentacam) in post-LASIK eyes.12 However, only a few studies have directly compared spectral-domain OCT and Scheimpflug imaging in post-LASIK eyes.24,33 Post-LASIK central corneal thickness measured with spectral-domain OCT (RTVue) and Scheimpflug imaging (Pentacam) was not significantly different in an observational study by Grewal and coworkers.33 On the contrary, a significant difference was noted between spectral-domain OCT (RTVue) and Scheimpflug imaging (Pentacam) in another study.24 In our study, the CCT measured with swept-source OCT was 10 μm thinner as compared with Scheimpflug imaging, which is lower than the reported difference of 15 μm between spectral-domain OCT and Scheimpflug imaging (Pentacam).24 A lower magnitude of difference (4 μm) in CCT measurements was found when comparing time-domain OCT (Visante) and Scheimpflug imaging (Pentacam).12 Huang et al reported an ICC of 0.997 for spectral-domain OCT and 0.985 for Scheimpflug imaging (Pentacam).24 Likewise, a significantly higher ICC for pachymetric measurements was observed using swept-source OCT in our cohort. Interestingly, we observed an increase in reliability, representing as an increase in ICC and decrease in RC, in postoperative pachymetry measurements using both devices.
Previously, we observed a significant forward shift of the posterior corneal surface on swept-source OCT within the first postoperative year following LASIK and photorefractive keratectomy.34 On the contrary, studies using Scheimpflug imaging failed to observe a significant change in the posterior cornea after laser refractive procedures.35,36 In line with our earlier findings, we demonstrated in the present study that the reliability of swept-source OCT was better than that of the Scheimpflug imaging for measurement of post-LASIK posterior BFS. It has been repeatedly stressed that the evaluation of posterior corneal elevation is particularly important for the early detection and monitoring of post-LASIK keratectasia.37
Although a direct comparison of swept source OCT and Scheimpflug imaging is not possible, we believe that swept source optical coherence tomography is able to image the post-LASIK corneas better than Scheimpflug photography mainly because of its shorter scanning time (0.3 vs. 2 seconds) and longer wavelength of light source (1310 vs. 475 nm). A shorter scanning time greatly reduces motion artifacts, while a longer wavelength allows better light penetration and less scatter through the LASIK flap interface. The excellent reliability with swept-source OCT is attributed to the precise delineation of the boundaries of the cornea because its images are minimally distorted by optical interference. The swept-source OCT also has an auto-alignment feature, wherein the head unit moves automatically and aligns the head by detecting the corneal center, possibly adding to the machine's high repeatability.
We analyzed the association of variance heterogeneity in our study. Notably, there was a significant association of variance heterogeneity for all parameters, except Ks and Km for Scheimpflug imaging. These results suggested that the reliability of corneal parameters measured with Scheimpflug imaging was less consistent across different age, spherical equivalent, and residual bed thickness measurements. An earlier study by Shankar et al found that Pentacam software specifically interpolated missing data, and such interpolation could be the reason for the reduced reliability in peripheral corneal thickness and posterior corneal surface.38 In another recent study, Hashemi et al pointed out inconsistencies in measurement error between keratoconus and healthy eyes with Schiempflug imaging (Pentacam).39
The present study is limited by its small sample size. Our study reported a better reliability of swept-source OCT for evaluation of corneal topography after LASIK compared with Scheimpflug imaging. It remains to be determined whether statistical significance would translate into clinical significance. Future studies comparing the reliability between swept-source OCT and newer models of Scheimpflug analyzers are warranted to further delineate corneal imaging technology in post-LASIK patients.
Footnotes
Abbreviations: CCT = central corneal thickness, IC = intraclass correlation coefficient, Kf = keratometry at flat axis, Ks = keratometry at steep axis, LASIK = laser in situ keratomileusis, OCT = optical coherence tomography, RBT = residual bed thickness, RC = repeatability coefficient, SEQ = spherical equivalent, TCT = thinnest corneal thickness.
