Abstract
HMG-CoA reductase inhibitors (also known as statins) are widely used as lipid-lowering agents in patients with rheumatoid arthritis (RA) to reduce their cardiovascular risk. However, whether they have an effect on RA disease activity is controversial.
This study aimed to investigate the effect of statins on disease activity in RA patients.
A systematic literature review was performed using the MEDLINE, EMBASE, Cochrane Library, ISI WEB of Knowledge, Scopus, and Clinical Trials Register databases.
Only prospective randomized controlled trials or controlled clinical trials comparing the efficacy of statins with placebo on adult RA patients were included. The efficacy was measured according to the ACR criteria, EULAR criteria, DAS28, HAQ score, ESR, or CRP.
The Jadad score was used for quality assessment. The inverse variance method was used to analyze continuous outcomes. A fixed-effects model was used when there was no significant heterogeneity; otherwise, a random-effects model was used. For stability of results, we performed leave-one-study-out sensitivity analysis by omitting individual studies one at a time from the meta-analysis. Publication bias was assessed using Egger test.
A total 13 studies involving 737 patients were included in the meta-analysis; 11 studies were included in the meta-analysis based on DAS28, while the other 2 studies were only included in the meta-analysis based on ESR or CRP. The standardized mean difference (SMD) in DAS28 between the statin group and the placebo group was −0.55 (95% CI [−0.83, −0.26], P = 0.0002), with an I2 value of 68%. Subgroup analysis showed that patients with more active disease tended to benefit more from statin therapy (SMD −0.73, P = 0.01) than patients with moderate or low disease activity (SMD −0.38, P = 0.03). Statin therapy also significantly reduced tender joint counts, swollen joint counts, ESR, and CRP compared with placebo, but the reduction in HAQ score and VAS was not significant (P > 0.05).
This meta-analysis suggested that statin therapy might be effective in the reduction of RA disease activity measured by DAS28, TJC, SJC, as well as ESR and CRP.
INTRODUCTION
3-Hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, also known as statins, are a class of drugs that are used to lower cholesterol levels by inhibiting the HMG-CoA reductase enzyme, which plays a central role in the production of cholesterol. Due to their cholesterol-lowering effects, statins are widely used in the treatment of cardiovascular diseases (CVDs), the latter accounts for approximately 30% of all deaths globally.1 Recent experimental and clinical evidence suggests that the beneficial effects of statins are pleiotropic, extending beyond their cholesterol-lowering effects.2 Statins have been shown to possess many anti-inflammatory and immunomodulatory properties that can influence multiple steps in the inflammatory process.3–6 Some clinical studies also support these findings. The JUPITER trial showed that statins are effective in the primary prevention of CVDs in patients with elevated CRP levels but relatively low cholesterol levels and other cardiovascular risks, suggesting a non-cholesterol-dependent effect of statins because the reduction in CRP by rosuvastatin was not related to the reduction in low-density lipoprotein (LDL) cholesterol.7 The recent COSMOS trial found that rosuvastatin significantly reduces intravascular ultrasound-detected intracoronary plaque volume, unrelated to the reduction in plasma LDL, supporting the idea of effects beyond cholesterol-lowering effects.8
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that primarily affects the synovial tissue, but it is also a systemic disease that can affect many systems. The etiology of RA is unclear. However, it is accepted that autoimmune responses, the dysregulation of T-helper 1-mediated immune responses in particular, play distinct roles in the pathogenesis of RA. Aberrant T-cell activation stimulates monocytes and macrophages to produce inflammatory cytokines and proteolytic enzymes, initiating the destruction.9
Recent studies show that in addition to their effects in reducing cardiovascular risks, statins may provide mild anti-inflammatory benefits in RA.10–12 These studies have suggested that statin treatment has a promising effect on disease activity in RA patients, which may be partly due to the immunomodulatory and anti-inflammatory properties. However, the results of these studies are controversial, and they consist largely of single-center studies with small sample sizes. Therefore, in this study, we conducted a comprehensive meta-analysis to evaluate the effect of statins on disease activity in RA patients.
METHODS
The meta-analysis was performed according to the recommendations of the Cochrane Collaboration,13 and the findings are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.14 As this study is a meta-analysis, ethical approval was not required.
Search Strategy
The following databases were searched on March 20, 2014: MEDLINE (PubMed), EMBASE (Ovid), the Cochrane Central Register of controlled trials, Scopus (Elsevier), and ISI Web of Knowledge. A combination of the following terms was used: “Rheumatoid arthritis”, AND “HMG-CoA reductase inhibitor”, “Anticholesteremic Agents”, statin, simvastatin, pravastatin, rosuvastatin, atorvastatin, fluvastatin, lovastatin, or pitavastatin. There was a limitation with regard to language in that we only considered English publications, but the year of publication was not limited. Type filters provided by the databases were not used during the search to ensure sensitivity. We also used references from the reports, clinical trial registers (ClinicalTrials.gov), and Internet browsers.
