Abstract
East Asian patients with diabetes have a higher risk for renal complications and strokes than Europeans. We aimed to evaluate the effect of methylenetetrahydrofolate reductase (MTHFR) gene 677C→T polymorphism, which was associated with a higher stroke risk and was common in the Chinese population, as well as homocysteine and estimated glomerular filtration rate (eGFR) levels on the risk of new-onset diabetes (NOD).
A total of 2422 subjects without diabetes were followed-up for 7 years. NOD was defined as fasting plasma glucose ≥7.0 mmol/L or self-reported physician diagnosis of diabetes.
Compared with subjects with MTHFR 677CC genotype, those with TT genotype had a higher risk of NOD in females (odds ratio 2.78, 95% confidence interval 1.39–5.56) but not in males (0.80, 0.40–1.61, P for interaction = 0.008). Furthermore, MTHFR 677C→T polymorphism was more strongly associated with the risk of NOD among females with higher body mass index (BMI, ≥23 vs <23 kg/m2, P for interaction = 0.009) or lower high-density lipoprotein-cholesterol (HDL-C, <1.3 vs ≥1.3 mmol/L, P for interaction = 0.015) levels. Hyperhomocysteinemia (≥16 vs <10 μmol/L) was not significantly associated with NOD in males (0.88, 0.42–1.85) or females (1.52, 0.65–3.57). However, mildly decreased eGFR (<90 vs 90–120 mL/min/1.73 m2) was associated with NOD mainly in males (1.96, 1.01–3.78; females, 0.74, 0.32–1.72, P for interaction = 0.134).
Females with MTHFR 677TT genotype had a significantly higher risk of NOD, particularly those with higher BMI or low HDL-C levels. The higher risk of NOD associated with mildly decreased eGFR also warrants more investigation. Our results provide insights into the ethnic differences of diabetic complications between East Asian patients and Europeans.
INTRODUCTION
Diabetes is now recognized as a worldwide public health problem. A recent national cross-sectional survey1 showed that the overall prevalence of diabetes was estimated to be 11.6% in the Chinese adult population. Diabetes is a progressive disease, due in part to the loss of β-cell function, with the reduction in function probably commencing 10 to 12 years prior to diagnosis and aggravated by increasing fasting plasma glucose (FPG) levels.2 Furthermore, East Asian patients with type 2 diabetes have a higher risk of developing renal complications than Europeans, and, with regard to cardiovascular complications, a predisposition for developing strokes.3 These results denote that more studies are needed to explain these interethnic differences, and, most importantly, effective strategies are required to facilitate earlier identification and prevention to combat these growing disease burdens, particularly in China.
Methylenetetrahydrofolate reductase (MTHFR) is the main regulatory enzyme for folate/homocysteine metabolism. MTHFR converts 5,10-methylene-tetrahydrofolate (THF) into 5-methyl-THF, the dominant circulating form of folate. The 5-methyl-THF product donates a methyl group to homocysteine in the generation of S-adenosylmethionine, a major source of methyl groups used for DNA methylation. Polymorphism of MTHFR 677C→T leads to a reduction in enzyme activity, resulting in increased concentrations of plasma homocysteine and lower levels of serum folate, and thereby confers a higher risk for stroke, particularly in those with low folate intake.4 A recent meta-analysis of case–control studies5 found that the homocysteine concentration in individuals with type 2 diabetes was significantly higher than that in control subjects (0.94 μmol/L, 95% confidence interval [CI] 0.40–1.48), and the odds ratio (OR) associated with type 2 diabetes for MTHFR 677TT relative to 677CC was 1.38 (95% CI 1.18–1.62).5 Our recent study also showed that participants with MTHFR 677TT genotype had a higher prevalence of diabetes than those with CC genotype.6 Furthermore, plasma homocysteine levels were significantly inversely associated with estimated glomerular filtration rate (eGFR), and MTHFR 677TT genotype was associated with a higher risk for decreased kidney function independent of homocysteine levels.7
However, no prospective studies investigating the association between MTHFR 677C→T polymorphism, homocysteine, renal function, and new-onset diabetes (NOD) have been conducted. Furthermore, the frequency of the MTHFR 677 TT genotype, which showed marked ethnic variations, was more common in China than in most of the European countries.8 The Chinese also had higher homocysteine levels,9 lower folate concentrations,10 and did not have a policy of mandatory folic acid fortification in food. These characteristics make the Chinese population particularly suited for testing the possible genotype (MTHFR 677C→T polymorphism) and phenotype (homocysteine and eGFR levels)-disease association using the same analytical framework. Therefore, in the current study, we aimed to evaluate the effect of MTHFR 677C→T polymorphism, homocysteine, and eGFR levels on the risk of NOD in a rural Chinese cohort, and to identify the possible effect modifiers. Our findings may possibly provide insights into the ethnic differences in diabetic complications seen between East Asian patients and Europeans.
