Abstract
Periodontitis and osteoporosis are primary concerns in public health and clinical management. The aim of this study was to investigate the association between periodontitis and osteoporosis by gender.
Data were retrieved from the National Health Insurance Research Database, Taiwan. A diagnosis of periodontitis was defined on the basis of subgingival curettage, periodontal flap operation, and gingivectomy (excluding those with restorative or aesthetic indications). Multiple logistic regression was used for analysis.
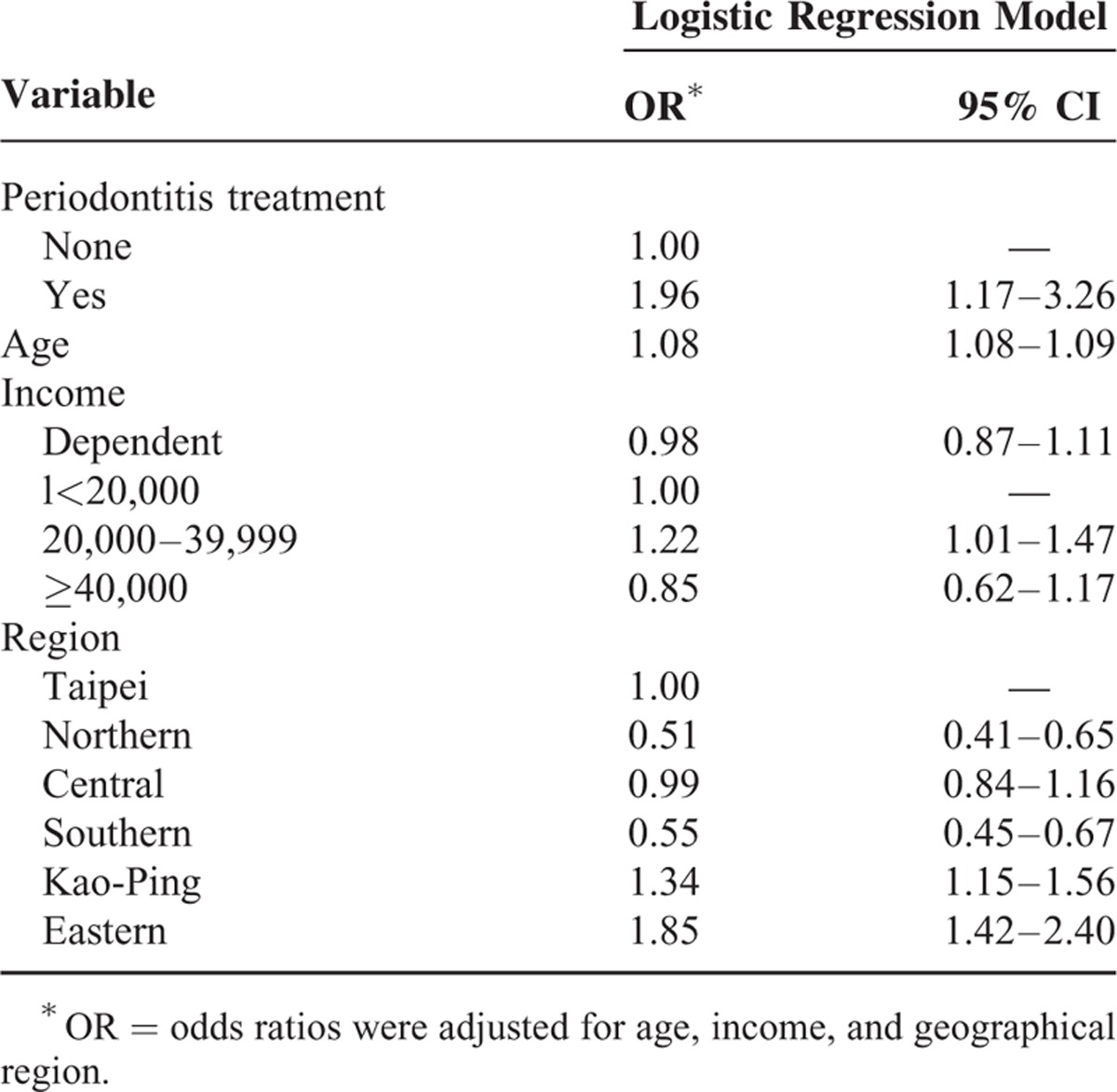
After adjusting for age, sex, income, and geographical region, there was a significant association between periodontitis and osteoporosis among women (odds ratio: 1.96; 95% confidence interval 1.17–3.26).