Inclusion and Exclusion Criteria
A study was included in the meta-analysis if it fulfilled the following criteria: the patients enrolled were adult (older than 16 years old) RA patients diagnosed using the American College of Rheumatology (ACR) criteria; the study had a prospective randomized controlled trial (RCT) or controlled clinical trial (CCT) design, in which the effects of statins were compared to placebo; and the efficacy was measured according to the American College of Rheumatology criteria (ACR20, ACR50, ACR70), European League Against Rheumatism (EULAR) criteria, disease activity score in 28 joints (DAS28), functionality assessment according to the health assessment questionnaire (HAQ), or inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). The definitions of RCT and CCT were based on the Cochrane Handbook; that is, a trial was eligible if the individuals followed in the trial were assigned prospectively to 1 of 2 (or more) alternative forms of intervention using either random allocation or some quasi-random method of allocation (such as alternation, date of birth, or case record number). Single-arm clinical trials, reviews, and reports involving only laboratory findings, underlying disease mechanisms, and treatment mechanisms were excluded.
Trial Selection and Data Extraction
The titles and abstracts of the trials were assessed independently by 2 of the authors (B.X. and L.Z.). The full-text version of all publications that potentially qualified for the review was assessed in detail based on the inclusion and exclusion criteria. Data extraction was performed independently by 2 of the authors (B.X. and Y.Y.). The following information was extracted from each study: the study design, patient characteristics, interventions, outcomes, and study duration. For studies with multiple time points, 1 time point was chosen for improved consistency with the other studies. For each outcome measure of interest, the following data were extracted from each intervention group: number of patients, the mean change in the outcome measure, and the standard deviation of the change. The standard deviations of continuous data were either extracted from the reports directly or calculated using a 95% CI interval, standard error, or the P value of the t-test reported using the statistical methods provided in the Cochrane Handbook.13 For studies presenting only baseline values and final values, the change values were imputed using the method provided in the Cochrane Handbook.13 The extracted data were compared. The protocol assumed that in the case of discrepancies between the investigators, another investigator would act as an arbiter until consensus was achieved.
Assessment of Trial Quality
