Abstract
The objective of this study was to assess the relationship between female hormone and menstrual factors and pancreatic cancer (PC) through a meta-analysis of observational studies.
We undertook a systematic literature search up to July 10, 2014 in PubMed and EMBASE databases. Combined relative risks (RRs) were estimated by random-effects models. Subgroup analysis was performed by study design, source of control, and geographic regions. Sensitivity analyses and publication bias were utilized to evaluate the robustness of our results.
A total of 27 case–control and cohort studies were retrieved for this meta-analysis. No significant associations were observed between the risk of PC and age at menarche (RR = 0.94, 95% confidence interval [CI] 0.83–1.07), age at menopause (RR = 0.98, 95% CI 0.85–1.13), hysterectomy (RR = 0.97, 95% CI 0.84–1.11), oophorectomy (RR = 1.02, 95% CI 0.82–1.26), hormone replacement therapy (RR = 0.97, 95% CI 0.87–1.08), and oral contraceptives (RR = 1.09, 95% CI 0.96–1.23).
This meta-analysis of observational studies does not support the hypothesis that exogenous hormone use and menstrual factors are associated with PC.
INTRODUCTION
Pancreatic cancer (PC) represents the fourth most common cause of cancer mortality and ranks 10th in incidence of all cancers in adults in the United States.1,2 The primary causes are poorly understood. Although cigarette smoking, obesity, a history of diabetes mellitus (DM), a history of pancreatitis, and not belonging to the O blood group have been shown to be risk factors for PC, they may only account for a small number of cases.3,4 Therefore, the question of what additional risk factors might influence the development of PC remains open.
A number of findings suggest that a cause and effect relationship may exist between PC risk and sex hormones. First, the incidence of PC is approximately 30% to 50% higher in men than in women.3 Second, biological experiments have confirmed the presence of steroid hormone receptors and sex-steroid biosynthetic enzymes in both normal and cancerous human pancreatic tissue.5–7 Third, exogenous estrogens can inhibit the development of PC in animal models.8,9 On the contrary, testosterone has been shown to strongly promote growth in experimental PCs.10 Fourth, the results of previous meta-analyses showed that parity (number of birth) is associated with a decreased risk of PC.3,11 Finally, obesity, one of the well-established risk factors for PC, provides a leading source of endogenous estrogen exposure in postmenopausal women.12 Therefore, these have raised interest in some exposures related with female hormones in the development of PC. Over the last 2 decades, many studies have examined hormonal and menstrual factors in relation to PC risk.4,13–38 However, findings regarding the association between hormonal and menstrual factors and PC risk have conflicted with each other. Thus, we tested the hypothesis that exogenous hormone use and menstrual factors are associated with PC by conducting a meta-analysis of case–control and cohort studies.
METHODS
Selection Criteria
We used the following inclusion criteria: the article described a case–control or cohort study that evaluated the relationship between hormonal and menstrual factors and PC risk; the article presented relative risk (RR) (ie, odds ratios [ORs], hazard ratios [HRs]) with corresponding 95% confidence intervals [CIs] or standard errors (SEs), or sufficient data to estimate them); if >1 article involving the same subjects was published, only the most informative study was included; reviews, meta-analyses, case reports, and conference abstracts were excluded.
Search Strategy
A systematic search of the PubMed and EMBASE databases (up to July 10, 2014) was performed without language limitations. We identified articles using the follows search terms: (“pancreatic cancer” OR “pancreatic neoplasms” OR “pancreatic tumors” OR “pancreatic adenocarcinoma”) AND (“hormone” OR “exogenous hormones” OR “exogenous hormones use” OR “hormone replacement therapy” OR “menopausal hormone therapy” OR “estrogen replacement therapy” OR “menopausal hormone use” OR “oral contraceptives” OR “reproductive factors” OR “reproductive history” OR “menstrual factors” OR “age at menarche” OR “menarche” OR “menstruation” OR “menopause” OR “age at menopause” OR “gravidity” OR “pregnancy” OR “breastfeeding” OR “miscarriage” OR “abortion” OR “fertility” OR “birth” OR “age at first birth” OR “climacteric” OR “parity” OR “ovariectomy” OR “oophorectomy” OR “hysterectomy”) AND (“risk” OR “risk factors” OR “risk assessment”). We also manually examined the references of relevant studies or reviews that assessed the association between menstrual factors and the risk of PC to identify additional studies.
Data Extraction
Two reviewers (B.T. and J.L.) independently extracted the following data from each eligible study: last name of the first author, year of publication, country, study period/follow-up years, study design, cases/cohort size (ie, controls), exposure variables, measurement of exposure, and RRs with corresponding 95% CIs or SEs, and raw data. Risk estimates that were adjusted for the maximum number of confounders were utilized in this meta-analysis; when these were unavailable, the raw data were used.