MATERIALS AND METHODS
This report followed the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement for cohort studies.
STUDY POPULATION AND DATA COLLECTION
Study participants were from an epidemiological study of metabolic syndrome conducted during 2003 to 2005 in rural communities (Dongzhi and Wangjiang) in Anqing, Anhui province of China. Detailed protocol and the details regarding “study population” and “data collection” have been previously described.11,12 Briefly, 6301 of the study subjects from Dongzhi community who received baseline screening examination were invited for a follow-up visit in 2011, and 2901 (46%) of them responded. The nonresponders did not differ from the responders substantially with respect to baseline characteristics (data not shown). This study was approved by the institutional review boards from the Nanfang Hospital in Guangzhou and the Institute of Biomedicine in the Anhui Medical University. Written informed consent was obtained from each study participant.
Baseline data was ascertained by trained research staff according to the standard operating procedures. Venous blood was drawn from the forearm of each participant in the fasting status. FPG, total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), and homocysteine were measured on the Hitachi 7020 Automatic Analyzer (Hitachi, Tokyo, Japan). Serum creatinine concentrations were determined using an enzymatic method (sarcosine oxidase-peroxidase–anti-peroxidase). Plasma insulin was measured using an enhanced chemiluminescence method on an Elecsys 2010 system (Roche, Basel, Switzerland). DNA was extracted from leukocytes in peripheral blood using standard techniques. MTHFR 677C→T genotype was determined by Taqman assay designed and manufactured by Applied Biosystems (Foster City, CA).
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from the fasting concentrations of insulin and glucose using the following formula: fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5.13 GFR was estimated using the equation according to the Chronic Kidney Disease Epidemiology Collaboration.14
OUTCOMES
We excluded participants with self-reported physician diagnosis of diabetes or FPG ≥7.0 mmol/L at baseline in the final analysis. NOD was defined as FPG ≥7.0 mmol/L or self-reported physician diagnosis of diabetes at follow-up year.
STATISTICAL ANALYSIS
Current smoking was defined as having smoked ≥10 packs in the last year. Current alcohol drinking was defined as drinking alcohol at least once per week in the last year. The question about insomnia was phrased as follows: “Do you frequently suffer from insomnia?”, and a choice of 3 responses (frequent: almost every week, medium: 1–3 times per month, and seldom) was provided.
Baseline characteristics are presented as mean or percentage, except for fasting insulin and homocysteine, which are presented as median (Q1–Q3) because of the skewed distribution. Between-group differences in baseline characteristics were tested using the Student t test, the signed rank test, or the χ2 test, accordingly. The effects of MTHFR 677C→T polymorphism (CC, CT, and TT), homocysteine (<10, 10–16, and ≥16 μmol/L), and eGFR (≥120, 90–120, <90 mL/min/1.73 m2) on the risks of NOD in males and females were estimated using logistic regression models with adjustment for baseline covariates including age (year), baseline FPG (≥5.6 vs <5.6 mmol/L), body mass index (BMI, ≥23 vs <23 kg/m2), blood pressure (<130/85, 130/85–140/90, ≥140/90 mm Hg), TG (≥1.7 vs <1.7 mmol/L), TC (≥5.2 vs <5.2 mmol/L), HDL-C (<1.3 [females]/1.0 [males] vs ≥1.3/1.0 mmol/L), current cigarette smoking, current alcohol drinking, and insomnia (frequent, medium, and seldom). We tested for effect modification with stratified analyses of the above major covariates. A 2-tailed P < 0.05 was considered statistically significant in all analyses. R software, version 2.15.1 (http://www.R-project.org) was used to perform all statistical analyses.