The methodology of the trials included in the review was assessed using the Jadad score.15 By definition, the scores ranged from 0 to 5, with higher scores indicating less likelihood of bias in the results.
Statistical Methods of Meta-Analysis
We calculated the standardized mean differences (SMD) for DAS28 to account for the possible use of different versions. Mean differences were calculated for other continuous data, such as ESR, CRP, HAQ, tender joint counts (TJC), swollen joint counts (SJC), and visual analog score (VAS). A mean difference lower than zero indicated that the patients from the experimental group had lower disease activity than those from the control group. By default, a fixed data model was applied. Heterogeneity of the trials was then assessed using the χ2 and I2 tests. When compared trials had a high heterogeneity (I2 > 50%), the random-effects model was applied instead. The results were considered statistically significant at a level of P < 0.05. RevMan 5.2 (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used to conduct the meta-analysis, and STATA 12.0 (StataCorp LP, College Station, TX) was used for the analysis of sensitivity and publication bias.
RESULTS
Characteristics of the Included Studies
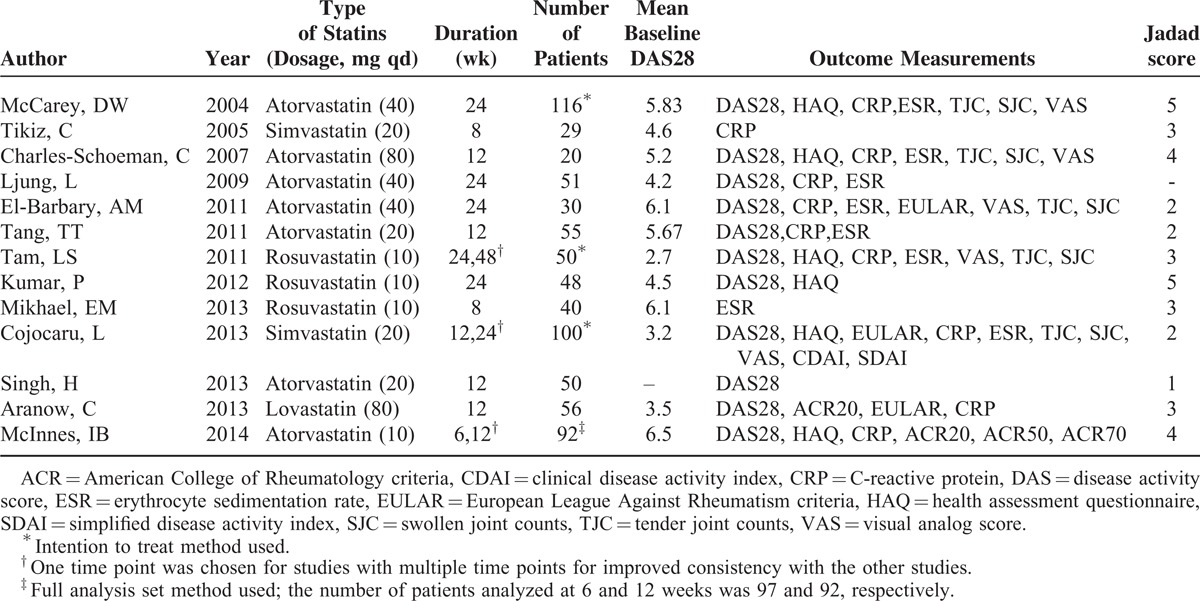
Figure 1 describes the flowchart of the literature search. A total of 13 studies (737 patients) were finally included in the meta-analysis: 10 were full-text articles,10–12,16–22 and 3 were abstracts.23–25 Attempts were done to contact the investigators of these 3 studies to gain more information. The risk of bias of the included studies was assessed using the Jadad score (Table 1). All studies included were single-center studies (Table 1), and the number of patients enrolled ranged from 20 to 116. Twelve studies enrolled RA patients with active disease, with a mean baseline DAS28 ranging from 3.5 to 6.5. One study enrolled stable RA patients, with a mean baseline DAS28 of 2.7. Atorvastatin was used in 7 studies (414 patients) at a dose ranging from 10 to 80 mg qd. Simvastatin was used in 2 studies (129 patients) at a dose of 20 mg qd. Rosuvastatin was used in 3 studies (138 patients) at a dose of 10 mg qd. Lovastatin was used in only 1 study (56 patients) at a dose of 80 mg qd. The duration of therapy ranged from 4 weeks to as long as 48 weeks. As 11 of the studies used DAS28 to measure the final outcome,10–12,16–20,23–25 we performed the meta-analysis mainly based on DAS28. HAQ, EULAR criteria, ACR criteria, CRP, and ESR were used to measure outcomes in some of the studies (shown in Table 1).
FIGURE 1.
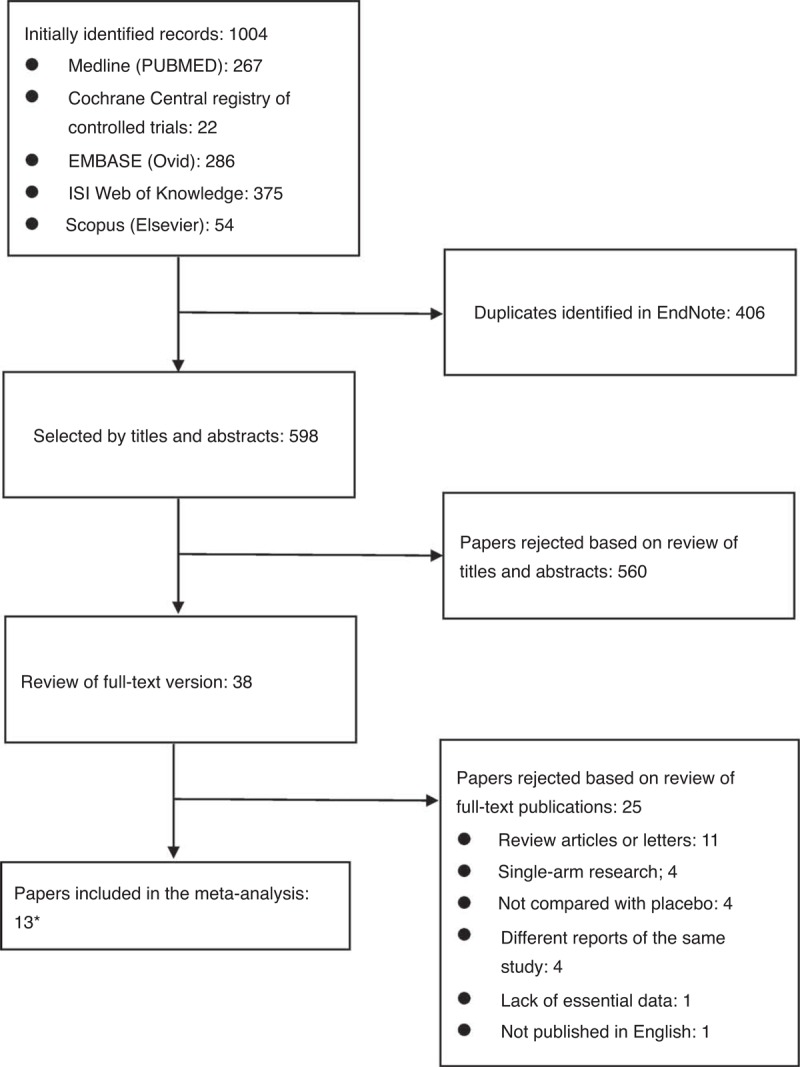
Flowchart of the study selection.∗3 of them have only abstracts available.