The association between periodontitis and osteoporosis was significant among women.
INTRODUCTION
Periodontitis and osteoporosis are both important issues in public health and clinical management.1 They are characterized by a reduction in bone mass and have also been associated with aging.2–4 In the case of osteoporosis, the bone loss is systemic whereas for periodontitis, it is localized and confined to the jaw bones, in the specific part related to the teeth.1 Some studies showed positive correlations2,5–24 whereas others have showed inverse associations.25–32 Some of the studies cited above have shown inconsistencies in results. The correlations between periodontal disease and osteoporosis have been hindered by small sample sizes.4,29 One of the studies that showed positive associations had a sample size of 11,655,13 whereas others ranged from 26 to 1341.8,9 On the contrary, studies with inverse associations had sample sizes of 80, 634, and 398, respectively.30,31 The aim of this study was to determine the associations between periodontal disease and osteoporosis by gender.
MATERIALS AND METHODS
Data were obtained from the National Health Insurance Research Database (NHIRD), a source database of the national health care system of Taiwan. The database contained detailed clinical records of every patient during each visit, primary and secondary diagnostic codes, as well as prescription orders. Samples were randomly retrieved from the Longitudinal Health Insurance Databases (LHID2005 and 2010) that contained all inpatient and outpatient medical claims as well as registration files of ∼2 million beneficiaries. Because the LHID comprise secondary data (which cannot be used to identify patients) released to the public for research purposes, this study was exempted from full review by the Institutional Review Board. The government encouraged and authorized researchers to make use of the data. The International Classification of Disease (ICD-9-CM) diagnostic guidelines were used to identify the diseases of interests.
DEFINITION OF PERIODONTITIS
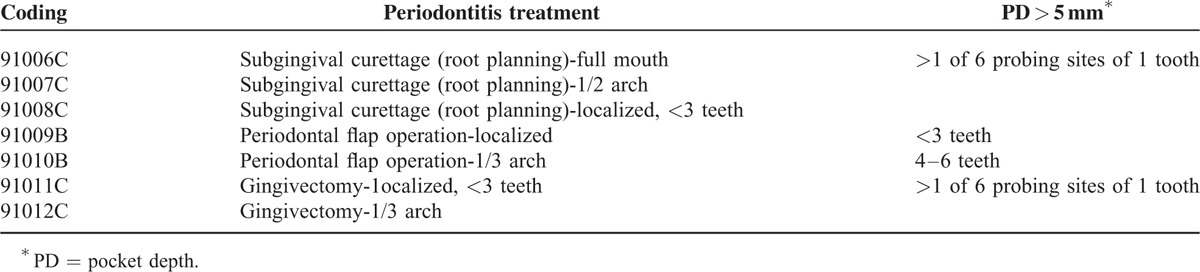
A diagnosis of periodontitis was defined on the basis of subgingival curettage, periodontal flap operation, and gingivectomy (excluding those with restorative or aesthetic indications) as prescribed by the National Health Insurance (NHI) system. The claim codes included 91006C, 91007C, 91008C, 91009B, 91010B, 91011C, and 91012C (Table 1). Information about the collection of pocket depth (PD) data in the patient's record has been described for each periodontal treatment.
TABLE 1.
Periodontitis Treatment Codes
DEFINITION OF OSTEOPOROSIS
Osteoporotic individuals were identified from data subsets CD or DD of the NHIRD based mainly on ICD-9-CM diagnostic code 733.0. CD indicated the ambulatory care expenditures by visit, and DD indicated the inpatient expenditures by admission.
MEASURES
Demographic characteristics (sex, insurance amount, and geographical region) were collected from the NHIRD registry. Monthly income was categorized as follows: <NT$20,000, NT$20,000 to NT$40,000, and >NT$40,000. Six main branches of the NHI have been categorized based on geographic and administrative districts: Taipei (Taipei City, Taipei County, Keelung City, Yilan County, Kinmen County, Lianjiang County), Northern (Taoyuan County, Hsinchu City, Hsinchu County, Miaoli County), Central (Taichung City, Taichung County, Changhua County, Nantou County), Southern (Tainan City, Tainan County, Chiayi City, Chiayi County, Yunlin County), Kao-Ping (Kaohsiung City, Kaohsiung County, Pingtung County, Penghu County), and Eastern region (Hualien County, Taitung County).