RESULTS
Overall, 2901 subjects were revisited. In this report, study participants with self-reported physician diagnosis of cardiovascular disease (CVD, n = 28), diabetes (n = 10), hypertension (n = 124), or with any missing data (n = 186) regarding baseline values for age, cigarette smoking status, alcohol drinking status, fasting glucose, homocysteine, MTHFR 677C→T polymorphism or insomnia, or with any missing data (n = 61) for FPG or self-reported physician diagnosis of diabetes at follow up, or who had an FPG value of ≥7.0 mmol/L at baseline(n = 70) were excluded. Our final analysis included 2422 participants.
The prevalence of MTHFR 677 CC, 677 CT, and 677 TT genotypes was 36.8%, 47.4%, and 15.8%, respectively. This population had no significant deviations in genotype distributions from expected Hardy–Weinberg equilibrium. Those with MTHFR 677 TT genotype had significantly higher homocysteine levels (median, 12.0 in males and 10.1 μmol/L in females) than those with CT (10.4 and 9.1 μmol/L) or CC (10.4 and 8.6 μmol/L) genotype (P < 0.05 for either of these genotypes in males or females). Furthermore, there was an inverse association between homocysteine and eGFR levels in males (r = −0.43, P < 0.001) and females (r = −0.63, P < 0.001).
The follow-up time ranged from 5.81 to 7.57 years, with a mean of 7.02 (SD 0.31) years. There were no significant differences in the follow-up time between subjects with and without NOD in males (6.97 [0.31] vs 7.02 [0.31], P = 0.137) and females (7.05 [0.34] vs 7.03 [0.32], P = 0.506). The baseline characteristics of the study subjects stratified by NOD status and sex are summarized in Table 1. NOD patients had significantly higher TG, BMI, insulin levels, and HOMA-IR compared with those without in both males and females. However, NOD patients had significantly higher MTHFR 677 TT genotype rates in females only (Table 1).
TABLE 1.
Baseline Characteristics According to NOD Status by Sex

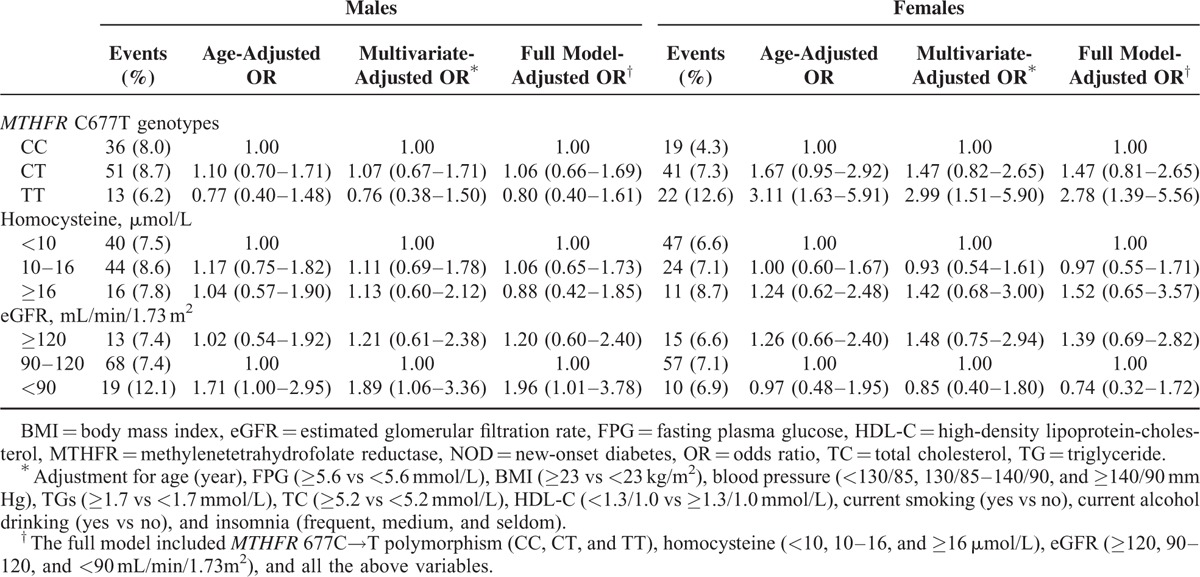
The incidence rates of NOD in males and females were 8.0% and 7.0%, respectively. Compared with subjects with MTHFR 677 CC genotype, those with TT genotype had a higher risk of NOD in females (OR 2.78, 95% CI 1.39–5.56) but not in males (0.80, 0.40–1.61, P for interaction = 0.008). Hyperhomocysteinemia (≥16 vs <10 μmol/L) was not associated with NOD in males (0.88, 0.42–1.85) or females (1.52, 0.65–3.57). However, mildly decreased eGFR (<90 vs 90–120 mL/min/1.73 m2) was associated with NOD mainly in males (1.96, 1.01–3.78; females, 0.74, 0.32–1.72, P for interaction = 0.134) (Table 2).