TABLE 1.
Characteristics of the Included Studies
The Effect of Statins Based on DAS28
A total of 11 studies used DAS28 to evaluate the effect of statins: 9 presented the DAS28 change from baseline,10–12,16,17,20,23–25 and 2 presented only baseline and final DAS28.18,19 The results are shown in Figure 2. The overall SMD of DAS28 was −0.55 (95% CI [−0.83,−0.26], P = 0.0002), which indicated that the DAS28 reduction in the statin group was 0.55 lower than that in the placebo group. This result was statistically significant. The I2 was 68%, suggesting a high heterogeneity. A subgroup analysis was conducted based on the statin used and the disease activity presented by baseline DAS28 score; the results showed that atorvastatin significantly reduced disease activity measured based on DAS28 (SMD −0.77, 95% CI [−1.17, −0.36], P = 0.0002, I2 = 71%) (shown in Figure 3A). No dose-dependent reduction of disease activity was observed. Furthermore, the patients with higher disease activity (defined as baseline DAS28 ≥5.1) tended to benefit more from statin therapy (SMD −0.73, 95% CI [−1.28, −0.18], P = 0.0007, I2 = 79%) than the patients with moderate and low activity (shown in Figure 3B).
FIGURE 2.
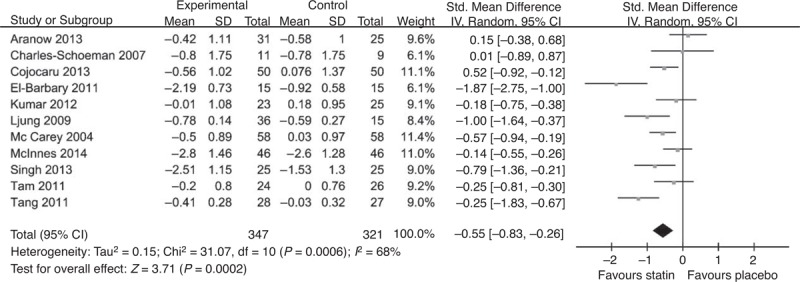
Forest graph of the meta-analysis of the effect of statins versus placebo on DAS28 in RA patients. The pooled standardized mean difference was −0.55, 95% CI [−0.83, −0.26], P = 0.0002, I2 = 68%. CI = confidence interval, SD = standard deviation.
FIGURE 3.
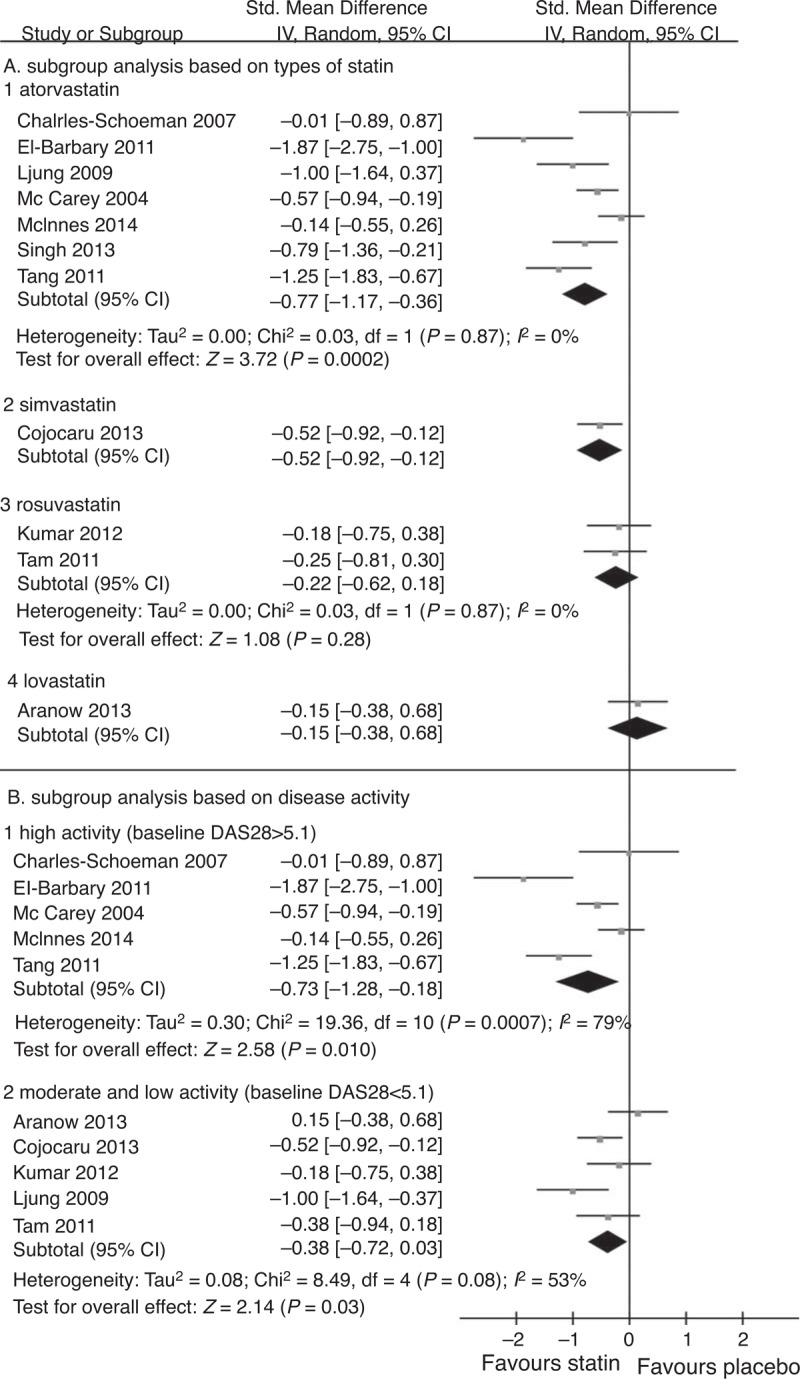
Subgroup analysis based on type of statin (A) and disease activity (B). A. Atorvastatin produced a greater reduction in DAS28 in RA patients (SMD −0.77, 95% CI [−1.77,−0.36], P = 0.002, I2 = 71%). B. Patients with highly active disease (defined as baseline DAS28 ≥5.1) tended to benefit more from statin therapy compared with patients with moderate and low disease activity (SMD −0.73, 95% CI [−1.28,−0.18], P = 0.01, I2 = 79%). CI = confidence interval, DAS28 = disease activity score in 28 joints, SMD = standardized mean difference.