STATISTICAL ANALYSES
All statistics were analyzed using SAS version 9.2 software (SAS Institute, Cary, NC). Osteoporosis was determined using the χ2 test. Demographic characteristics included sex, insurance amount, and geographical region; t test was used to determine the difference between cohort and the comparison groups. Multivariate logistic regression was used to analyze the association between periodontitis and osteoporosis. Differences were considered significant at P value <0.05.
RESULTS
A total of 1,878,401 individuals were sampled (Figure 1). In general, 4535 women and 4770 men were diagnosed with periodontal diseases from 2001 to 2003, and 1266 women and 243 men were diagnosed with osteoporosis from 2004 to 2010. Among the periodontal disease cohorts, 15 women and 4 men were diagnosed with osteoporosis. Osteoporotic individuals had a significantly higher mean age (65.81, n = 1509) than their nonosteoporotic counterparts (35.03, n = 1,876,892, P < 0.0001) as shown in Table 2. Variables such as sex, insurance amount, geographical region, and periodontal disease were significantly different between osteoporotic and nonosteoporotic individuals (Table 2). Results from multiple logistic regressions are shown in Table 3. The risk for osteoporosis was higher among patients who received periodontal treatment (odds ratio [OR]: 2.30; 95% confidence interval [CI] 1.29–3.20). Tables 4 and 5 show the OR of osteoporosis categorized by sex. The risk of developing osteoporosis was more significant among women (OR: 1.96; 95% CI 1.17–3.26) than men (OR: 2.37; 95% CI 0.88–6.39).
FIGURE 1.
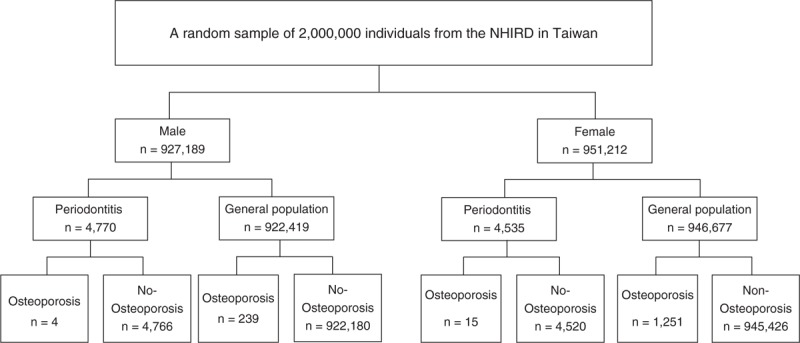
Flow chart of subjects selected from the National Health Insurance Research Database.
TABLE 2.
Baseline Characteristics of Study Subjects
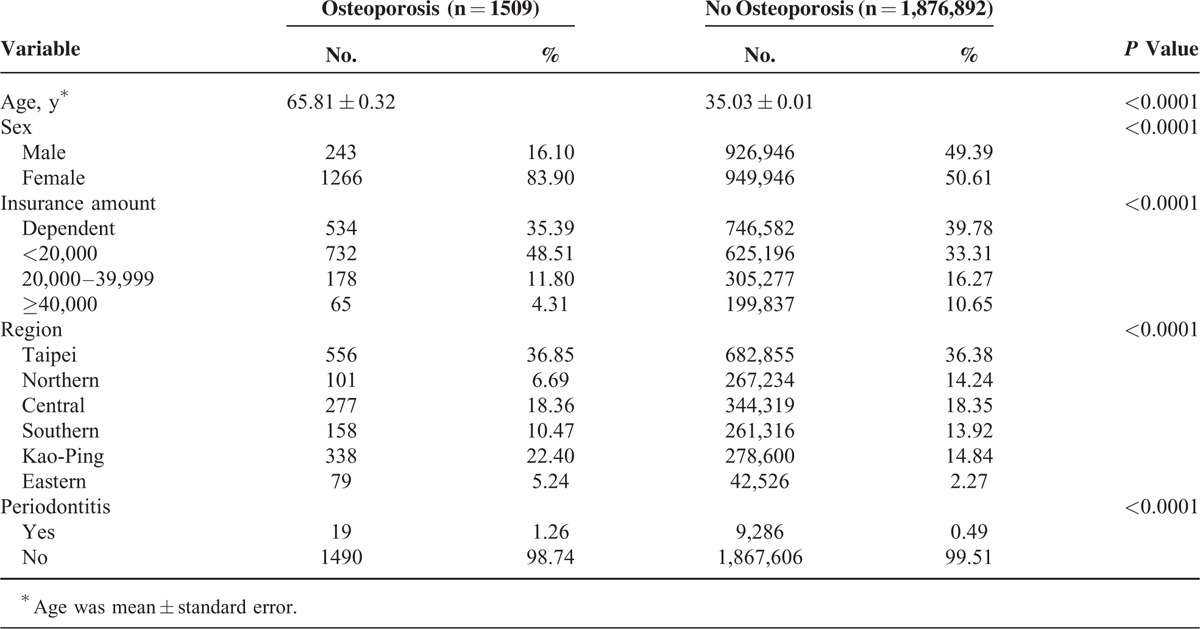
TABLE 3.
