Abstract
Meta-analyses have found conflicting results with respect to the use of progesterone or progesterone plus estrogen as luteal phase support for in vitro fertilization (IVF) protocols involving gonadotropins and/or gonadotropin-releasing hormone analogs. The aim of the present study was to perform an updated meta-analysis on the efficacy of progesterone versus progesterone plus estrogen as luteal phase support.
We searched the MEDLINE, Cochrane Library, and Google Scholar databases (up to March 18, 2014). The search terms were (estrogen OR estradiol OR oestradiol) AND (progesterone) AND (IVF OR in vitro fertilization) AND (randomized OR prospective). We did not limit the form of estrogen and included subjects who contributed more than 1 cycle to a study. The primary outcome was clinical pregnancy rate. Secondary outcomes were ongoing pregnancy rate, fertilization rate, implantation rate, and miscarriage rate.
A total of 11 articles were included in the present analysis, with variable numbers of studies assessing each outcome measure. Results of statistical analyses indicated that progesterone plus estrogen treatment was more likely to result in clinical pregnancy than progesterone alone (pooled odds ratio 1.617, 95% confidence interval 1.059–2.471; P = 0.026). No significant difference between the 2 treatment regimens was found for the other outcome measures.
Progesterone plus estrogen for luteal phase support is associated with a higher clinical pregnancy rate than progesterone alone in women undergoing IVF, but other outcomes such as ongoing pregnancy rate, fertilization rate, implantation rate, and miscarriage rate are the same for both treatments.
INTRODUCTION
Most stimulation protocols for assisted reproductive technology result in a defective luteal phase. The mechanisms underlying the insufficient function of the corpus luteum in this context may include supraphysiologic estradiol level, decreased luteinizing hormone level, inhibition of the corpus luteum, and asynchronization of estradiol and progesterone.1,2 Luteal phase support (LPS) is commonly used in in vitro fertilization (IVF) involving gonadotropin-releasing hormone (GnRH) analogs, and options include human chorionic gonadotropin, progesterone, estradiol, and GnRH agonists, as well as cytokines (eg, granulocyte colony-stimulating factor and lymphocyte immunotherapy).3 However, there is still controversy in the types of hormones used for LPS, as well as their dosage, duration, and timing.4
With respect to the use of progesterone or progesterone plus estrogen as LPS, prior meta-analyses have not included a large number of studies and/or reported conflicting results. Although a 2002 meta-analysis by Pritts and Atwood5 included 3 studies, of which only one study reported an increase in the implantation rate with the addition of oral estrogen to progesterone. A 2011 Cochrane review6 (updated from 20047) evaluated 7 studies and found that combining transdermal estrogen and progesterone would improve the clinical pregnancy rate, but the addition of estrogen did not affect other outcomes including ongoing pregnancy, fertilization, implantation, and miscarriage rates. Prior meta-analyses, such as those by Kolibianakis et al8 (4 studies) and Gelbaya et al9 (10 studies), found no beneficial effect of a progesterone/estrogen combination on the pregnancy rates, and their findings were further supported by a 2010 meta-analysis performed by Jee et al.10 The aim of this study was to perform a meta-analysis on the efficacy of progesterone versus progesterone plus estrogen of any form for LPS during IVF.
METHODS
Search Strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.11 Meta-analyses do not involve patients, and thus do not require institutional review board approval. We searched the MEDLINE, Cochrane Library, and Google Scholar databases up to March 18, 2014. The search terms were (estrogen OR estradiol OR oestradiol) AND (progesterone) AND (IVF OR in vitro fertilization) AND (randomized OR prospective). Abstracts were reviewed, and reference lists of relevant studies were also searched for relevant studies. This study did not involve human subjects, so informed consent was not required. In addition, no approval was required from an institutional review board.
Inclusion criteria for the meta-analysis were as follows: randomized controlled trial; women undergoing IVF stimulated with gonadotropins and/or GnRH analogs; at least 1 of the treatment arms including the combination of progesterone + estrogen (P + E) for LPS; a control arm including progesterone alone (P) for LPS; and reported outcomes of clinical pregnancy rate, ongoing pregnancy rate, fertilization rate, implantation rate, and/or miscarriage rate. Non-English and non-Chinese publications, case reports, comments, editorials, and letters were excluded.
Study Selection and Data Extraction
Studies were identified via the search strategy by 2 independent reviewers, with a third reviewer being consulted if there was uncertainty regarding eligibility. The following information was extracted from studies that met the inclusion criteria: name of the first author, year of publication, study design, basic information of the subjects (number of patients in each group, age of each group, body mass index of each group, duration of infertility), characteristics of treatment protocols, intervention for each group (type, dosage, timing of initiation, duration of administration), and primary and secondary outcomes (clinical pregnancy rate, ongoing pregnancy rate, fertilization rate, implantation rate, miscarriage rate). Data extraction was also performed by 2 independent reviewers, with a third reviewer being consulted in case of any uncertainty. The Delphi list was used to assess the included studies.12 Quality assessment was also performed by 2 independent reviewers, with a third reviewer being consulted in cases of uncertainty.
Statistical Analysis
Data are presented as mean ± standard deviation or number (%). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for both primary outcome (clinical pregnancy rate) and secondary outcomes of subjects treated with P + E compared with P. Heterogeneity among the studies was assessed using the Cochran Q and the I2 statistics. Either a Q statistic with P < 0.1013 or an I2 statistic >50%14 indicates that heterogeneity exists among the studies, and in this case a random-effects model (DerSimonian–Laird method)15 of analysis was used; otherwise, a fixed-effects model (Mantel–Haenszel method) was used. Sensitivity analysis was performed using the leave-1-out approach. A 1-sided Egger test was performed and funnel plots were created to evaluate publication bias.16 A P value <0.05 was considered statistically significant. Homogeneity tests, pooled estimates, and sensitivity analyses were performed using the Comprehensive Meta-Analysis Version 2.0 (Biostat, Englewood, NJ).
