Abstract
To investigate the association between macular thickness and peripapillary retinal nerve fiber layer (RNFL) thickness in Chinese children.
This cross-sectional study recruited consecutive cases of healthy pediatric subjects aged 4 to 18 from Caritas Medical Centre in Hong Kong Special Administrative Region, China, from 2013 to 2014. Subjects with only eye, ocular tumors, congenital glaucoma, congenital cataract, congenital nystagmus, microphthalmos, optic nerve or retinal disease, active ocular infections, corneal scars, and severe visual impairment of any cause were excluded. Peripapillary RNFL thickness and macular thickness at 1-mm-diameter fovea center (C1), 3-mm-diameter temporal quadrant (T3), and 3-mm- diameter nasal quadrant (N3) were measured with optical coherence tomography. Best-corrected visual acuity, axial length, and cycloplegic refraction were also recorded. Spearman correlation was used to analyze the association between T3, C1, and N3 with each of the following: average and quadrant RNFL thickness, axial length, and spherical equivalent.
In 179 subjects, the mean age was 7.9 ± 3.6 years. There were 90 male and 89 female subjects, all of Chinese ethnicity. The mean spherical equivalent was −0.1 ± 3.1 D and mean axial length was 22.9 ± 1.4 mm. There were significant and positive correlations of the average (T3: r = 0.20, P = 0.04; N3: r = 0.2, P = 0.005), superior (T3: r = 0.20, P = 0.03; N3: r = 0.2, P = 0.03), and inferior (T3: r = 0.20, P = 0.02; N3: r = 0.2, P = 0.01) peripapillary RNFL thicknesses with the T3 and N3 macular thicknesses but not C1. The nasal peripapillary RNFL thickness was also positively correlated with T3 (r = 0.20, P = 0.01). There were no significant associations between the macular thickness (T3, C1, N3) with neither the spherical equivalent (P > 0.2) nor the axial length (P > 0.3).
The macular thickness was positive correlated with the peripapillary RNFL thickness in a population of healthy Chinese children.
INTRODUCTION
Glaucoma is characterized by a progressive loss of the peripapillary retinal nerve fiber layer (RNFL). RNFL thickness imaging is an important diagnostic and monitoring modality for glaucoma. However, in those with optic nerve head deformities, tilted discs, peripapillary atrophy, and high myopia, the peripapillary RNFL may be of limited use as the normative database of most imaging programs do not account for these optic nerve head variants. The prevalence of myopia varies among different populations but is particularly high in East Asian regions.1–3 The macular region contains almost half of the retinal ganglion cells in the eye.4 Previous studies have demonstrated significant correlations between the macular thickness and the peripapillary RNFL thickness in adults.4–11 Furthermore, macular thickness measurement was suggested to be useful in the early detection of glaucoma.4,12,13 Optical coherence tomography (OCT) is a noninvasive imaging modality capable of objectively measuring the macular thickness and peripapillary RNFL thickness in children as young as the age of 3.14–16 The purpose of this study was to investigate the association between macular thickness and peripapillary RNFL thickness in Chinese children.
METHODS
The study was conducted in accordance with the Declaration of Helsinki and no patient personal data were disclosed in the study. Study approval was obtained from the Institutional Review Board of the Hospital Authority of Hong Kong. Informed consent was obtained from the parents or legal guardians of the subjects. The authors declare no financial or proprietary interests.
This cross-sectional study recruited consecutive cases of pediatric subjects aged 4 to 18, attending the ophthalmology specialist outpatient clinic of Caritas Medical Centre in Hong Kong Special Administrative Region, China, from 2013 to 2014. Subjects with only eye, ocular tumors, congenital glaucoma, congenital cataract, congenital nystagmus, microphthalmos, optic nerve or retinal disease, active ocular infections, corneal scars, amblyopia, and extremes of refractive errors (spherical equivalent <−10 or >+10 diopters [D]) were excluded. Parts of the methodology have been discussed in other related studies by the authors.14
All subjects underwent a complete ophthalmological examination including ocular alignment and motility assessments as well as anterior and posterior segment examinations after pupil dilatation with a tropicamide 1% and phenylephrine hydrochloride 2.5% ophthalmic solution (Mydrin-P, Santen Pharmaceutical, Osaka, Japan).