Logistic Regression Model of Risk Factors of Osteoporosis Among Periodontal Disease Patients
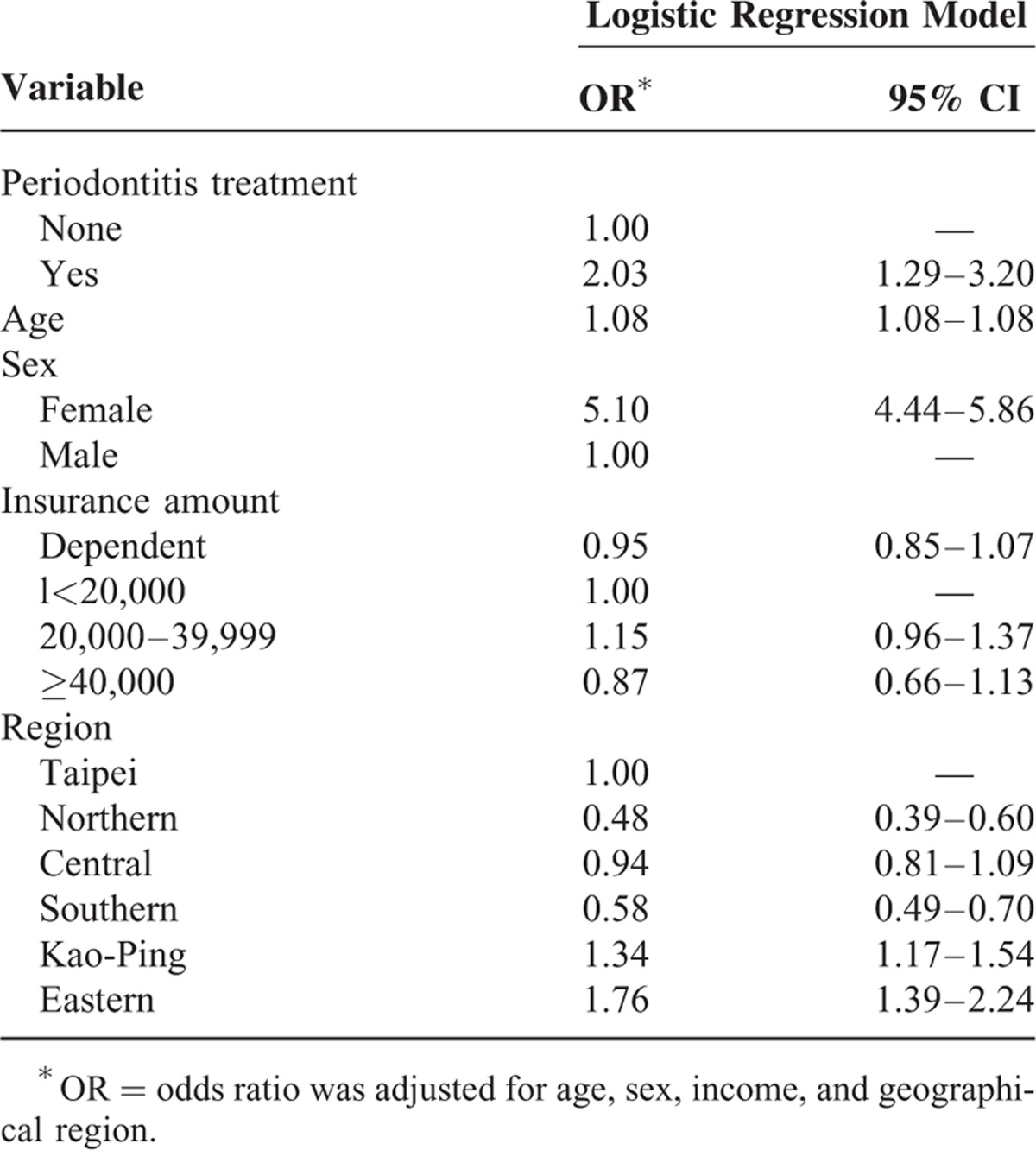
TABLE 4.