RESULTS
The initial search identified 315 articles (Figure 1). We identified abstracts with full-text articles, and performed manual search of relevant reference lists but did not identify additional articles. A total of 296 articles were excluded, and 19 were subjected to full-text review. Eight more articles were excluded for the following reasons: not performed in women stimulated with gonadotropins and/or GnRH analogs (n = 3), having no outcome of interest (n = 2), no progesterone-alone group (n = 1), not a randomized controlled trial (n = 1), and having no retrievable article (n = 1) (Supplemental ). Thus, 11 articles17–27 were included in the meta-analysis.
Figure 1.
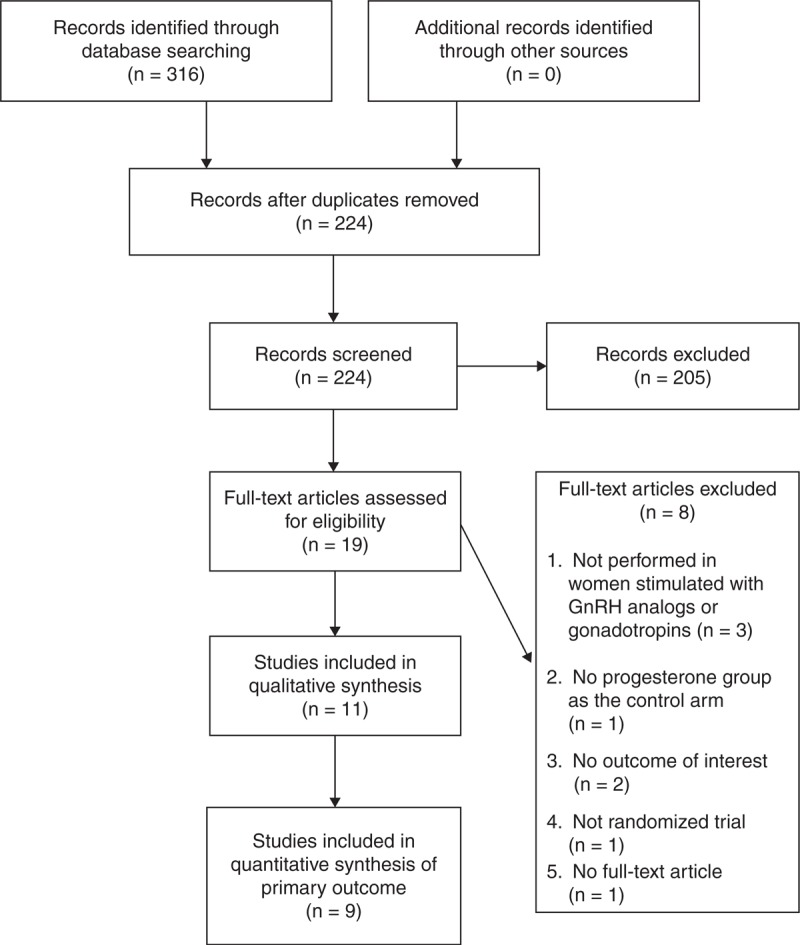
Flow diagram of study selection. GnRH = gonadotropin-releasing hormone.
Quality Assessment
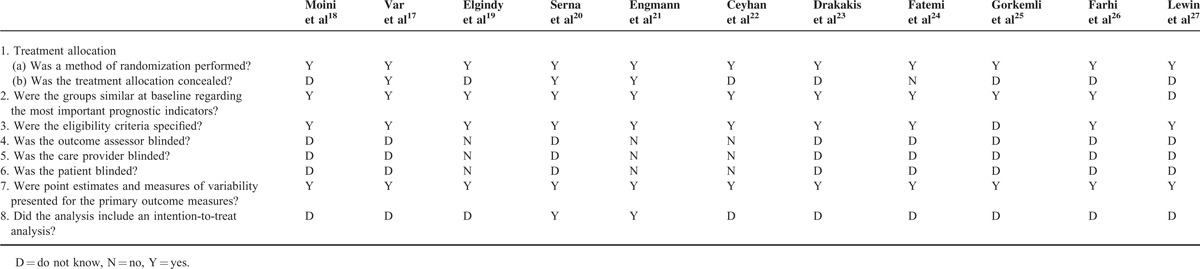
Table 1 shows the results of the Delphi quality assessment. All 11 studies were randomized, with 10 studies meeting specified eligibility criteria, and had similar group characteristics at baseline. However, most of the included studies did not conceal treatment allocation, and did not address whether the analysis was intent-to-treat. None of the studies addressed or performed blinding.
Table 1.
Delphi Quality Assessment for the Included Studies
Study and Subject Characteristics
The 11 studies included a total of 1756 subjects. The mean age of subjects ranged from 28.7 ± 5.4 to 35.8 ± 5.3 years; mean body mass index, when reported, ranged from 22.0 ± 2.8 to 32.1 ± 40.9 kg/m2; and the mean duration of infertility, when reported, ranged from 2.3 ± 1.4 to 9.8 ± 6.4 years (Table 2). Details regarding overall treatment protocols and progesterone and estradiol interventions are summarized in Tables 3 and 4, respectively. Oral estrogen was administered in 7 studies, transdermal estrogen was administered in 4 studies, and vaginal estrogen was administered in 2 studies (1 study included oral or vaginal estrogen,19 and the other study included oral and transdermal estrogen23). Table 5 summarizes the primary and secondary outcomes after intervention (P + E vs P).
Table 2.