TABLE 2.
Relationship of MTHFR 677C→T Polymorphism, Baseline Homocysteine Levels, and eGFR With the Risk of NOD
In the full models, we observed that higher TG, BMI, FPG levels, and insomnia, in addition to MTHFR C677T polymorphism in females, and higher FPG levels and insomnia in addition to lower eGFR levels in males remained independent risk factors for the risk of NOD (data not shown).
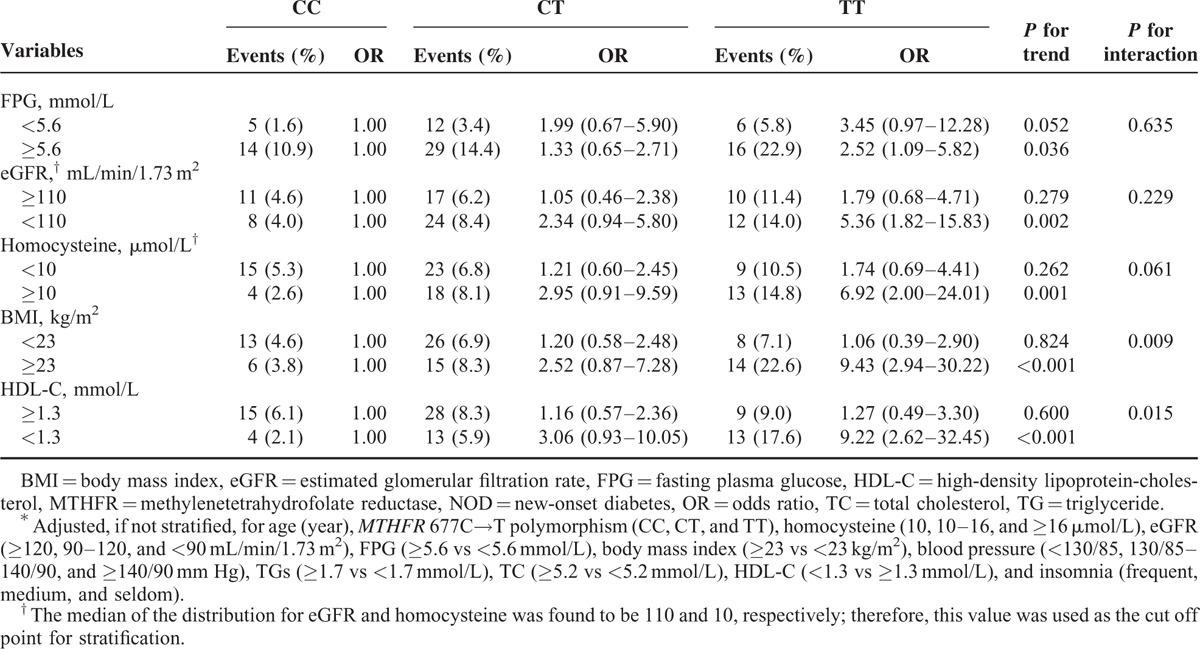
In the stratified analyses, the MTHFR 677C→T polymorphism (CC, CT, and TT genotypes) was more strongly associated with the risk of NOD among females with higher BMI (≥23 vs <23 kg/m2, P for interaction = 0.009) or lower HDL-C levels (<1.3 vs ≥1.3 mmol/L, P for interaction = 0.015). However, the association of MTHFR 677C→T polymorphism with increased risks of NOD in females appeared to be similar among subgroups classified according to baseline homocysteine (≥10 vs <10 μmol/L, P for interaction = 0.061), eGFR (<110 vs ≥110 mL/min/1.73 m2, P for interaction = 0.229), FPG (≥5.6 vs <5.6 mmol/L, P for interaction = 0.635), TG levels (≥1.7 vs <1.7 mmol/L, P for interaction = 0.189), or insomnia (frequent vs medium or seldom, P for interaction = 0.831). Furthermore, similar trends for eGFR category and increased risk of NOD in males were observed among subgroups stratified by insomnia (P for interaction = 0.946), baseline FPG (P for interaction = 0.977), homocysteine levels (P for interaction = 0.573), or MTHFR 677C→T polymorphism (P for interaction = 0.880) (Table 3).