The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Author contributions: concept and design (V.J.); analysis and interpretation (M.Y.); writing the article (T.C., S.B., M.Y., V.J.); critical revision of the article (T.C., V.J.); final approval of the article (T.C., S.B., M.Y., V.J.); data collection (S.B.); provision of materials, patients, or resources (V.J.); statistical expertise (M.Y.); literature research (T.C., S.B., V.J.); and administrative, technical, or logistic support (S.B., V.J.).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Savini G, Barboni P, Zanini M. Intraocular lens power calculation after myopic refractive surgery: theoretical comparison of different methods. Ophthalmology 2006; 113:1271–1282. [DOI] [PubMed] [Google Scholar]
- 2.Randleman JB, Woodward M, Lynn MJ, et al. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology 2008; 115:37–50. [DOI] [PubMed] [Google Scholar]
- 3.Rio-Cristobal A, Martin R. Corneal assessment technologies: current status. Surv Ophthalmol 2014; 59:599–614. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed S, Lee GK, Rao SK, et al. Repeatability and reproducibility of pachymetric mapping with Visante anterior segment-optical coherence tomography. Invest Ophthalmol Vis Sci 2007; 48:5499–5504. [DOI] [PubMed] [Google Scholar]
- 5.Bourges JL, Alfonsi N, Laliberte JF, et al. Average 3-dimensional models for the comparison of Orbscan II and Pentacam pachymetry maps in normal corneas. Ophthalmology 2009; 116:2064–2071. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda S, Kawana K, Yasuno Y, et al. Anterior ocular biometry using 3-dimensional optical coherence tomography. Ophthalmology 2009; 116:882–889. [DOI] [PubMed] [Google Scholar]
- 7.McAlinden C, Khadka J, Pesudovs K. A comprehensive evaluation of the precision (repeatability and reproducibility) of the Oculus Pentacam HR. Invest Ophthalmol Vis Sci 2011; 52:7731–7737. [DOI] [PubMed] [Google Scholar]
- 8.Tang M, Chen A, Li Y, et al. Corneal power measurement with Fourier-domain optical coherence tomography. J Cataract Refract Surg 2010; 36:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford AZ, Patel DV, McGhee CN. Comparison and repeatability of keratometric and corneal power measurements obtained by Orbscan II, Pentacam, and Galilei corneal tomography systems. Am J Ophthalmol 2013; 156:53–60. [DOI] [PubMed] [Google Scholar]
- 10.Jhanji V, Yang B, Yu M, et al. Corneal thickness and elevation measurements using swept-source optical coherence tomography and slit scanning topography in normal and keratoconic eyes. Clin Exp Ophthalmol 2013; 41:735–745. [DOI] [PubMed] [Google Scholar]
- 11.Prisant O, Calderon N, Chastang P, et al. Reliability of pachymetric measurements using orbscan after excimer refractive surgery. Ophthalmology 2003; 110:511–515. [DOI] [PubMed] [Google Scholar]
- 12.Ho T, Cheng AC, Rao SK, et al. Central corneal thickness measurements using Orbscan II, Visante, ultrasound, and Pentacam pachymetry after laser in situ keratomileusis for myopia. J Cataract Refract Surg 2007; 33:1177–1182. [DOI] [PubMed] [Google Scholar]
- 13.Cheng AC, Rao SK, Lau S, et al. Central corneal thickness measurements by ultrasound, Orbscan II, and Visante OCT after LASIK for myopia. J Refract Surg 2008; 24:361–365. [DOI] [PubMed] [Google Scholar]
- 14.Ciolino JB, Khachikian SS, Belin MW. Comparison of corneal thickness measurements by ultrasound and scheimpflug photography in eyes that have undergone laser in situ keratomileusis. Am J Ophthalmol 2008; 145:75–80. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Choi SK, Lee D, et al. Corneal thickness measurement using Orbscan, Pentacam, Galilei, and ultrasound in normal and post-femtosecond laser in situ keratomileusis eyes. Cornea 2012; 31:978–982. [DOI] [PubMed] [Google Scholar]
- 16.Hashemi H, Mehravaran S. Central corneal thickness measurement with Pentacam, Orbscan II, and ultrasound devices before and after laser refractive surgery for myopia. J Cataract Refract Surg 2007; 33:1701–1707. [DOI] [PubMed] [Google Scholar]
- 17.Kim SW, Byun YJ, Kim EK, et al. Central corneal thickness measurements in unoperated eyes and eyes after PRK for myopia using Pentacam, Orbscan II, and ultrasonic pachymetry. J Refract Surg 2007; 23:888–894. [DOI] [PubMed] [Google Scholar]