Basic Data of the Subjects in the Included Studies
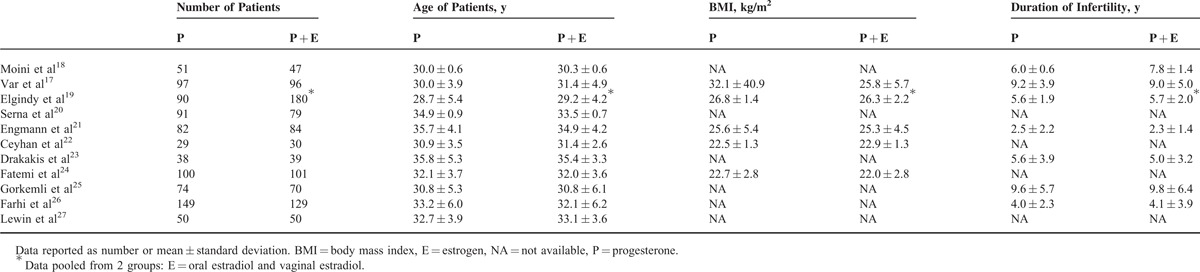
Table 3.
Treatment Protocols
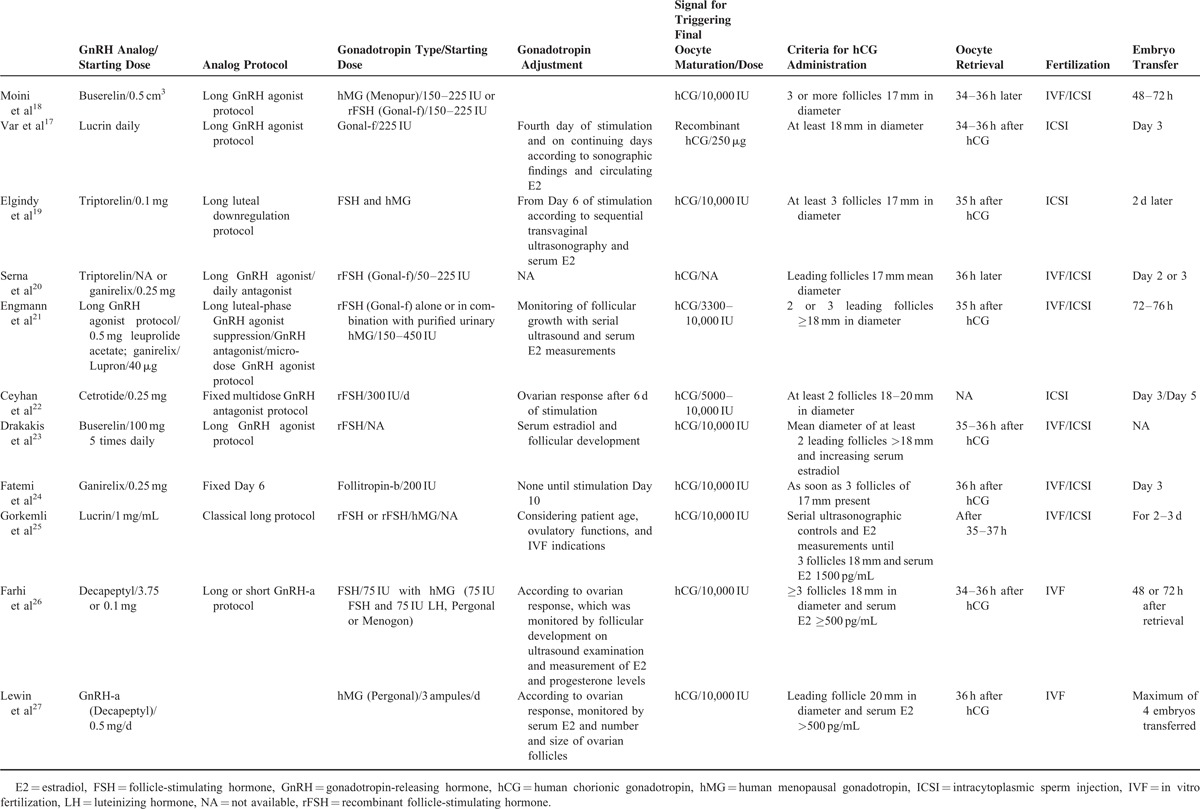
Table 4.
Progesterone and Estradiol Interventions
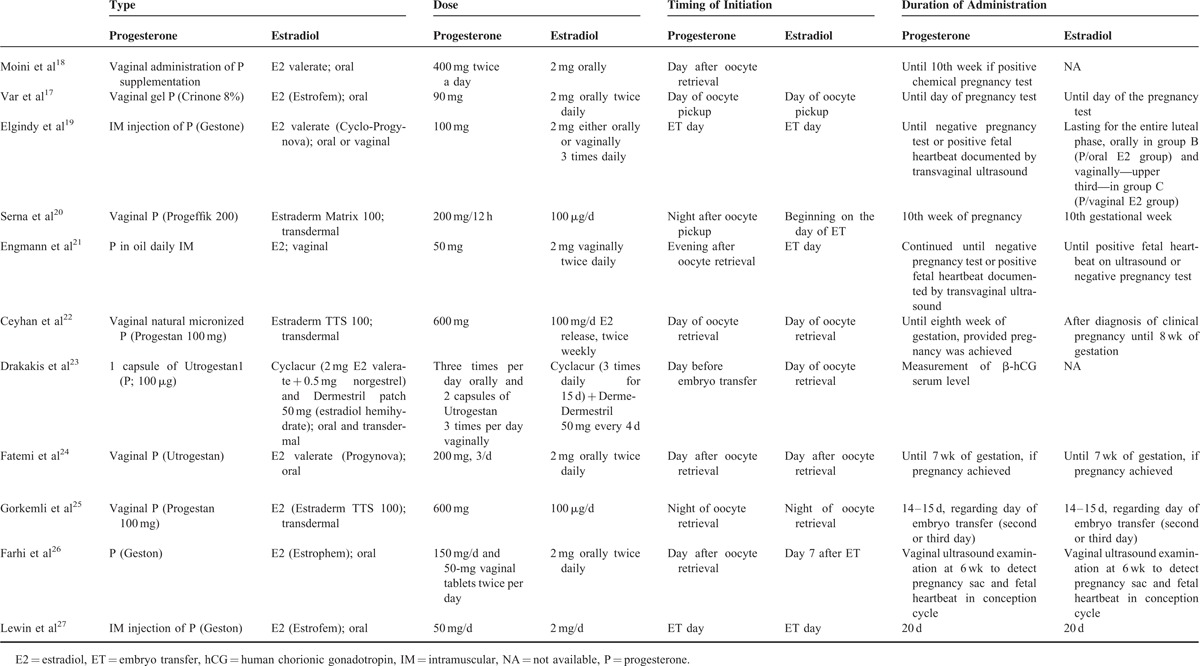
Table 5.