The Effect of Statins Based on Other Measures
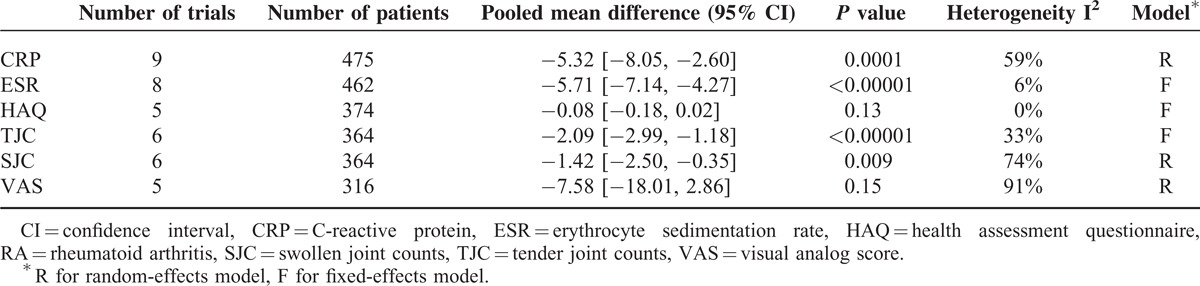
Other outcome measurements, including CRP, ESR, TJC, SJC, HAQ, and pain evaluated based on VAS, were also analyzed as continuous outcomes using the inverse variance method. The results are shown in Table 2. The fixed-effects model or the random-effects model was chosen according to the heterogeneity. The results showed that statin therapy significantly reduced CRP (mean difference −5.32, 95% CI [−8.05, −2.60], P = 0.0001), ESR (mean difference −5.71, 95% CI [−7.14, −4.27], P < 0.00001), TJC (mean difference −2.09, 95% CI [−2.99, −1.18], P < 0.00001), and SJC (mean difference −1.42, 95% CI [−2.50, −0.35], P = 0.009) (Table 2). Although statin therapy also reduced the HAQ score (mean difference −0.08, 95% CI [−0.18, 0.02], P = 0.13) and VAS (mean difference −7.58, 95% CI [−18.01, 2.86], P = 0.15), the reductions were not significant (Table 2).
TABLE 2.
Meta-Analysis of the Effect of Statins on Other Measures in RA Patients
Analysis of Sensitivity and Publication Bias
Analyses of sensitivity and publication bias were conducted using the Stata 12.0 software. The sensitivity analysis showed that the sequential omission of individual studies did not alter the overall effect (upper limit of 95% CI interval lower than 0 in all cases). The results are shown in Figure 4. The publication bias was evaluated using a funnel plot, which showed no significant evidence of asymmetry (Figure 5). We also performed an Egger test to quantify the publication bias, and the P value was 0.324, suggesting no significant bias of the analysis (Figure 5).
FIGURE 4.