Spherical Equivalent and Axial Length
All subjects received cycloplegic refraction with 3 drops of cyclopentolate hydrochloride 1% (Bausch & Lomb, Rochester, NY) administered 5 minutes apart to relieve all accommodative components. After at least 30 minutes, postcycloplegic autorefraction with a keratorefractometer (Topcon KR-8900 by Topcon Europe Medical B.V., Essebaan 11, 2908 LJ, Capelle a/d Ijssel, the Netherlands) was performed by an optometrist with at least 5 years of experience with pediatric assessment. The spherical equivalent was calculated in diopters. Axial length measurements in millimeters (mm) were obtained with the noncontact optical biometry (IOL Master, Carl Zeiss Meditec AG, Max-Dohrn-Straße 8–10 10589 Berlin, German). Axial length measurements were performed 3 times by a single technician who was masked to subjects’ clinical information, and the average of the 3 values was recorded. Poor signal values as well as values that differed by more than 0.1 mm were rejected and the measurement was repeated.
Optical Coherence Tomography Imaging
The Spectralis Spectral Domain OCT (Heidelberg Engineering, Carlsbad, CA) was performed after cycloplegia, by a single, imaging technician who was masked to subjects’ clinical information.
Peripapillary RNFL Thickness
Scans were centered on the optic disc with a scanning diameter of 3.5 mm and 768 A scans were obtained using the High Speed (HS) mode. To improve image quality, Automatic Real Time (ART) function was used to obtain multiple frames during scanning and to optimize images by noise reduction. Scans were repeated 3 times and assessed for signal strength and centration. Scans with signal strength quality ≤16 or poor centration were excluded. RNFL thickness was analyzed with the RNFL Single Exam Report OU with fovea-to-disc technology. The RNFL thickness of each of the 4 quadrants and the global RNFL thickness were recorded in micrometers (μm). To adjust for the OCT magnification based on differences in axial length, the Littmann formula was used to calculate the Littmann-adjusted RNFL = 3.382 × 0.01306 × (axial length − 1.82) × unadjusted RNFL.17–21
Macular Thickness Measurement
The Spectralis OCT has an axial image resolution of 7 μm, a lateral resolution of 14 μm, and a scanning velocity up to 40,000 A scans per second. Macular measurements were acquired using a dense (25-line) horizontal Raster Scan protocol, centered on the fovea with a distance of 240 μm between the horizontal scans. The TruTrack active eye tracking system was used to increase scan quality. The following 3 parameters were recorded in this study (Figure 1):
FIGURE 1.
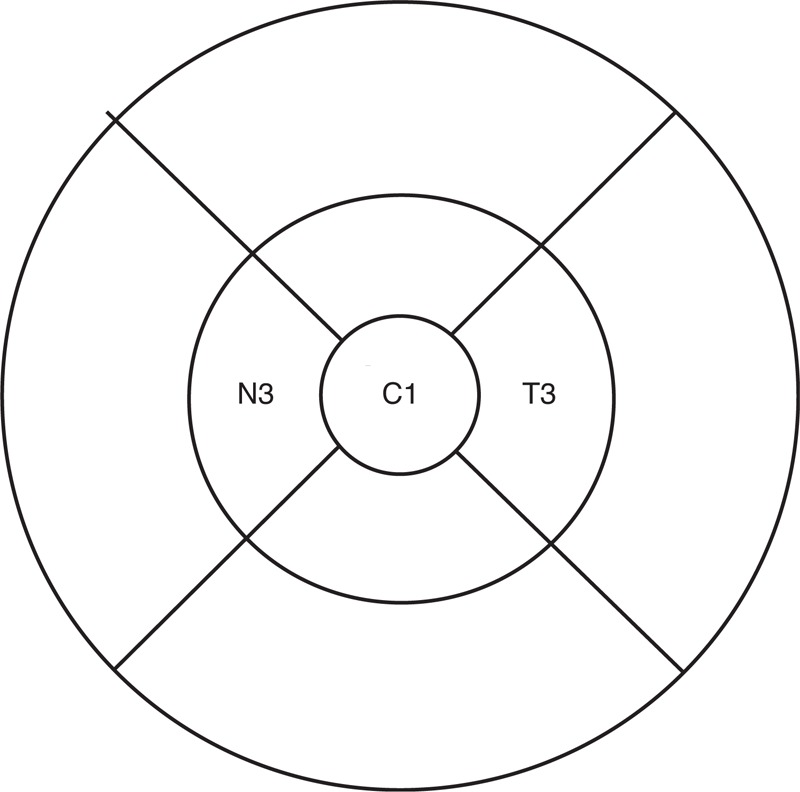
ETDRS grid showing T3, C1, and N3.