Summary of Primary and Secondary Outcomes
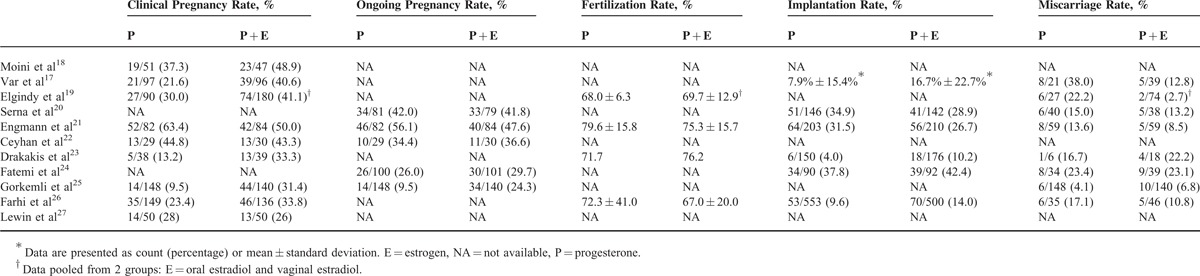
Significantly More Clinical Pregnancies With P + E Versus P
Of the 11 studies, 9 studies reported clinical pregnancy rate (Table 5).17–19,21–23,25–27 P + E was more likely to result in a clinical pregnancy than P alone (pooled OR = 1.617, 95% CI 1.059–2.471; P = 0.026) (Figure 2A). A random-effects model was used, as there was heterogeneity among the studies (Q = 25.45, P = 0.001; I2 = 68.57). Pooled ORs remained >1.0 as each study was removed in turn. In 5 instances, the pooled ORs became nonsignificant after each of those 5 studies was removed, but since their P values were borderline and near the threshold with points in the same direction, influence from any of these 5 studies on the overall pooled OR (without study removal) is negligible (Figure 2B). The funnel plot with the Egger test (Figure 2C) was performed to evaluate publication bias in these studies, and with an estimated intercept of −0.157, and a 1-tailed P = 0.477, there is no significant asymmetry or bias (Figure 2C).
Figure 2.
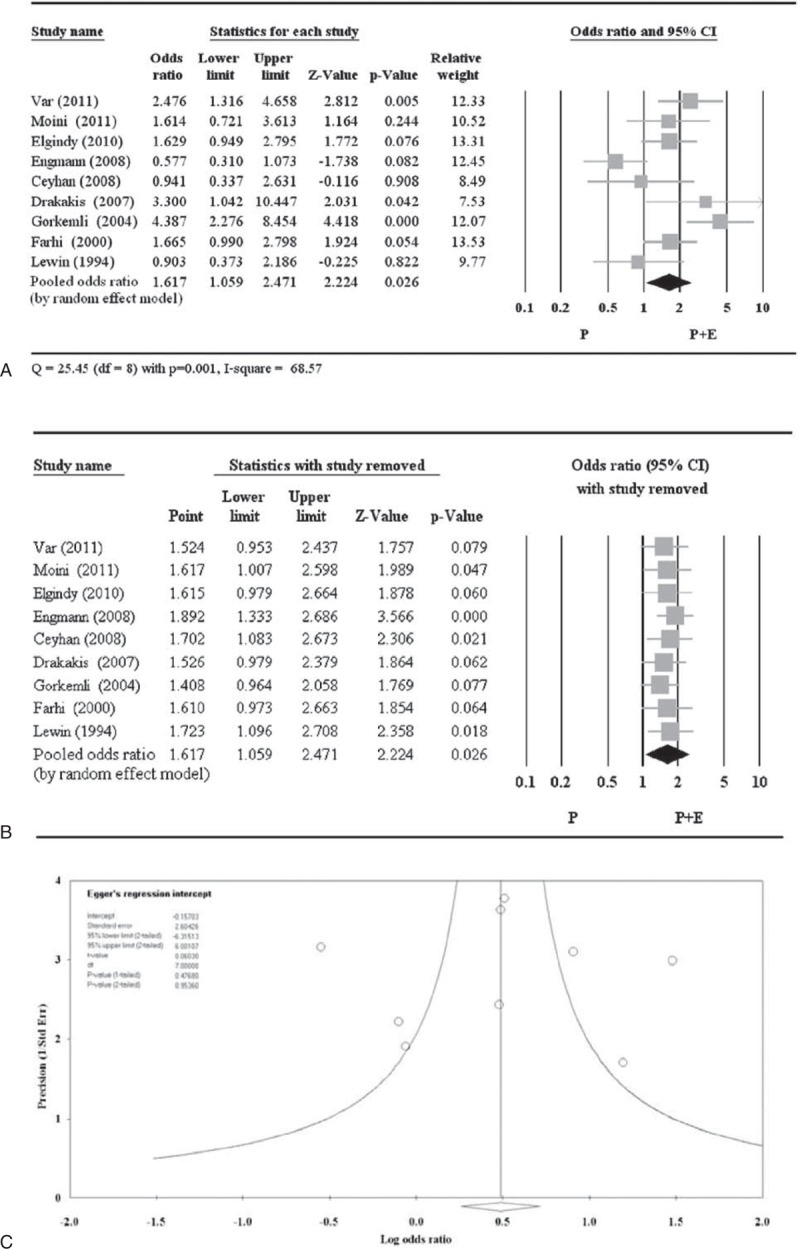
Meta-analysis (A), sensitivity analysis (B), and funnel plot (C) for odds ratio of clinical pregnancy. CI = confidence interval.