Logistic Regression Model of Risk Factors of Osteoporosis in Men
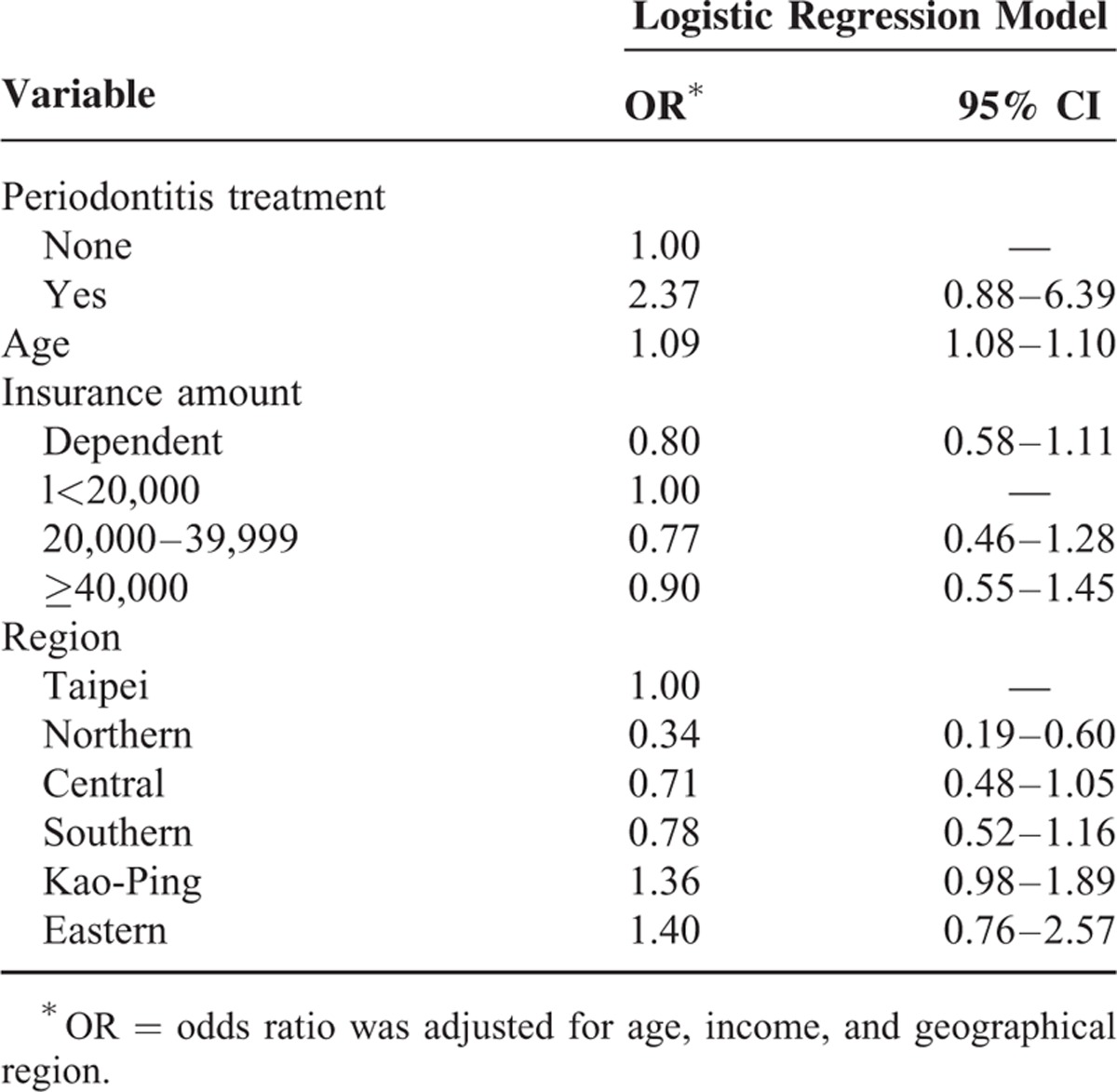
TABLE 5.