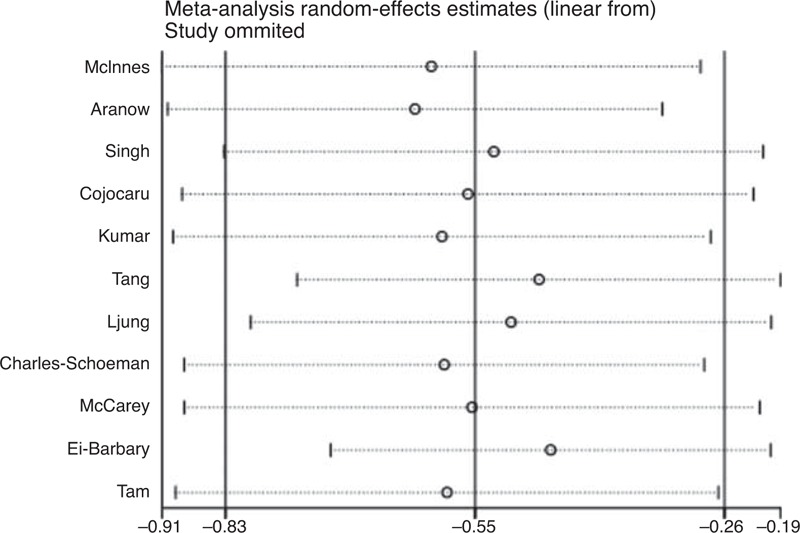
Sensitivity analysis on the effect of statins versus placebo on DAS28 in RA patients. The results show that omitting any single study did not change the results of the meta-analysis.
FIGURE 5.
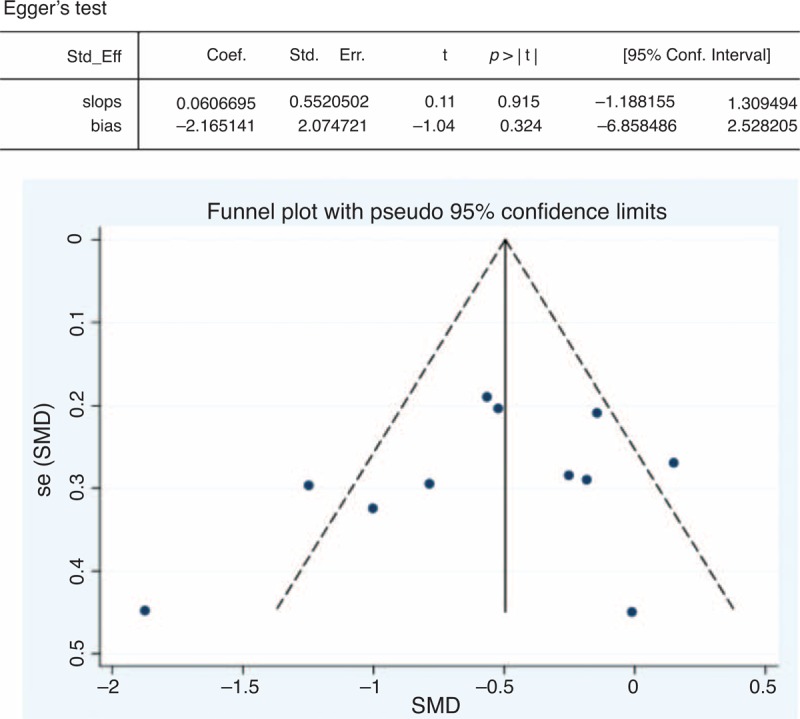
Overall analysis of publication bias on the effect of statins versus placebo on DAS28 in RA patients. Egger linear regression test was performed to quantify publication bias (P = 0.324); the funnel plot shows no significant evidence of asymmetry. RA = rheumatoid arthritis, SMD = standardized mean difference.
DISCUSSION
Hyperlipidemia, together with other cardiovascular risk factors, plays important roles in the pathogenesis of CVD.26 Stains are used in RA patients with hyperlipidemia for the prevention of CVD. RA is associated with an increased risk of CVD, which can only be partially explained by traditional cardiac risk factors.27 RA and atherosclerosis share many inflammatory mechanisms,28 and the extent of inflammation associated with RA predicts both CVD and overall mortality rates in RA.29 This meta-analysis aimed to answer the question of whether statins benefit RA patients more than originally thought via other than lipid-lowering effects. The previous studies on the effect of statins on disease activity in RA patients are almost all single-center studies with small sample sizes, and the results are controversial. Our results showed that compared to placebo, statins lowered disease activity measured by DAS28. Statins also significantly reduced the inflammatory index, such as ESR and CRP, as well as TJC and SJC, compared to placebo. However, the effect of statins on the improvement of either the HAQ score or the pain score measured by VAS was not significant. The subgroup analysis showed that patients with more active disease appeared to benefit more from statin therapy, as the estimated effect was more obvious in patients with highly active disease (defined as a mean baseline DAS28 ≥5.1) compared with patients with less active disease.