TABLE 3.
Stratified Analysis of Multivariate ORs for NOD Among 1172 Women According to MTHFR 677C→T Polymorphism∗
Further adjustment for HOMA-IR (n = 2144) did not substantially change the relationship of MTHFR 677C→T polymorphism (TT vs CC, 2.20; 1.02–4.73) in females or eGFR levels (<90 vs 90–120 mL/min/1.73 m2, 1.85; 0.92–3.71) in males with the risk of NOD.
DISCUSSION
Previous studies have shown conflicting results regarding the homocysteine levels in patients with diabetes. A recent meta-analysis of 14 case–control studies found that the mean homocysteine concentration was greater in patients with type 2 diabetes than in control subjects.5 However, the 3 studies15–17 that contributed most to the overall estimate included patients with type 2 diabetes accompanied by varying degrees of kidney disorders. These results suggest that it may be renal dysfunction but not diabetes that mostly explains the difference in homocysteine levels between type 2 diabetes patients and control subjects in this meta-analysis.5 In fact, in our present prospective study, we did not find significant associations between homocysteine levels and NOD in males or females.
However, consistent with this meta-analysis,5 female subjects with TT genotype in the current study had a higher risk of NOD (TT vs CC genotype, 2.78; 1.39–5.56). To explain the significant relationship of MTHFR gene 677C→T polymorphism (but not homocysteine levels) with the risk of NOD, we speculate that, first, the homocysteine levels (median 10.7 μmol/L in males and 9.1 μmol/L in females) in the present study was possibly not high enough to cause obvious organ damage. Second, plasma homocysteine may just serve as a reliable functional marker of folate status, and folate may have other actions, including antioxidant actions, effects on cofactor availability, or direct interactions with the enzyme endothelial NO synthase, in addition to homocysteine lowering that influence health status.18 A previous report19 showed that folic acid given to patients with coronary heart disease resulted in improvement in endothelial function without any change in plasma homocysteine concentration. Unfortunately, we were not able to directly examine the association between MTHFR 677C→T polymorphism, folate, and the risk of NOD in the current study due to the lack of baseline folate data. Additional studies are required to further address this topic and to evaluate the role of folic acid supplementation in reducing the risk of diabetes, particularly in populations without folic acid fortification. Meanwhile, we did not have enough information to explain the sex-specific effect of MTHFR 677C→T polymorphism, which may relate to the possible role of hormones in folate/homocysteine metabolism.20 This topic also needs to be confirmed and investigated in future studies.