Logistic Regression Model of Risk Factors of Osteoporosis in Women
DISCUSSION
To our knowledge, this is the first large-scale population-based cohort study investigating the association between periodontal disease and osteoporosis by gender. In this study, a significant association was observed between periodontitis and osteoporosis specifically in women. Different definitions of periodontal diseases might be attributed to the inconsistencies of results obtained in the previous studies.33,34 Three categories of periodontal treatments have been prescribed by the Bureau of National Health Insurance, Taiwan (Table 1). The first is subgingival curettage (root planning) that is performed based on the codes 91006C (full mouth), 91007C (1/2 arch), and 91008C (localized 3 teeth and less). The second is periodontal flap operation: codes 91009B (localized, <3 teeth with PD >5 mm) and 91010B (1/3 arch, 4–6 teeth with PD ≥5 mm). The third is gingivectomy or gingivoplasty—91011C (localized, <3 teeth affected) and 91012C (1/3 arch). However, gingivectomy with restorative or aesthetic indications was excluded. Individuals receiving the first or third treatments ought to have at least 1 of 6 probing sites of 1 tooth with PD >5 mm. The Taiwanese NHI system allows beneficiaries to receive tooth scaling twice a year. The ICD codes recorded in Taiwan's NHI program may not truly indicate the presence of severe periodontitis and because of that, periodontal treatment codes (3 categories used in Taiwan) were considered more reliable than the ICD-9 code.35 The periodontal status among individuals registered in NHIRD might be different from those diagnosed through general clinical examination.
PD is one of the major clinical indices of periodontitis as defined by the NHI Professional Peer Review Committee (Table 1). Dentists are required to assess PD at 6 sites per tooth (distobuccal, distolingual, mesiobuccal, mesiolingual, midbuccal, and midlingual), with at least 1 site having a PD of ≥5 mm for all the periodontal treatments listed in Table 1. Radiographs were taken to assist in diagnosis and treatment. The British Society of Periodontology suggests periodontal disease where a basic periodontal examination score of ≥5.5 mm is found.29 This is similar to that defined by the NHI program as mentioned earlier and which has been used in this study. Some studies defined periodontitis as having >2 interproximal sites and with PD ≥5 mm, radiographic bone loss, and clinical attachment loss (CAL) >6 mm.6 Several studies have used PD as one of the variables to evaluate the severity of periodontal disease and have also reported positive association between PD and osteoporosis.10,21,24 Recently, a report by the OSTEODENT (Diagnostic validity of dental radiography techniques for identifying osteoporotic patients) multicenter research project found greater CAL in osteoporotic than normal women. However, there was no difference in PD between these 2 groups.21 PDs of 2.15 to 2.43 mm have been used to define periodontitis in Hellenic women. The pockets used in this study were of ≥5 mm as shown in all the periodontal treatments (Table 1).
A hospital-based cross-sectional study that enrolled 34 postmenopausal Taiwanese women aged 50 to 59 years reported a significant relationship between PD and osteoporosis at interproximal instead of the faciolingual sites.16 A significant association also existed between the mandibles, but not the maxilla at both the interproximal and faciolingual sites. The statistical power might have reduced when the PD values recorded at the 6 sites of each tooth were considered.28,29,36 PD might be one of the valuable parameters to assess the relationship between periodontal disease and osteoporosis. Selection of sites might be another vital issue that requires further investigation. This study has shown an association between periodontal disease and osteoporosis although the prevalence of osteoporosis had been underestimated in NHIRD.37 However, the OR of osteoporosis in periodontal and nonperiodontal individuals may have been influenced by nondifferential misclassifications, hence, producing a bias toward the null. Unlike other studies, this study made use of large sample size. The mean PD of osteoporotic individuals (2.43 mm) have been reported to be higher than that of normal bone mineral density (2.15 mm) in Hellenic women.21 The trend of PD was similar to that of CAL, but the results were insignificant as a result of small sample size. Similar results were obtained by a well-defined age cohort in Linköping community in Sweden, but such results ought to be interpreted with caution.28
Age is an important confounder.29 The incidence of osteoporosis and periodontitis has been reported to increase with age.4 In our study, the mean age of osteoporotic individuals was 65.8 (Table 2) and the age effect was statistical significant for all individuals (OR: 1.09; CI 1.08–1.10 for males, OR: 1.08; CI 1.08–1.09 for females, and OR: 1.08; CI 1.08–1.08 for total individuals (Tables 3–5). These results are similar to those described in the previous studies.6,24,38 Age and other potential confounding factors were also adjusted in other studies.2,7 This study suggests that age is significantly associated with periodontal disease and osteoporosis. This is in agreement with some of the aforementioned studies. A previous study did not find significant relationships between bone mass measurement and periodontal parameters.25 Other studies have reported mean ages of 46 to 55 years (younger age group) that probably explain why no association was found between the diseases.39 More studies should center on the association between age and periodontal diseases.