In our meta-analysis, we only included prospective and placebo-controlled studies. To ensure credibility, single-arm studies were not included. Kanda et al treated 24 RA patients with simvastatin 10 mg per day in an open-label 12-week study and reported improvement in multiple clinical measures, including DAS28, CRP, and ESR.30 However, the RCTs with simvastatin included in our research failed to show an advantage over placebo.10 Van Doornum et al tested the effect of 20 mg qd of atorvastatin in 29 RA patients and did not report any change in DAS after 12 weeks of treatment, partially due to the small sample size, lower dosage, and relatively mild disease activity.31
Our meta-analysis showed that statins may have disease-modifying activity in RA patients, but the mechanism of that remains unclear. There have been 2 RCTs comparing statins to other lipid-lowering drugs: ezetimibe and fenofibrate.32,33 The results of these 2 studies showed no difference between statins and the other lipid-lowering drugs with regard to changes in most of the outcome measurements (ESR, CRP, and DAS28). Fenofibrate has also been reported to have anti-inflammatory activity,34 whereas ezetimibe is a drug that only acts locally and is not absorbed into the circulation. The results suggested that cholesterol reduction per se may result in an anti-inflammatory effect. However, other studies with statins and ezetimibe reached a different conclusion. One recent study compared the cholesterol-lowering efficacy of high-dose statin monotherapy with the same statin at a lower dose plus ezetimibe. The high-dose statins alone improved flow-mediated dilation more than dual therapy with low-dose statins and ezetimibe despite comparable reductions in LDL cholesterol.35 Therefore, whether the disease-modifying activity of statins is due to their pleiotropic effects or lipid-lowering effects is still difficult to determine.
LIMITATIONS
This meta-analysis has limitations. Only 13 studies qualifying for the inclusion criteria were finally included. However, the literature search was comprehensive, and efforts were taken to obtain more data. Therefore, it is reasonable to draw conclusions based on this meta-analysis. The studies included are almost all single-center studies with a small number of patients. The types of statins used in each study, the difference in the dosage, the duration of the treatment, and the disease activity also added to the heterogeneity. In the 7 studies about atorvastatin, the dosage varied from 10 to 80 mg per day,11,12,16,19,20,23,25 and did not seem to correlate with DAS28 reduction. The different baseline disease activity and disease-modifying antirheumatic drugs (DMARDs) therapy applied in different studies also complicated the effect of statin dosage on DAS28 reduction. Random-effects models may not fully account for the heterogeneity. Therefore, caution should be taken when interpreting the results of this meta-analysis. The results of our analysis should be confirmed with new and larger studies in the future. The TRACE-RA (Trial of Atorvastatin in the Primary Prevention of Cardiovascular Endpoints in Rheumatoid Arthritis) study, which was a multicenter, placebo-controlled study that aimed to enroll over 3000 patients with RA, tried to investigate whether atorvastatin could reduce the occurrence of CVDs. Although this trial has been stopped because of low number of cardiovascular endpoints, its substudy, TRACE RA DAS, tried to answer the question of whether atorvastatin could reduce inflammation and disease activity in RA patients, and its results merit expectation.
CONCLUSIONS
In summary, our meta-analysis suggested that statin therapy might be effective in the reduction of RA disease activity measured by DAS28, TJC, SJC, as well as ESR and CRP.
Footnotes
Abbreviations: ACR = American College of Rheumatology, CRP = C-reactive protein, DAS = disease activity score, ESR = erythrocyte sedimentation rate, EULAR = The European League Against Rheumatism, HAQ = health assessment questionnaire, HMG-CoA = 3-hydroxy-3-methylglutaryl-coenzyme A, RA = arthritis rheumatoid, SJC = swollen joint counts, TJC = tender joint counts, VAS = visual analog scale.
BX, YY, and LZ contributed equally to this study.