Statistical Analysis
RR was used to measure of the association between hormonal and menstrual factors and PC risk. ORs and HRs were deemed equivalent to RRs because the prevalence of PC is rare.39 The RRs with corresponding 95% CIs were pooled using the DerSimonian and Laird40 random-effects model (random-effects models consider both within-study and between-study variability). Both the I2 test (values of 25%, 50%, and 75% were considered to represent low, moderate, and high heterogeneity, respectively) and Cochran Q statistic (a P value <0.1 was considered indicative of statistically significant heterogeneity) were calculated to quantify the extent of heterogeneity across studies.41,42 Subgroup analyses were performed according to study design (case–control vs cohort studies), source of control (population-based vs hospital-based case–control studies), and geographic regions (North America vs Europe vs Asia). Sensitivity analyses were performed to evaluate the stability of the results by excluding one study at a time. In addition, we performed an alternative sensitivity analysis to investigate whether the overall results were influenced by potential confounders or not. Egger test was used to detect potential publication bias (P < 0.05 was considered statistically significant).43 When publication bias was detected by Egger test, the Duval and Tweedie44 nonparametric trim-and-fill procedure was used to further evaluate the robustness of our results. All statistical analyses were conducted using STATA software, version 12.0 (STATA Corporation, College Station, TX).
For age at menarche and age at menopause, we performed a meta-analysis of the comparison of the highest versus lowest category in each study. For hysterectomy and oophorectomy, we compared women undergoing gynecologic surgery with those that did not have gynecologic surgery. For hormone replacement therapy (HRT) and oral contraceptives (OCs), an analysis of comparison of users versus nonusers was performed. When >1 estimate in a study fell into the exposure level considered, we recalculated a pooled risk estimate using the method proposed by Hamling et al.45
RESULTS
Literature Search and Study Characteristics
Figure 1 shows the process used for the literature search and study selection. A total of 441 publications were identified from databases. Seven studies were additionally identified from the references of other relevant studies. To begin with, 122 duplicate records were excluded. Next, we reviewed the titles and abstracts, and 285 articles were further removed. Finally, 41 articles with full-text were assessed for eligibility. Of these 41 articles, 14 were further excluded because they provided insufficient data,46 were reviews or meta-analysis,3,10,11,47,48 involved parity,49–53 and had overlapping data.54–56 Thus, in total, 27 articles listed in Table 1 were included in the present analysis.4,13–38 All studies were published in English. The first study dated back to 1966. The latest article was published in 2013. Fourteen of 27 studies were case–control studies,13–19,22,23,25,29,34,36,37 the remainders were cohort studies.4,20,21,24,26–28,30–33,35,38 In 7 of case–control studies, controls were recruited randomly from hospitals in 3 studies;13–15,18,29,36,37 in the other 7, they were drawn from the general population.16,17,19,22,23,25,34 All studies were published in Western countries except 2 from East Asia and 1 from Africa.19,28,29 Cases were ascertained by means of computerized record linkages to cancer registries, histopathology, and medical records (eg, health insurance records, death certificates, radiological images). Assessment tools to collect data on exposure variables consisted of interviewer-administered questionnaire, self-administered questionnaire, prescription registry, hospital records, and mass screening registry.
FIGURE 1.
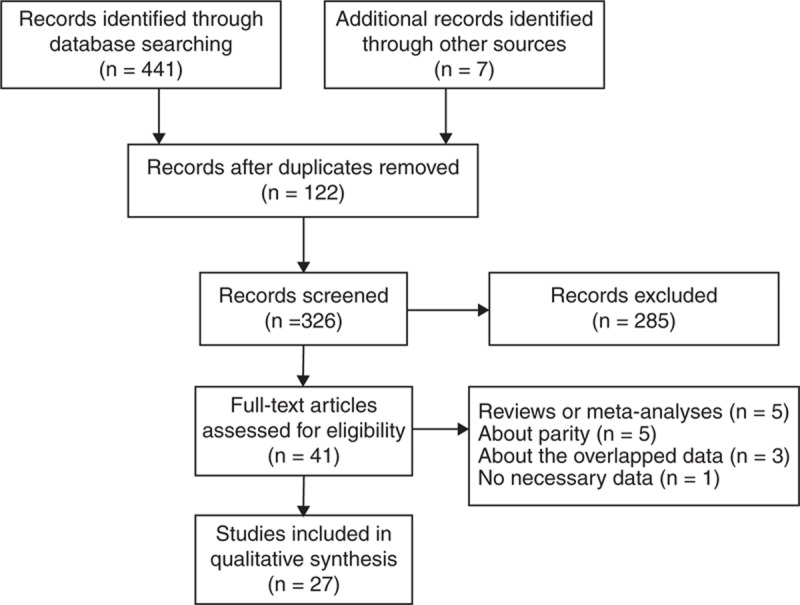
Flow diagram of literature search and selection.