Our current study detected a detrimentally interactive effect between the MTHFR 677C→T polymorphism and higher BMI (≥23 vs <23 kg/m2), or lower HDL-C (<1.3 vs ≥1.3 mmol/L) levels on the risk of NOD among females. Obese subjects without diabetes have been shown to exhibit an enhanced rate of glucose production.21 We hypothesize that “a higher BMI state” may augment the MTHFR 677C→T polymorphism-mediated risk of NOD. Furthermore, MTHFR 677C→T polymorphism appeared to modify the efficacy of the drug pravastatin in reducing risk of cardiovascular events. A significantly protective effect against coronary heart disease (hazard ratio [HR] 0.71, 95% CI 0.58–0.87) was shown in subjects with CC genotype but not in subjects with CT (HR 1.25, 95% CI 0.97–1.61) or TT genotype (HR 0.80, 95% CI 0.50–1.28, P for interaction = 0.004).22 Consistently, we also observed an interaction between the MTHFR 677C→T polymorphism and HDL-C levels on the risk of NOD. These results suggest that an investigation of the possible modifying effect of MTHFR 677C→T polymorphism on CVD associated with HDL-C increasing therapy maybe provide some clues regarding the conflicting results gathered from these kinds of trials.23 Overall, our results indicate that the MTHFR 677TT genotype, along with homocysteine, BMI, and HDL-C levels, may help to identify apparently healthy females at increased risk for diabetes.
Menon et al24 reported that hyperhomocysteinemia did not appear to be a risk factor for all-cause or CVD mortality, and prior studies demonstrating an association between homocysteine and CVD risk may have inadequately adjusted for the confounding effects of kidney function. We also observed that mildly decreased eGFR (<90 mL/min/1.73 m2), but not homocysteine, was associated with the risk of new-onset disease. Nevertheless, more studies are needed to verify if our findings can be generalized to other populations or ethnicities, particularly individuals with lower eGFR levels or obvious chronic kidney disease.
The strengths of our study include the 7-year prospective follow-up of middle-aged rural Chinese men and women, and the comprehensive adjustments for the major traditional risk factors for NOD, including baseline FPG and IR. Our study also has several limitations. First, we did not measure glycosylated hemoglobin levels or perform glucose-tolerance tests. Our analyses were mainly based on the diabetes defined by FPG levels. Second, we did not have information regarding family history of diabetes. However, further adjustment for family history of diabetes did not change the significant association between MTHFR 677C→T polymorphism and prevalence of diabetes in our previous study.6 Lastly, previous studies have shown that nonalcoholic fatty liver disease (NAFLD) was strongly associated with IR and diabetes.25 Unfortunately, we were not able to examine this effect in the current study due to the lack of data on NAFLD. Additional studies are required to further address this topic.
In conclusion, we found that individuals with MTHFR 677TT genotype had a significantly higher risk of NOD among females, particularly in those with higher BMI or lower HDL-C levels. The higher risk of NOD associated with mildly decreased eGFR (<90 mL/min/1.73 m2) also warrants more investigation. Our study findings, if further confirmed, will provide new strategies to identify apparently healthy population at increased risk for diabetes. Furthermore, considering the higher frequency of MTHFR 677TT genotype in China and the higher stroke risk associated with TT genotype, our results also provide some explanations for the ethnic differences in diabetic complications seen between East Asian patients and Europeans.
Footnotes
Abbreviations: BMI = body mass index, CVD = cardiovascular disease, eGFR = estimated glomerular filtration rate, FPG = fasting plasma glucose, HDL-C = high-density lipoprotein-cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, MTHFR = methylenetetrahydrofolate reductase, NAFLD = nonalcoholic fatty liver disease, NOD = new-onset diabetes, OR = odds ratio, TC = total cholesterol, TG = triglyceride.
YL and XQ contributed equally to this article.
Design of the study: Xiping X, XQ, YL, DX, GT, BW, XW, Xin X, FH. Conduct of the study: Xiping X, XQ, YL, HY, DX, GT, BW. Data collection and analysis: Xiping X, XQ, YL, Xin X. Data interpretation and manuscript writing: Xiping X, XQ, YL, HY, DX, GT, BW, XW, Xin X, FH. All authors read and approved the final manuscript.