C1 = The average thickness in the central 1 mm diameter of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid,22 representing the foveal central subfield thickness in a 0.5 mm radius.
T3 = The average thickness in the temporal quadrant of a concentric ring with an inner diameter of 1 mm and an outer diameter of 3 mm of ETDRS grid. This represents the temporal macular thickness up to a 1.5 mm radius from the foveal center.
N3 = The average thickness in the nasal quadrant of a concentric ring with an inner diameter of 1 mm and an outer diameter of 3 mm of ETDRS grid. This represents the nasal macular thickness up to a 1.5 mm radius from the foveal center.
Statistics
Only the right eye of each subject was used for statistical analysis. Statistical significance was considered when P < 0.05. Means were expressed with standard deviations.
Normality testing was performed using the D’Agostino & Pearson omnibus normality test. Spearman correlation was used to analyze the correlations of C1, T3, and N3 with each of the following: peripapillary RNFL thickness (average, superior, inferior, nasal, temporal), axial length, and spherical equivalent.
RESULTS
Of the 179 subjects eligible for the study, the mean age was 7.9 ± 3.6 years (range: 4.0–18.0, median: 7.0). There were 90 male and 89 female subjects, all of Chinese ethnicity. The mean cycloplegic spherical equivalent was −0.1 ± 3.1 D (range: −10.0–8.9 D, median: 0.13 D) and the mean axial length was 22.9 ± 1.4 mm (range: 20.2–27.4 mm, median: 22.9 mm). The mean best-corrected Snellen visual acuity was 0.8 ± 0.5 (range: 0.1–1.2, median: 0.8). Myopia (spherical equivalent <−1 D) was found in 31.8% (57/179) of subjects, hypermetropia (spherical equivalent >+1 D) accounted for 34.6% (62/179), and emmetropia (spherical equivalent ≥−1 D to ≤+1 D) was seen in 33.5% (60/179) of subjects. There were no significant correlations between the macular thickness (T3, C1, N3) with neither the spherical equivalent (P > 0.2) nor the axial length (P > 0.3).
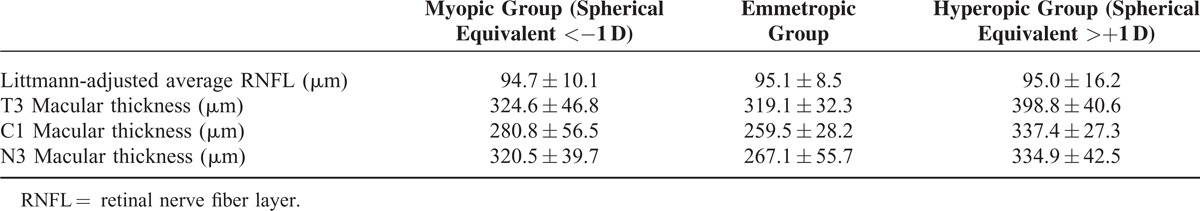
The peripapillary RNFL measurements (mean, range, and median) both with and without Littmann adjustments are summarized in Table 1. The macular thicknesses were as follows: T3 (321.0 ± 39.8 μm) (range: 172.0–353.0 μm, median: 323.0 μm), C1 (268.9 ± 48.7 μm) (range: 115.0–577.0 μm, median: 264.0 μm), and N3 (337.0 ± 37.4 μm) (range: 201–549 μm, median: 338.0 μm). The distribution of the Littmann-adjusted RNFL and macular thickness among the refractive groups has been summarized in Table 2.
TABLE 1.