This work was supported by the grants from the National Natural Science Foundation of China (81325019, 81172859 and 81273312), Beijing Municipal Natural Science Foundation (7141008), the Research Special Fund for Public Welfare Industry of Health (2013202017), and the Capital Health Research and Development of Special Fund (2011-4001-02).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. J Cardiovasc Dis 2013; 1:1–2. [Google Scholar]
- 2.Zhou Q, Liao JK. Pleiotropic effects of statins: basic research and clinical perspectives. Circ J 2010; 74:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito T, Ikeda U, Shimpo M, et al. HMG-CoA reductase inhibitors reduce interleukin-6 synthesis in human vascular smooth muscle cells. Cardiovasc Drugs Ther 2002; 16:121–126. [DOI] [PubMed] [Google Scholar]
- 4.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 2001; 7:687–692. [DOI] [PubMed] [Google Scholar]
- 5.Romano M, Diomede L, Sironi M, et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest 2000; 80:1095–1100. [DOI] [PubMed] [Google Scholar]
- 6.Weber C, Erl W, Weber KS, et al. HMG-CoA reductase inhibitors decrease CD11b expression and CD11b-dependent adhesion of monocytes to endothelium and reduce increased adhesiveness of monocytes isolated from patients with hypercholesterolemia. J Am Coll Cardiol 1997; 30:1212–1217. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 8.Takayama T, Hiro T, Yamagishi M, et al. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ J 2009; 73:2110–2117. [DOI] [PubMed] [Google Scholar]
- 9.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001; 344:907–916. [DOI] [PubMed] [Google Scholar]
- 10.Cojocaru L, Rusali AC, Suta C, et al. The role of simvastatin in the therapeutic approach of rheumatoid arthritis. Autoimmune Dis 2013; 2013: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang TT, Song Y, Ding YJ, et al. Atorvastatin upregulates regulatory T cells and reduces clinical disease activity in patients with rheumatoid arthritis. J Lipid Res 2011; 525:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarey DW, McInnes IB, Madhok R, et al. Trial of atorvastatin in rheumatoid arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 2004; 363:2015–2021. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Oxford: Cochrane Collaboration; 2011. [Google Scholar]
- 14.Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–94. [DOI] [PubMed] [Google Scholar]
- 15.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 16.McInnes IB, Kim HY, Lee SH, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis 2014; 73:124–131. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P, Kennedy G, Khan F, et al. Rosuvastatin might have an effect on C-reactive protein but not on rheumatoid disease activity: Tayside randomized controlled study. Scott Med J 2012; 57:80–83. [DOI] [PubMed] [Google Scholar]
- 18.Tam LS, Li EK, Shang Q, et al. Effects of rosuvastatin on subclinical atherosclerosis and arterial stiffness in rheumatoid arthritis: a randomized controlled pilot trial. Scand J Rheumatol 2011; 40:411–421. [DOI] [PubMed] [Google Scholar]
- 19.El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol 2011; 38:229–235. [DOI] [PubMed] [Google Scholar]
- 20.Charles-Schoeman C, Khanna D, Furst DE, et al. Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J Rheumatol 2007; 34:1459–1464. [PubMed] [Google Scholar]
- 21.Tikiz C, Utuk O, Pirildar T, et al. Effects of Angiotensin-converting enzyme inhibition and statin treatment on inflammatory markers and endothelial functions in patients with longterm rheumatoid arthritis. J Rheumatol 2005; 32:2095–2101. [PubMed] [Google Scholar]
- 22.Mikhael EM, Gorial FI, Majeed IA. Effect of rosuvastatin as adjuvant therapy to methotrexate on hematological parameters in patients with moderately-highly active rheumatoid arthritis. J Exp Integr Med 2013; 3:127–131. [Google Scholar]
- 23.Singh H, Talapatra P, Mathur R, et al. Role of atorvastatin on disease activity of rheumatoid arthritis patients. IJR 2013; 8:S30. [Google Scholar]
- 24.Aranow C, Cush JJ, Bolster MB, et al. A double-blind randomized placebo-controlled trial of lovastatin in patients with rheumatoid arthritis. Arthritis Rheum 2013; 65:S1181–S1182. [Google Scholar]
- 25.Ljung L, Leirisalo-Repo M, Yki-Jarvinen H, et al. Improvement of cardiovascular risk markers with atorvastatin treatment in rheumatoid arthritis. Arthritis Rheum 2009; 60:430. [Google Scholar]
- 26.Santulli G. Coronary heart disease risk factors and mortality. JAMA 2012; 307:1137. [DOI] [PubMed] [Google Scholar]
- 27.del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001; 44:2737–2745. [DOI] [PubMed] [Google Scholar]
- 28.Pasceri V, Yeh ET. A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation 1999; 100:2124–2126. [DOI] [PubMed] [Google Scholar]
- 29.Wallberg-Jonsson S, Johansson H, Ohman ML, et al. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol 1999; 26:2562–2571. [PubMed] [Google Scholar]
- 30.Kanda H, Yokota K, Kohno C, et al. Effects of low-dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Mod Rheumatol 2007; 17:364–368. [DOI] [PubMed] [Google Scholar]
- 31.Van Doornum S, McColl G, Wicks IP. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann Rheum Dis 2004; 63:1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto M. A comparative study of anti-inflammatory and antidyslipidemic effects of fenofibrate and statins on rheumatoid arthritis. Mod Rheumatol 2010. 1–6. [DOI] [PubMed] [Google Scholar]
- 33.Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007; 50:852–858. [DOI] [PubMed] [Google Scholar]
- 34.H O, N K. Successful treatment with fenofibrate, a peroxisome proliferator activated receptor alpha ligand, for a patient with rheumatoid arthritis. Ann Rheum Dis 2004; 63:1002–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostad MA, Eggeling S, Tschentscher P, et al. Flow-mediated dilation in patients with coronary artery disease is enhanced by high dose atorvastatin compared to combined low dose atorvastatin and ezetimibe: Results of the CEZAR study. Atherosclerosis 2009; 205:227–232. [DOI] [PubMed] [Google Scholar]