The most important limitation of this study is that the information retrieved from the database did not contain health-related behaviors or status such as smoking, tooth brushing, alcohol consumption, dietary calcium, and number of teeth or tooth loss as well as social and ecologic parameters. Nevertheless, possible effects due to confounding bias from above factors were probably minimized by adjusting for age, gender, insurance cost, and geographical region.
CONCLUSION
The association between periodontitis and osteoporosis was significant in Taiwanese women.
Acknowledgements
This study is based in part on the data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration or the National Health Research Institutes.
Footnotes
Abbreviations: NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, PD = pocket depth.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Reddy MS, Morgan SL. Decreased bone mineral density and periodontal management. Periodontology 2000 2013; 61:195–218. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoat M. The association between osteoporosis and oral bone loss. J Periodontol 2005; 76 suppl 11:2125–2132. [DOI] [PubMed] [Google Scholar]
- 3.Jeffcoat MK, Lewis CE, Reddy MS, et al. Post-menopausal bone loss and its relationship to oral bone loss. Periodontology 2000 2000; 23:94–102. [DOI] [PubMed] [Google Scholar]
- 4.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol 2001; 6:197–208. [DOI] [PubMed] [Google Scholar]
- 5.Brennan RM, Genco RJ, Hovey KM, et al. Clinical attachment loss, systemic bone density, and subgingival calculus in postmenopausal women. J Periodontol 2007; 78:2104–2111. [DOI] [PubMed] [Google Scholar]
- 6.Al Habashneh R, Alchalabi H, Khader YS, et al. Association between periodontal disease and osteoporosis in postmenopausal women in jordan. J Periodontol 2010; 81:1613–1621. [DOI] [PubMed] [Google Scholar]
- 7.Brennan-Calanan RM, Genco RJ, Wilding GE, et al. Osteoporosis and oral infection: independent risk factors for oral bone loss. J Dental Res 2008; 87:323–327. [DOI] [PubMed] [Google Scholar]
- 8.Wactawski-Wende J, Hausmann E, Hovey K, et al. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol 2005; 76 suppl 11:2116–2124. [DOI] [PubMed] [Google Scholar]
- 9.von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol 1994; 65:1134–1138. [DOI] [PubMed] [Google Scholar]
- 10.Mohammad AR, Brunsvold M, Bauer R. The strength of association between systemic postmenopausal osteoporosis and periodontal disease. Int J Prosthodont 1996; 9:479–483. [PubMed] [Google Scholar]
- 11.Mohammad AR, Hooper DA, Vermilyea SG, et al. An investigation of the relationship between systemic bone density and clinical periodontal status in post-menopausal Asian-American women. Int Dental J 2003; 53:121–125. [DOI] [PubMed] [Google Scholar]
- 12.Payne JB, Reinhardt RA, Nummikoski PV, et al. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporosis Int 1999; 10:34–40. [DOI] [PubMed] [Google Scholar]
- 13.Ronderos M, Jacobs DR, Himes JH, et al. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from NHANES III. J Clin Periodontol 2000; 27:778–786. [DOI] [PubMed] [Google Scholar]
- 14.Tezal M, Wactawski-Wende J, Grossi SG, et al. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki K, Kurosu Y, Kamiya T, et al. Low metacarpal bone density, tooth loss, and periodontal disease in Japanese women. J Dental Res 2001; 80:1818–1822. [DOI] [PubMed] [Google Scholar]
- 16.Shen EC, Gau CH, Hsieh YD, et al. Periodontal status in post-menopausal osteoporosis: a preliminary clinical study in Taiwanese women. J Chin Med Assoc 2004; 67:389–393. [PubMed] [Google Scholar]
- 17.Gomes-Filho IS, Passos Jde S, Cruz SS, et al. The association between postmenopausal osteoporosis and periodontal disease. J Periodontol 2007; 78:1731–1740. [DOI] [PubMed] [Google Scholar]
- 18.Nicopoulou-Karayianni K, Tzoutzoukos P, Mitsea A, et al. Tooth loss and osteoporosis: the OSTEODENT Study. J Clin Periodontol 2009; 36:190–197. [DOI] [PubMed] [Google Scholar]