Mean, Range, and Median of Peripapillary RNFL Thickness With and Without Littmann Adjustment
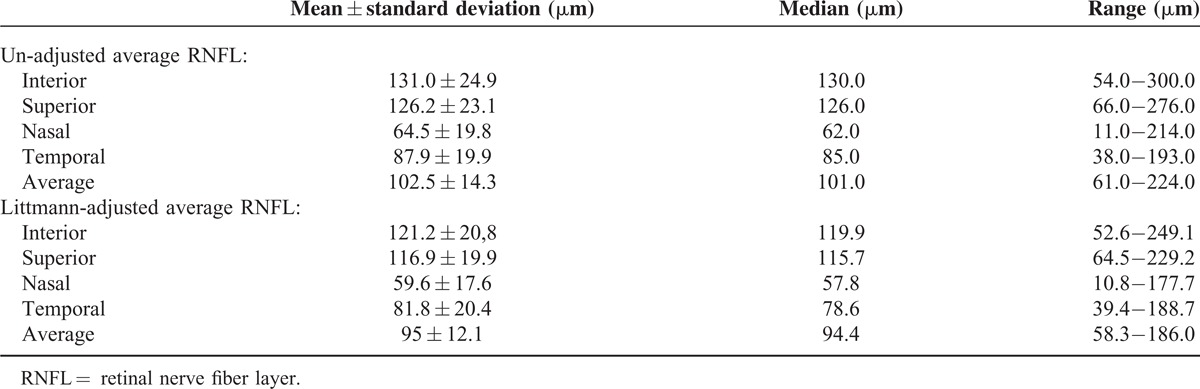
TABLE 2.
Littmann-Adjusted RNFL and Macular Thickness Among Different Refractive Groups
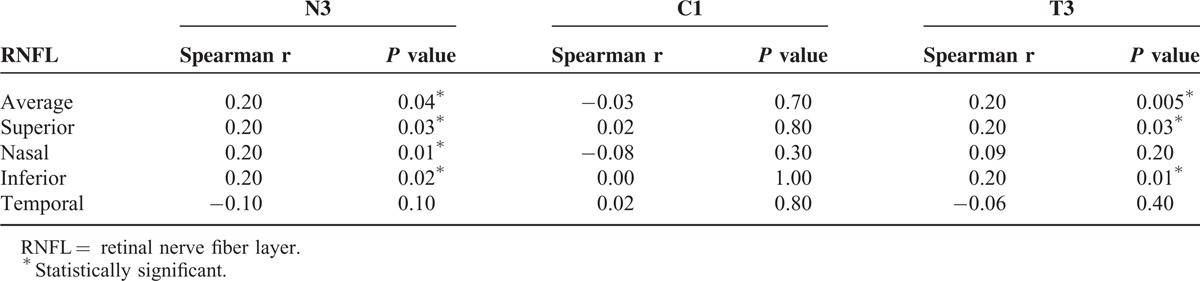
There were significant and positive correlations of the average, superior, and inferior peripapillary RNFL thicknesses with the T3 and N3 macular thicknesses but not C1. The nasal peripapillary RNFL thickness was also positively correlated with T3. There was no significant correlation of the macular thickness with the temporal peripapillary RNFL thickness (P > 0.1) (Table 3).
TABLE 3.
Spearman Correlation of Peripapillary RNFL Thickness With Macular Thickness
After Littmann adjustment of the RNFL, there continued to be significant and positive correlations of the average and superior peripapillary RNFL thicknesses with the T3 and N3 macular thicknesses but not C1. There were also significant and positive correlations of the nasal and inferior peripapillary RNFL thicknesses with the T3 but not the N3 or C1 macular thicknesses. There was no significant correlation of the macular thickness with the temporal peripapillary RNFL thickness (P > 0.1) (Table 4).
TABLE 4.
Spearman Correlation of Peripapillary RNFL Thickness With Macular Thickness After Littmann Adjustment
Linear regression showed a significant linear relationship of the T3 (r2 = 0.03, P = 0.02) and N3 (r2 = 0.04, P = 0.01) macular thickness with the average peripapillary, Littmann-adjusted, RNFL thickness (Figure 2).
FIGURE 2.
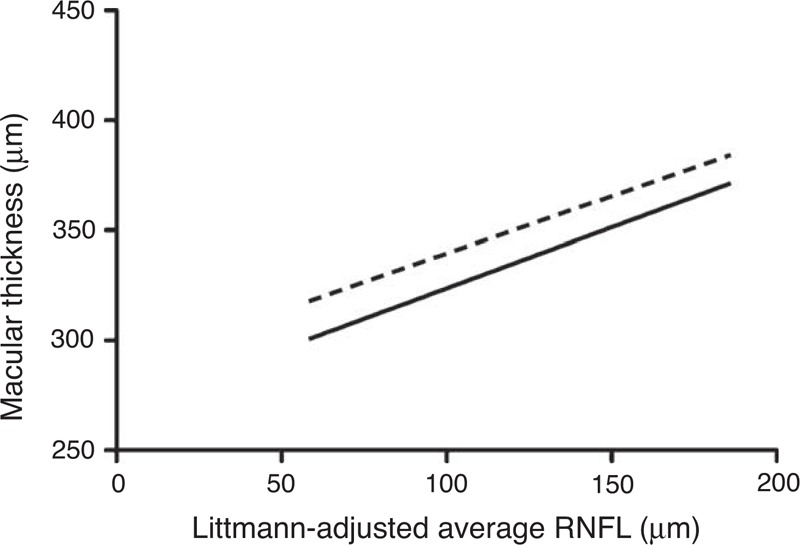
Linear regression of macular thickness with average peripapillary RNFL thickness (Littmann-adjusted). RNFL = retinal nerve fiber layer, dotted line = N3, solid line = T3.