No Significant Difference in Ongoing Pregnancy Rate for P + E Versus P
A total of 5 of the 11 studies reported ongoing pregnancy rate (Table 5).20–22,24,25 There was no significant difference between P + E and P treatments with respect to ongoing pregnancy rates (pooled OR = 1.232, 95% CI 0.743–2.044; P = 0.419) (Figure 3A). A random-effects model was used, as there was heterogeneity among the studies (Q = 10.679, P = 0.030; I2 = 62.54). All pooled ORs remained nonsignificant after each study was removed in turn, indicating no obvious influence of any individual study on the pooled estimate (Figure 3B). The Egger test showed an estimated intercept of 0.957, with a 1-tailed P = 0.426, indicating no significant asymmetry or bias (Figure 3C).
Figure 3.
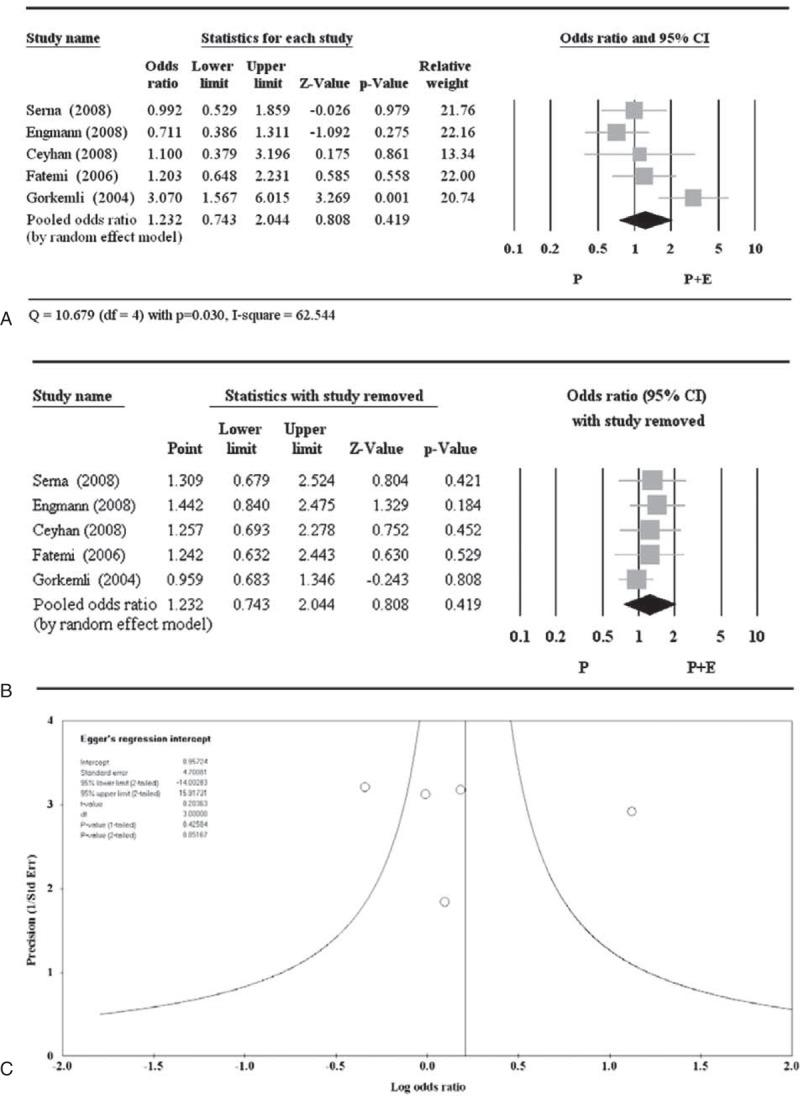
Meta-analysis (A), sensitivity analysis (B), and funnel plot (C) for odds ratio of ongoing pregnancy. CI = confidence interval.
No Significant Difference in Fertilization Rate for P + E Versus P
Of the 11 studies, only 4 reported fertilization rate (Table 5).19,21,23,26 But among those 4 studies, the study by Drakakis et al23 did not report standard deviation, and therefore was not included in the analysis. There was no significant difference between P + E and P with respect to the fertilization rate (pooled difference in means −1.912, 95% CI −6.807 to 2.983; P = 0.444) (Figure 4A). A random-effects model was used, as there was heterogeneity among the studies (Q = 6.197, P = 0.045; I2 = 67.72). Of the 3 included studies, pooled OR was significant when the study by Elgindy et al19 was removed but the overall pooled OR was nonsignificant, indicating influence of that particular study on the overall pooled estimate (Figure 4B). Nevertheless, point estimate of the study by Elgindy et al was in the same direction as that of the other 2 studies. The Egger test was not performed because more than 5 studies are needed to observe publication bias.
Figure 4.