Table 1.
Characteristics of the Included Studies
Results of Overall Meta-Analyses
Late Versus Early Age at Menarche
Seventeen studies have examined the association between age at menarche and PC risk.4,17–19,22–28,30,34–38 The pooled RR for the oldest age compared with the youngest age was 0.94 (95% CI 0.83–1.07, I2 = 55.1%, PQ = 0.003) (Figure 2).
FIGURE 2.
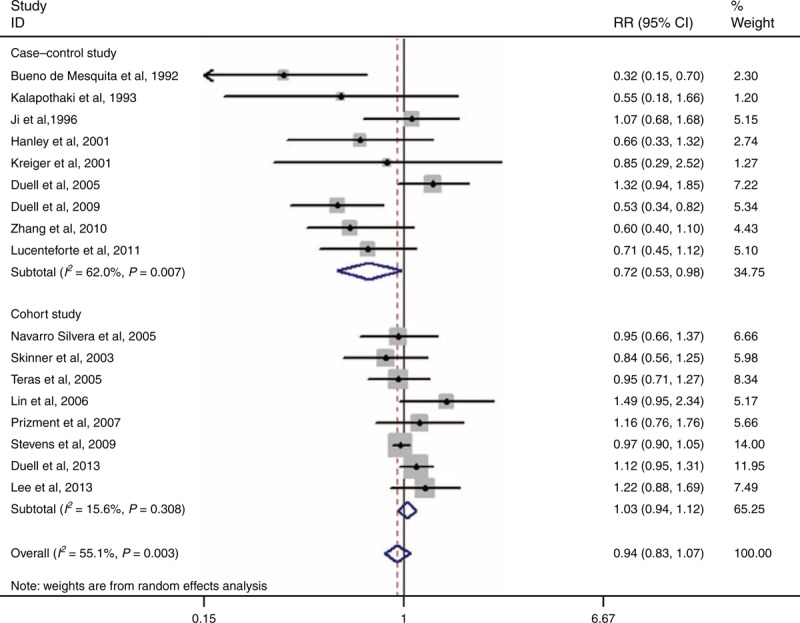
Forest plots of age at menarche and PC risk. CI = confidence interval, PC = pancreatic cancer, RR = relative risk.
Late Versus Early Age at Menopause
The effect of age at menopause on the risk of PC has been evaluated by 16 articles.4,17–19,23–25,27,28,30,32,34–38 The summary RR for the oldest age versus the youngest age was 0.98 (95% CI 0.85–1.13, I2 = 46.3%, PQ = 0.022) (Figure 3).
FIGURE 3.
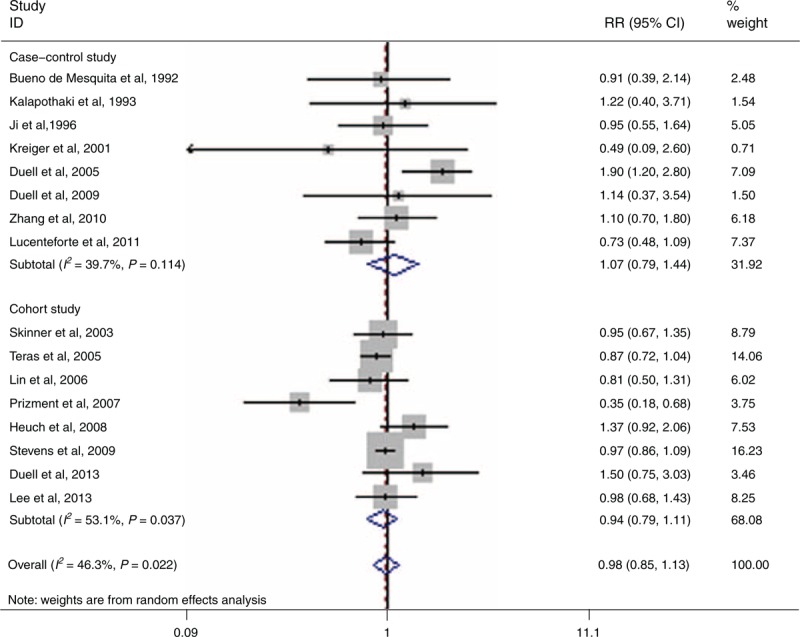
Forest plots of age at menopause and PC risk. CI = confidence interval, PC = pancreatic cancer, RR = relative risk.