DISCUSSION
The diagnosis of glaucoma in children poses a particular challenge because the majority of normative database of peripapillary RNFL thickness found in imaging machines is based on the adult population. Furthermore, young children are also unable to produce reliable visual fields. Thus, clinical examination and stereoscopic optic disc photography has been the mainstay of diagnosis and monitoring in pediatric glaucoma. However, OCT is an objective and reproducible tool that can assess the macula thickness and peripapillary RNFL thickness even in young children.14,23 Recently, a study by Cingu et al has reported that RNFL measurements were a useful tool to diagnose and follow up glaucoma in children with vernal keratoconjunctivitis under corticosteroid treatment.24 Garas et al have also demonstrated using a Fourier-domain OCT that the ganglion cell complex parameters (RNFL, the retinal ganglion cell layer and the inner-plexiform layer) had moderate sensitivity, high specificity, positive predictive value, and positive likelihood ratio for glaucoma.25
The peripapillary RNFL thickness in children can vary greatly depending on their axial length and refractive status.26,27 Genetics and long hours of near work make East Asian children more susceptible to myopia than their Caucasian counterparts.28,29 Thus, in myopic Asian children, there is no effective and reliable way to benchmark the amount of ganglion cell loss in those suspected to have glaucoma. In our population of healthy Chinese children, over a third was myopic, which was in line with Fan et al, who also reported a myopic prevalence of 36.71% ± 2.87% in a similar Chinese pediatric population.30 More than half of the retinal ganglion cells are located within 4 to 5 mm of the fovea with the thickest cell density (4–6 cell bodies) located between 750 and –1100 μm from the foveal center.4,12 Thus, in this study, we have chosen to assess the nasal and temporal macular thicknesses up to a 1.5 mm radius from the foveal center. This shortens the OCT scanning time, making it possible to perform the scan even in young children with limited attention span while still acquiring the essential information.
Previously, macular thickness was found to be correlated with the refractive status31–34 and axial length35,36 in children. The independent association of macular thickness and foveal thickness with amblyopia have been explored by Yalcin et al and Wu et al, reporting a thicker peripapillary RNFL thickness and foveal thickness in amblyopic eyes.37,38 In adults, studies have demonstrated that thinning of the inner macular layers was observed in early and established glaucoma.4–11,39–42 Hess et al were one of the first to report that the macular volume was significantly reduced in children with glaucoma versus controls.16
To the best of our knowledge, this is one of the first studies using the Spectralis OCT machine to investigate the correlation between the macular thickness and the peripapillary RNFL thickness in a healthy pediatric population. In our study, the nasal and temporal 3-mm macular thickness was positively correlated with the average, superior, and nasal peripapillary RNFL thickness and both the nasal and temporal thickness demonstrated a positive linear relationship with the average peripapillary RNFL thickness. There was however, no association between the macular thickness with either axial length or spherical equivalent unlike in adults where a thinner macular thickness was correlated with a longer axial length.35,43 Furthermore, changes in the axial length or refractive status did not seem to correlate with the T3 and N3 macular thickness (both P > 0.2), which is important as both parameters will change with age in children, suggesting that the macular thickness is independent of the axial length and refractive status of the child.
Our study had its limitations. Firstly, it would have been ideal to record all 9 segments of the macular thickness and including up to a 6 mm diameter from the fovea center. However, this would imply a longer scanning time and excess eye movements in young children can lead to artifacts and unreliable scans so the authors decided to focus on the central 3-mm macular thickness which should contain the highest concentration of ganglion cells. Secondly, we did not account for the effects of the internal limiting membrane on the macular thickness so the macular thickness may not be a direct representation of the RNFL at the macula but nevertheless, diseases affecting just the internal limiting membrane thickness are not common so measurement of the macular thickness served its purpose in this study. Thirdly, this study only recruited healthy children and it be would be vital to investigate in a pediatric glaucoma population whether the positive correlation between macular thickness and peripapillary RNFL thickness still holds. Fourthly, although the correlations were statistically significant in the Spearman test, the “r” values were low, representing weak relationships only. Lastly, the study sample was from a healthy Chinese pediatric population so that results may not be generalizable populations with different demographics.