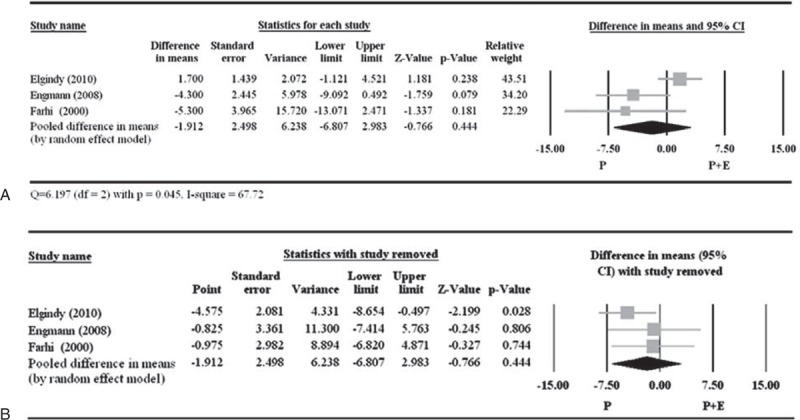
Meta-analysis (A) and sensitivity analysis (B) for the difference in fertilization rate between the 2 treatment groups. The study by Drakakis et al23 did not report standard deviation and was excluded from the meta-analysis. CI = confidence interval.
No Significant Difference in Implantation Rate for P + E Versus P
A total of 6 of the 11 studies reported implantation rate (Table 5).17,20,21,23,24,26 However, the study by Var et al17 used a different definition of implantation rate compared with the other studies, and therefore was excluded from meta-analysis with respect to this parameter. There was no significant difference between P + E and P with respect to implantation rate (pooled OR 1.150, 95% CI 0.779–1.699; P = 0.482) (Figure 5A). A random-effects model was used, as there was heterogeneity among the studies (Q = 11.09, P = 0.026; I2 = 63.93). All pooled ORs remained >1.0, and were nonsignificant when each study was removed in turn, indicating no obvious influence of any individual study on the pooled estimate (Figure 5B). The Egger test had an estimated intercept of 1.837, with a 1-tailed P = 0.291, indicating no significant asymmetry or bias (Figure 5C).
Figure 5.
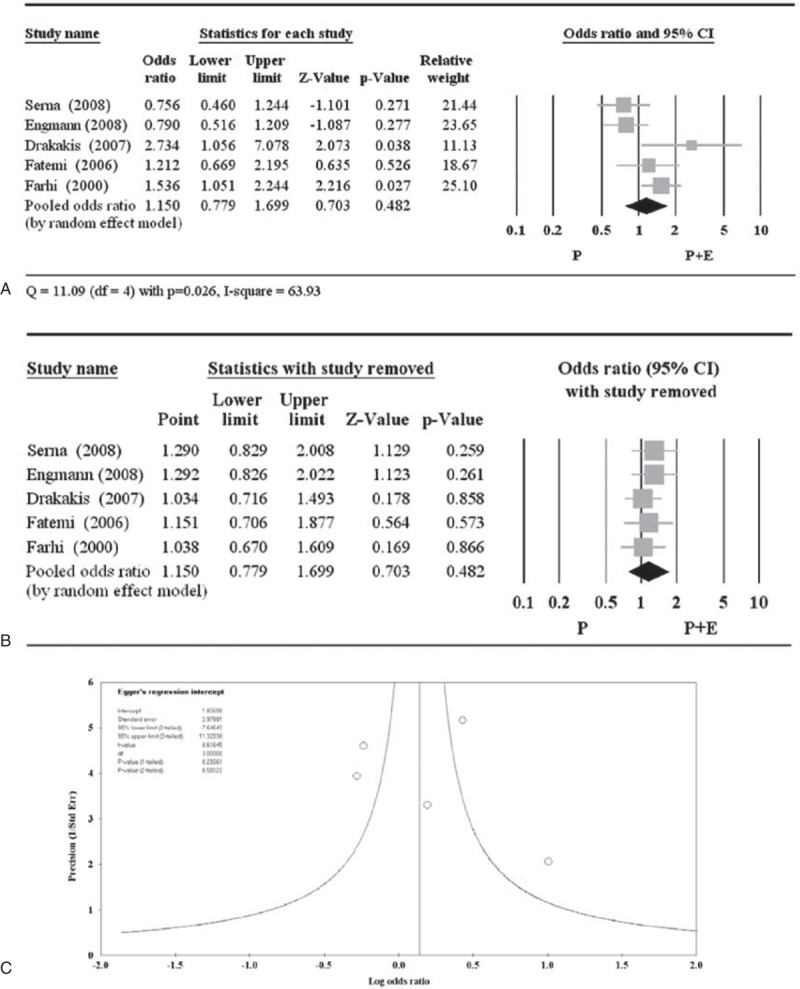
Meta-analysis (A), sensitivity analysis (B), and funnel plot (C) for the odds ratio of implantation. The study by Var et al17 used a different definition of implantation rate and was excluded from the meta-analysis. CI = confidence interval.
No Significant Difference in Miscarriage Rate for P + E Versus P
A total of 8 of the 11 studies reported miscarriage rate data (Table 5).17,19,20,21,23–26 There was no significant difference between P + E and P treatments with respect to miscarriage rate (pooled OR 0.633, 95% CI 0.342–1.172; P = 0.146) (Figure 6A). A random-effects model was used, as there was heterogeneity among the studies (Q = 12.191, P = 0.094; I2 = 42.58). With exception of the study by Gorkemli et al,25 all other pooled ORs remained <1.0 and were nonsignificant when each study was removed in turn, indicating no obvious influence on the overall pooled estimate from any of those remaining 7 studies (Figure 6B). The study by Gorkemli et al (point estimate 0.523, P = 0.031) might influence the pooled estimate but was not removed since its point estimate is in the same direction as the overall pooled OR. The Egger test showed an estimated intercept of −2.15, with a 1-tailed P = 0.182, indicating no significant asymmetry or bias (Figure 6C).