The study was supported by the Major State Basic Research Development Program of China (973 program) (No. 2012CB517703), the Public Welfare and Health Sector Research Project (201002010), Major Scientific and Technological Planning Project of Guangzhou City (2010U1-E00821), and the National Nature and Science Grant (No. 81202280, No. 81402735, No. 81473052, and No. 81441091). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310:948–959. [DOI] [PubMed] [Google Scholar]
- 2.Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract 1998; 40 Suppl:S21–25. [DOI] [PubMed] [Google Scholar]
- 3.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet 2011; 378:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang T, Ren J, Huang J, et al. Association of homocysteine with type 2 diabetes: a meta-analysis implementing Mendelian randomization approach. BMC Genomics 2013; 14:867.doi: 10.1186/1471-2164-14-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin X, Li J, Zhang Y, et al. Prevalence and associated factors of diabetes and impaired fasting glucose in Chinese hypertensive adults aged 45 to 75 years. PLoS One 2012; 7:e42538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Q, Tang G, He M, et al. Methylenetetrahydrofolate reductase C677T polymorphism is associated with estimated glomerular filtration rate in hypertensive Chinese males. BMC Med Genet 2012; 13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilcken B, Bamforth F, Li Z, et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas worldwide. J Med Genet 2003; 40:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin X, Li J, Cui Y, et al. MTHFR C677T and MTR A2756G Polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensives Chinese adults. Nutr J 2012; 11:2.doi: 10.1186/1475-2891-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin X, Li J, Cui Y, et al. Effect of folic acid intervention on the change of serum folate level in hypertensive Chinese adults: do methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms affect therapeutic responses? Pharmacogenet Genomics 2012; 22:421–428. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Hong X, Li Z, et al. Prevalence of metabolic syndrome and its relation to body composition in a Chinese rural population. Obesity (Silver Spring) 2006; 14:2089–2098. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Xie D, Qin X, et al. Metabolic syndrome, but not insulin resistance, is associated with an increased risk of renal function decline. Clin Nutr 2014; doi: 10.1016/j.clnu.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000; 23:57–63. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emoto M, Kanda H, Shoji T, et al. Impact of insulin resistance and nephropathy on homocysteine in type 2 diabetes. Diabetes Care 2001; 24:533–538. [DOI] [PubMed] [Google Scholar]
- 16.Ozmen B, Ozmen D, Turgan N, et al. Association between homocysteinemia and renal function in patients with type 2 diabetes mellitus. Ann Clin Lab Sci 2002; 32:279–286. [PubMed] [Google Scholar]
- 17.Tessari P, Coracina A, Kiwanuka E, et al. Effects of insulin on methionine and homocysteine kinetics in type 2 diabetes with nephropathy. Diabetes 2005; 54:2968–2976. [DOI] [PubMed] [Google Scholar]
- 18.Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol 2002; 22:6–13. [DOI] [PubMed] [Google Scholar]
- 19.Doshi SN, McDowell IF, Moat SJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation 2002; 105:22–26. [DOI] [PubMed] [Google Scholar]
- 20.Brown CA, McKinney KQ, Young KB, et al. The C677T methylenetetrahydrofolate reductase polymorphism influences the homocysteine-lowering effect of hormone replacement therapy. Mol Genet Metab 1999; 67:43–48. [DOI] [PubMed] [Google Scholar]
- 21.Gastaldelli A, Miyazaki Y, Pettiti M, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab 2004; 89:3914–3921. [DOI] [PubMed] [Google Scholar]
- 22.Maitland-van der Zee AH, Lynch A, Boerwinkle E, et al. Interactions between the single nucleotide polymorphisms in the homocysteine pathway (MTHFR 677C>T, MTHFR 1298 A>C, and CBSins) and the efficacy of HMG-CoA reductase inhibitors in preventing cardiovascular disease in high-risk patients of hypertension: the GenHAT study. Pharmacogenet Genomics 2008; 18:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 24.Menon V, Sarnak MJ, Greene T, et al. Relationship between homocysteine and mortality in chronic kidney disease. Circulation 2006; 113:1572–1577. [DOI] [PubMed] [Google Scholar]
- 25.Machado MV, Cortez-Pinto H. Non-alcoholic fatty liver disease: what the clinician need to know. World J Gastroenterol 2014; 20:12956–12980. [DOI] [PMC free article] [PubMed] [Google Scholar]