CONCLUSION
In a population of healthy Chinese children, the macular thickness was positively correlated with the peripapillary RNFL thickness.
Acknowledgments
The authors would like to thank Mr Ernest Kwan for his expertise in performing all the Optical Coherence Tomography scans in this study and for commitment to research.
Footnotes
Abbreviations: μm = micrometer, C1 = macular thickness at area of 1 mm diameter from foveal center, D = diopter, IOP = intraocular pressure, N3 = nasal quadrant macular thickness at area of 1.5 mm radius from foveal center, OCT = optical coherence tomography, RNFL = retinal nerve fiber layer, T3 = temporal quadrant macular thickness at area of 1.5 mm radius from foveal center.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Katz J, Tielsch JM, Sommer A. Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci 1997; 38:334–340. [PubMed] [Google Scholar]
- 2.Wong TY, Foster PJ, Hee J, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci 2000; 41:2486–2494. [PubMed] [Google Scholar]
- 3.Wang Q, Klein BE, Klein R, et al. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 1994; 35:4344–4347. [PubMed] [Google Scholar]
- 4.Giovannini A, Amato G, Mariotti C. The macular thickness and volume in glaucoma: an analysis in normal and glaucomatous eyes using OCT. Acta Ophthalmol Scand Suppl 2002; 236:34–36. [DOI] [PubMed] [Google Scholar]
- 5.Bagga H, Greenfield DS, Knighton RW. Macular symmetry testing for glaucoma detection. J Glaucoma 2005; 14:358–363. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol 2005; 139:44–55. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield DS, Bagga H, Knighton RW. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol 2003; 121:41–46. [DOI] [PubMed] [Google Scholar]
- 8.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am J Ophthalmol 2004; 138:218–225. [DOI] [PubMed] [Google Scholar]
- 9.Ojima T, Tanabe T, Hangai M, et al. Measurement of retinal nerve fiber layer thickness and macular volume for glaucoma detection using optical coherence tomography. Jpn J Ophthalmol 2007; 51:197–203. [DOI] [PubMed] [Google Scholar]
- 10.Wollstein G, Ishikawa H, Wang J, et al. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol 2005; 139:39–43. [DOI] [PubMed] [Google Scholar]
- 11.Sakata LM, Deleon-Ortega J, Sakata V, et al. Optical coherence tomography of the retina and optic nerve—a review. Clin Experiment Ophthalmol 2009; 37:90–99. [DOI] [PubMed] [Google Scholar]
- 12.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol 1990; 300:5–25. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitaki V, Tsilimbaris MK, Pallikaris A, et al. Macular retinal and nerve fiber layer thickness in early glaucoma: clinical correlations. Middle East Afr J Ophthalmol 2012; 19:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Yau GS, Woo TT, et al. The anterior chamber depth and retinal nerve fiber layer thickness in children. ScientificWorldJournal 2014; 2014:538283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Dairi MA, Asrani SG, Enyedi LB, et al. Optical coherence tomography in the eyes of normal children. Arch Ophthalmol 2009; 127:50–58. [DOI] [PubMed] [Google Scholar]
- 16.Hess DB, Asrani SG, Bhide MG, et al. Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am J Ophthalmol 2005; 139:509–517. [DOI] [PubMed] [Google Scholar]
- 17.Leung CK, Mohamed S, Leung KS, et al. Retinal nerve fiber layer measurements in myopia: An optical coherence tomography study. Invest Ophthalmol Vis Sci 2006; 47:5171–5176. [DOI] [PubMed] [Google Scholar]
- 18.Littmann H. Determination of the real size of an object on the fundus of the living eye. Klin Monbl Augenheilkd 1982; 180:286–289. [DOI] [PubMed] [Google Scholar]
- 19.Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol 1994; 232:361–367. [DOI] [PubMed] [Google Scholar]
- 20.Kang SH, Hong SW, Im SK, et al. Effect of myopia on the thickness of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci 2010; 51:4075–4083. [DOI] [PubMed] [Google Scholar]
- 21.Savini G, Barboni P, Parisi V, et al. The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectral-domain OCT. Br J Ophthalmol 2012; 96:57–61. [DOI] [PubMed] [Google Scholar]