Figure 6.
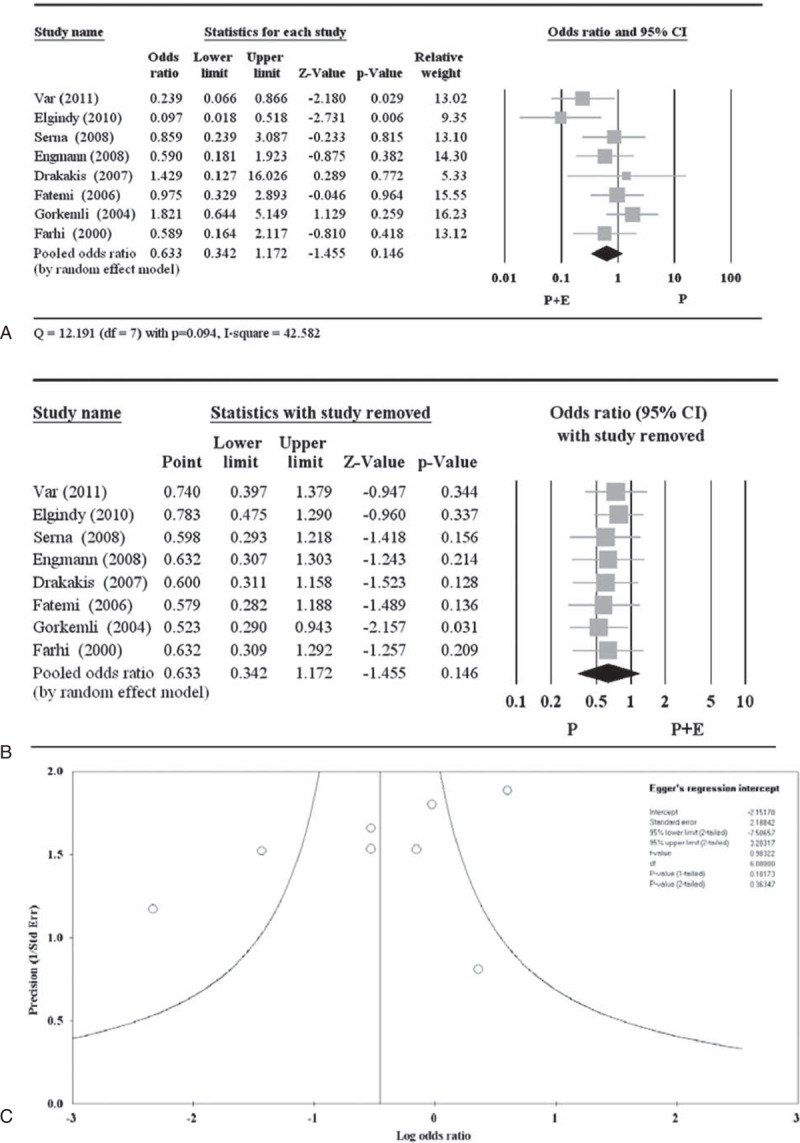
Meta-analysis (A), sensitivity analysis (B), and funnel plot (C) for the odds ratio of miscarriage. CI = confidence interval.
DISCUSSION
The aim of the present study was to perform a meta-analysis examining the efficacy of progesterone plus estrogen versus progesterone alone as LPS during IVF. A search of the literature identified 11 articles. A risk of bias was present given that none of the articles addressed or performed blinding.
A meta-analysis of the 11 articles (1756 subjects with variable numbers of articles/subjects analyzed for each outcome measure) showed a significant benefit for progesterone plus estrogen compared with that for progesterone alone only for the primary outcome of clinical pregnancy. No significant difference was found between the 2 treatment groups for any of the secondary outcomes including the ongoing pregnancy rate, fertilization rate, implantation rate, and miscarriage rate. These results support findings of the 2011 Cochrane review (9 articles; 1571 subjects, also with variable numbers of articles/subjects analyzed for each outcome measure).9 But in that analysis, the significant benefit of progesterone plus estrogen over progesterone alone was based on a subgroup analysis of transdermal estrogen (and transdermal and oral estrogen in 1 study), while our analysis included estrogen supplementation in oral, vaginal, and transdermal forms. Our analysis also included a new article by Moini et al18 and the 2 articles that were excluded from the 2011 Cochrane review.17,26
Potential limitations of this study include the limited sample size (1756 subjects), the inclusion of different forms and dosages of estrogen supplementation, and the inclusion of subjects who contributed more than 1 cycle to a study. Furthermore, while the live birth rate may be the more appropriate outcome, no trial has yet reported this outcome, so our meta-analysis is limited by the design of included studies and appears less than optimal. Nonetheless, the use of estrogen as a supplement to progesterone in LPS does not appear to be significantly beneficial. Additional large randomized controlled trials are necessary to clarify the role of estrogen supplementation in addition to progesterone for LPS in IVF, and to definitively show any beneficial effect of estrogen with respect to outcome measures other than clinical pregnancy. Other than estrogen forms and dosages, factors such as subject age28 or GnRH agonist protocol29 may be relevant and warrant further investigation. The adoption of standardized terminology in assisted reproductive technology30 will also be helpful in future studies.
Footnotes
Abbreviations: CI = confidence interval, E = estrogen, GnRH = gonadotropin-releasing hormone, IVF = in vitro fertilization, LPS = luteal phase support, OR = odds ratio, P = progesterone.
X-MZ and FL contributed equally to this study.