- 19.Megson E, Kapellas K, Bartold PM. Relationship between periodontal disease and osteoporosis. Int J Evid Based Healthc 2010; 8:129–139. [DOI] [PubMed] [Google Scholar]
- 20.Grocholewicz K, Bohatyrewicz A. Oral health and bone mineral density in postmenopausal women. Arch Oral Biol 2012; 57:245–251. [DOI] [PubMed] [Google Scholar]
- 21.Pepelassi E, Nicopoulou-Karayianni K, Archontopoulou AD, et al. The relationship between osteoporosis and periodontitis in women aged 45–70 years. Oral Dis 2012; 18:353–359. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi O, Yoshihara A, Nakamura K, et al. Association between periodontitis and systemic bone mineral density in Japanese community-dwelling postmenopausal women. J Dentistry 2012; 40:304–311. [DOI] [PubMed] [Google Scholar]
- 23.Gondim V, Aun J, Fukuda CT, et al. Severe loss of clinical attachment level: an independent association with low hip bone mineral density in postmenopausal females. J Periodontol 2013; 84:352–359. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Sugita N, Yoshihara A, et al. Peroxisome proliferator-activated receptor (PPAR) polymorphism, vitamin D, bone mineral density and periodontitis in postmenopausal women. Oral Dis 2013; 19:501–506. [DOI] [PubMed] [Google Scholar]
- 25.Elders PJ, Habets LL, Netelenbos JC, et al. The relation between periodontitis and systemic bone mass in women between 46 and 55 years of age. J Clin Periodontol 1992; 19:492–496. [DOI] [PubMed] [Google Scholar]
- 26.Klemetti E, Collin HL, Forss H, et al. Mineral status of skeleton and advanced periodontal disease. J Clin Periodontol 1994; 21:184–188. [DOI] [PubMed] [Google Scholar]
- 27.Weyant RJ, Pearlstein ME, Churak AP, et al. The association between osteopenia and periodontal attachment loss in older women. J Periodontol 1999; 70:982–991. [DOI] [PubMed] [Google Scholar]
- 28.Lundstrom A, Jendle J, Stenstrom B, et al. Periodontal conditions in 70-year-old women with osteoporosis. Swed Dental J 2001; 25:89–96. [PubMed] [Google Scholar]
- 29.Marjanovic EJ, Southern HN, Coates P, et al. Do patients with osteoporosis have an increased prevalence of periodontal disease? A cross-sectional study. Osteoporosis Int 2013; 24:1973–1979. [DOI] [PubMed] [Google Scholar]
- 30.Sultan N, Rao J. Association between periodontal disease and bone mineral density in postmenopausal women: a cross sectional study. Med Oral Patol Oral Cir Bucal 2011; 16:e440–e447. [DOI] [PubMed] [Google Scholar]
- 31.Famili P, Cauley J, Suzuki J, et al. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 2005; 76:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilgram TK, Hildebolt CF, Dotson M, et al. Relationships between clinical attachment level and spine and hip bone mineral density: data from healthy postmenopausal women. J Periodontol 2002; 73:298–301. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Maestre MÁ, González-Cejudo C, Machuca G, et al. Periodontitis and osteoporosis: a systematic review. Climacteric 2010; 13:523–529. [DOI] [PubMed] [Google Scholar]
- 34.Passos JdS, Gomes-Filho IS, Vianna MIP, et al. Outcome measurements in studies on the association between osteoporosis and periodontal disease. J Periodontol 2010; 81:1773–1780. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z-Y, Chiang C-H, Huang C-C, et al. The association of tooth scaling and decreased cardiovascular disease: a nationwide population-based study. Am J Med 2012; 125:568–575. [DOI] [PubMed] [Google Scholar]
- 36.Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosth Dentistry 1990; 63:218–222. [DOI] [PubMed] [Google Scholar]
- 37.Yang N-P, Deng C-Y, Chou Y-J, et al. Estimated prevalence of osteoporosis from a Nationwide Health Insurance database in Taiwan. Health Policy 2006; 75:329–337. [DOI] [PubMed] [Google Scholar]
- 38.Moedano DE, Irigoyen ME, Borges-Yáñez A, et al. Osteoporosis, the risk of vertebral fracture, and periodontal disease in an elderly group in Mexico City. Gerodontology 2011; 28:19–27. [DOI] [PubMed] [Google Scholar]
- 39.Geurs NC, Lewis CE, Jeffcoat MK. Osteoporosis and periodontal disease progression. Periodontology 2000 2003; 32:105–110. [DOI] [PubMed] [Google Scholar]