- 18.Prakash G, Agarwal A, Jacob S, et al. Comparison of Fourier-domain and time-domain optical coherence tomography for assessment of corneal thickness and intersession repeatability. Am J Ophthalmol 2009; 148:282–290. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda S, Kawana K, Yasuno Y, et al. Repeatability and reproducibility of anterior ocular biometric measurements with 2-dimensional and 3-dimensional optical coherence tomography. J Cataract Refract Surg 2010; 36:1867–1873. [DOI] [PubMed] [Google Scholar]
- 20.Nam SM, Im CY, Lee HK, et al. Accuracy of RTVue optical coherence tomography, Pentacam, and ultrasonic pachymetry for the measurement of central corneal thickness. Ophthalmology 2010; 117:2096–2103. [DOI] [PubMed] [Google Scholar]
- 21.Ishibazawa A, Igarashi S, Hanada K, et al. Central corneal thickness measurements with Fourier-domain optical coherence tomography versus ultrasonic pachymetry and rotating Scheimpflug camera. Cornea 2011; 30:615–619. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Huang J, Wen D, et al. Measurement of central corneal thickness by high-resolution Scheimpflug imaging, Fourier-domain optical coherence tomography and ultrasound pachymetry. Acta Ophthalmol 2012; 90:449–455. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Ding X, Savini G, et al. A comparison between Scheimpflug imaging and optical coherence tomography in measuring corneal thickness. Ophthalmology 2013; 120:1951–1958. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Pesudovs K, Yu A, et al. A comprehensive comparison of central corneal thickness measurement. Optom Vis Sci 2011; 88:940–949. [DOI] [PubMed] [Google Scholar]
- 25.Karnowski K, Kaluzny BJ, Szkulmowski M, et al. Corneal topography with high-speed swept source OCT in clinical examination. Biomed Opt Express 2011; 2:2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda N. Optical coherence tomography for corneal diseases. Eye Contact Lens 2010; 36:254–259. [DOI] [PubMed] [Google Scholar]
- 27.Ye C, Yu M, Jhanji V. Stromal bed thickness measurement during laser in situ keratomileusis using intraoperative optical coherence tomography. Cornea 2015; 34:387–391. [DOI] [PubMed] [Google Scholar]
- 28.Yuen LH, Chan WK, Koh J, et al. A 10-year prospective audit of LASIK outcomes for myopia in 37,932 eyes at a single institution in Asia. Ophthalmology 2010; 117:1236–1244. [DOI] [PubMed] [Google Scholar]
- 29.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991; 254:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wegener A, Laser-Junga H. Photography of the anterior eye segment according to Scheimpflug's principle: options and limitations—a review. Clin Exp Ophthalmol 2009; 37:144–154. [DOI] [PubMed] [Google Scholar]
- 31.Jain R, Dilraj G, Grewal SP. Repeatability of corneal parameters with Pentacam after laser in situ keratomileusis. Indian J Ophthalmol 2007; 55:341–347. [PMC free article] [PubMed] [Google Scholar]
- 32.McNabb RP, Farsiu S, Stinnett SS, et al. Optical coherence tomography accurately measures corneal power change from laser refractive surgery. Ophthalmology 2015; 122:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grewal DS, Brar GS, Grewal SP. Assessment of central corneal thickness in normal, keratoconus, and post-laser in situ keratomileusis eyes using Scheimpflug imaging, spectral domain optical coherence tomography, and ultrasound pachymetry. J Cataract Refract Surg 2010; 36:954–964. [DOI] [PubMed] [Google Scholar]
- 34.Chan TC, Liu D, Yu M, et al. Longitudinal evaluation of posterior corneal elevation after laser refractive surgery using swept-source optical coherence tomography. Ophthalmology 2014; 122:687–692. [DOI] [PubMed] [Google Scholar]
- 35.Ciolino JB, Khachikian SS, Cortese MJ, et al. Long-term stability of the posterior cornea after laser in situ keratomileusis. J Cataract Refract Surg 2007; 33:1366–1370. [DOI] [PubMed] [Google Scholar]
- 36.Grewal DS, Brar GS, Grewal SP. Posterior corneal elevation after LASIK with three flap techniques as measured by Pentacam. J Refract Surg 2011; 27:261–268. [DOI] [PubMed] [Google Scholar]
- 37.Baek T, Lee K, Kagaya F, et al. Factors affecting the forward shift of posterior corneal surface after laser in situ keratomileusis. Ophthalmology 2001; 108:317–320. [DOI] [PubMed] [Google Scholar]
- 38.Shankar H, Taranath D, Santhirathelagan CT, et al. Anterior segment biometry with the Pentacam: comprehensive assessment of repeatability of automated measurements. J Cataract Refract Surg 2008; 34:103–113. [DOI] [PubMed] [Google Scholar]
- 39.Hashemi K, Guber I, Bergin C, et al. Reduced precision of the Pentacam HR in eyes with mild to moderate keratoconus. Ophthalmology 2015; 122:211–212. [DOI] [PubMed] [Google Scholar]