This study was supported by National Natural Science Foundation of China (81100421/81260109/H0425), Jiangsu Health International Exchange Program, Top Six Talent Peaks Program of Jiangsu (2014-WSW-080) and National Science Foundation of Yangzhou (YZ2014050).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pabuccu R, Akar ME. Luteal phase support in assisted reproductive technology. Curr Opin Obstet Gynecol 2005; 17:277–281. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz E, Taboas E, Portela S, et al. Treatment of luteal phase defects in assisted reproduction. Curr Drug Targets 2013; 14:832–842. [DOI] [PubMed] [Google Scholar]
- 3.Check JH. Luteal phase support for in vitro fertilization–embryo transfer—present and future methods to improve successful implantation. Clin Exp Obstet Gynecol 2012; 39:422–428. [PubMed] [Google Scholar]
- 4.Aboulghar M. Luteal support in reproduction: when, what and how? Curr Opin Obstet Gynecol 2009; 21:279–284. [DOI] [PubMed] [Google Scholar]
- 5.Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod 2002; 17:2287–2299. [DOI] [PubMed] [Google Scholar]
- 6.van der Linden M, Buckingham K, Farquhar C, et al. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2011; 10:CD009154. [DOI] [PubMed] [Google Scholar]
- 7.Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database Syst Rev 2004; 3:CD004830. [DOI] [PubMed] [Google Scholar]
- 8.Kolibianakis EM, Venetis CA, Papanikolaou EG, et al. Estrogen addition to progesterone for luteal phase support in cycles stimulated with GnRH analogues and gonadotropins for IVF: a systematic review and meta-analysis. Hum Reprod 2008; 23:1346–1354. [DOI] [PubMed] [Google Scholar]
- 9.Gelbaya TA, Kyrgiou M, Tsoumpou I, et al. The use of estradiol for luteal phase support in in vitro fertilization/intracytoplasmic sperm injection cycles: a systematic review and meta-analysis. Fertil Steril 2008; 90:2116–2125. [DOI] [PubMed] [Google Scholar]
- 10.Jee BC, Suh CS, Kim SH, et al. Effects of estradiol supplementation during the luteal phase of in vitro fertilization cycles: a meta-analysis. Fertil Steril 2010; 93:428–436. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998; 51:1235–1241. [DOI] [PubMed] [Google Scholar]
- 13.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127:820–826. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 16.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000; 320:1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Var T, Tonguc EA, Doğanay M, et al. A comparison of the effects of three different luteal phase support protocols on in vitro fertilization outcomes: a randomized clinical trial. Fertil Steril 2011; 95:985–989. [DOI] [PubMed] [Google Scholar]
- 18.Moini A, Zadeh Modarress S, Amirchaghmaghi E, et al. The effect of adding oral oestradiol to progesterone as luteal phase support in ART cycles—a randomized controlled study. Arch Med Sci 2011; 7:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elgindy EA, El-Haieg DO, Mostafa MI, et al. Does luteal estradiol supplementation have a role in long agonist cycles? Fertil Steril 2010; 93:2182–2188. [DOI] [PubMed] [Google Scholar]
- 20.Serna J, Cholquevilque JL, Cela V, et al. Estradiol supplementation during the luteal phase of IVF-ICSI patients: a randomized, controlled trial. Fertil Steril 2008; 90:2190–2195. [DOI] [PubMed] [Google Scholar]
- 21.Engmann L, DiLuigi A, Schmidt D, et al. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study. Fertil Steril 2008; 89:554–561. [DOI] [PubMed] [Google Scholar]
- 22.Ceyhan ST, Basaran M, Kemal Duru N, et al. Use of luteal estrogen supplementation in normal responder patients treated with fixed multidose GnRH antagonist: a prospective randomized controlled study. Fertil Steril 2008; 89:1827–1830. [DOI] [PubMed] [Google Scholar]
- 23.Drakakis P, Loutradis D, Vomvolaki E, et al. Luteal estrogen supplementation in stimulated cycles may improve the pregnancy rate in patients undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer. Gynecol Endocrinol 2007; 23:645–652. [DOI] [PubMed] [Google Scholar]
- 24.Fatemi HM, Kolibianakis EM, Camus M, et al. Addition of estradiol to progesterone for luteal supplementation in patients stimulated with GnRH antagonist/rFSH for IVF: a randomized controlled trial. Hum Reprod 2006; 21:2628–2632. [DOI] [PubMed] [Google Scholar]
- 25.Gorkemli H, Ak D, Akyurek C, et al. Comparison of pregnancy outcomes of progesterone or progesterone + estradiol for luteal phase support in ICSI-ET cycles. Gynecol Obstet Invest 2004; 58:140–144. [DOI] [PubMed] [Google Scholar]
- 26.Farhi J, Weissman A, Steinfeld Z, et al. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization–embryo transfer cycles. Fertil Steril 2000; 73:761–766. [DOI] [PubMed] [Google Scholar]
- 27.Lewin A, Benshushan A, Mezker E, et al. The role of estrogen support during the luteal phase of in vitro fertilization–embryo transplant cycles: a comparative study between progesterone alone and estrogen and progesterone support. Fertil Steril 1994; 62:121–125. [DOI] [PubMed] [Google Scholar]
- 28.Gleicher N, Brown T, Dudkiewicz A, et al. Estradiol/progesterone substitution in the luteal phase improves pregnancy rates in stimulated cycles—but only in younger women. Early Pregnancy 2000; 4:64–73. [PubMed] [Google Scholar]
- 29.Fatemi HM, Popovic-Todorovic B, Papanikolaou E, et al. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update 2007; 13:581–590. [DOI] [PubMed] [Google Scholar]
- 30.Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92:1520–1524. [DOI] [PubMed] [Google Scholar]