- 22.Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98 (5 suppl):786–806. [PubMed] [Google Scholar]
- 23.Massin P, Vicaut E, Haouchine B, et al. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol 2001; 119:1135–1142. [DOI] [PubMed] [Google Scholar]
- 24.Cingu AK, Cinar Y, Turkcu FM, et al. Evaluation of retinal nerve fiber layer thickness in vernal keratoconjunctivitis patients under long-term topical corticosteroid therapy. Cutan Ocul Toxicol 2014; 33:184–188. [DOI] [PubMed] [Google Scholar]
- 25.Garas A, Vargha P, Hollo G. Diagnostic accuracy of nerve fibre layer, macular thickness and optic disc measurements made with the RTVue-100 optical coherence tomograph to detect glaucoma. Eye (Lond) 2011; 25:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung MM, Huang RY, Lam AK. Retinal nerve fiber layer thickness in normal Hong Kong Chinese children measured with optical coherence tomography. J Glaucoma 2010; 19:95–99. [DOI] [PubMed] [Google Scholar]
- 27.Tas M, Oner V, Turkcu FM, et al. Peripapillary retinal nerve fiber layer thickness in hyperopic children. Optom Vis Sci 2012; 89:1009–1013. [DOI] [PubMed] [Google Scholar]
- 28.French AN, Morgan IG, Mitchell P, et al. Patterns of myopigenic activities with age, gender and ethnicity in Sydney schoolchildren. Ophthalmic Physiol Opt 2013; 33:318–328. [DOI] [PubMed] [Google Scholar]
- 29.French AN, Morgan IG, Mitchell P, et al. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology 2013; 120:2100–2108. [DOI] [PubMed] [Google Scholar]
- 30.Fan DS, Lam DS, Lam RF, et al. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci 2004; 45:1071–1075. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, He X, Zhu J, et al. Macular measurements using optical coherence tomography in healthy Chinese school age children. Invest Ophthalmol Vis Sci 2011; 52:6377–6383. [DOI] [PubMed] [Google Scholar]
- 32.Huynh SC, Wang XY, Rochtchina E, et al. Distribution of macular thickness by optical coherence tomography: findings from a population-based study of 6-year-old children. Invest Ophthalmol Vis Sci 2006; 47:2351–2357. [DOI] [PubMed] [Google Scholar]
- 33.Wakitani Y, Sasoh M, Sugimoto M, et al. Macular thickness measurements in healthy subjects with different axial lengths using optical coherence tomography. Retina 2003; 23:177–182. [DOI] [PubMed] [Google Scholar]
- 34.Yau GSK, Lee JWY, Woo TWY, Wong RLM, Wong IYH. Central macular thickness in children with myopia, emmetropia, and hyperopia: an optical coherence tomography study. Biomed Res Int 2015; Article ID 847694, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam DS, Leung KS, Mohamed S, et al. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci 2007; 48:376–382. [DOI] [PubMed] [Google Scholar]
- 36.Wong AC, Chan CW, Hui SP. Relationship of gender, body mass index, and axial length with central retinal thickness using optical coherence tomography. Eye (Lond) 2005; 19:292–297. [DOI] [PubMed] [Google Scholar]
- 37.Yalcin E, Balci O. Peripapillary retinal nerve fiber layer and foveal thickness in hypermetropic anisometropic amblyopia. Clin Ophthalmol 2014; 8:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu SQ, Zhu LW, Xu QB, et al. Macular and peripapillary retinal nerve fiber layer thickness in children with hyperopic anisometropic amblyopia. Int J Ophthalmol 2013; 6:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan O, Li G, Lu AT, et al. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology 2008; 115:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa H, Stein DM, Wollstein G, et al. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci 2005; 46:2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guedes V, Schuman JS, Hertzmark E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology 2003; 110:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lederer DE, Schuman JS, Hertzmark E, et al. Analysis of macular volume in normal and glaucomatous eyes using optical coherence tomography. Am J Ophthalmol 2003; 135:838–843. [DOI] [PubMed] [Google Scholar]
- 43.Wu PC, Chen YJ, Chen CH, et al. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye (Lond) 2008; 22:551–555. [DOI] [PubMed] [Google Scholar]