Ever Versus Never Hysterectomy
Twelve reports have evaluated the correlation between hysterectomy and PC risk.4,13–17,21,25,27,30,34,37 The cumulative risk estimates for ever having had a hysterectomy versus never having had 1 was 0.97 (95% CI 0.84–1.11, I2 = 33.4%, PQ = 0.123) (Figure 4).
FIGURE 4.
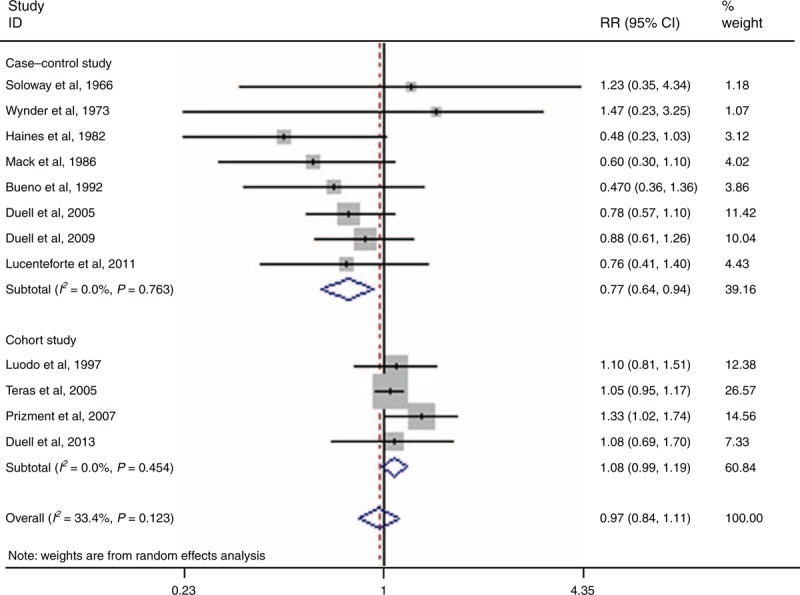
Forest plots of hysterectomy and PC risk. CI = confidence interval, PC = pancreatic cancer, RR = relative risk.
Ever Versus Never Oophorectomy
Nine articles have assessed the link between oophorectomy and PC risk.4,14–17,25,30,34,37 The pooled RR for ever having had an oophorectomy versus never having had 1 was 1.02 (95% CI 0.82–1.26, I2 = 27.8%, PQ = 0.197) (Figure 5). In a further analysis that account for type of oophorectomy (ie, bilateral vs unilateral procedures), the pooled RRs were 1.10 (95% CI 0.83–1.46, I2 = 0.0%, PQ = 0.524) for unilateral procedures and 1.06 (95% CI 0.78–1.43, I2 = 40.5%, PQ = 0.168) for bilateral ones.
FIGURE 5.
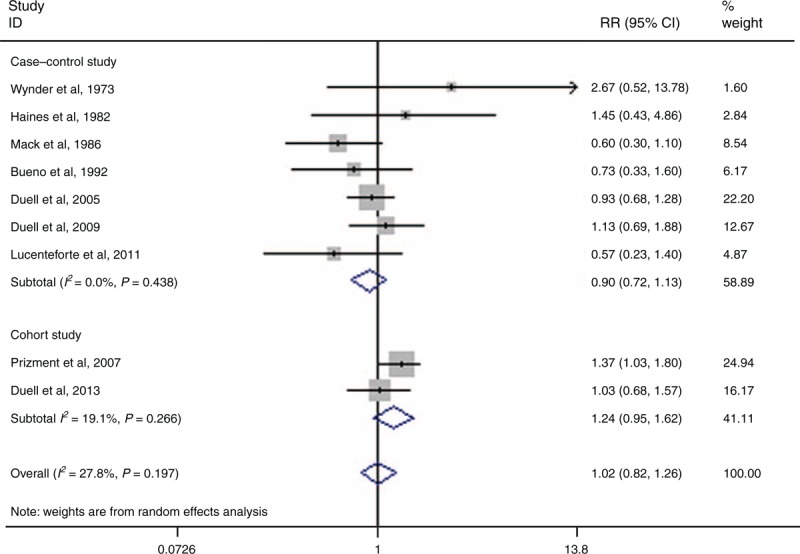
Forest plots of oophorectomy and PC risk. CI = confidence interval, PC = pancreatic cancer, RR = relative risk.
Ever Versus Never OC Use
Fourteen studies have investigated the relationship between OC and PC risk.4,17,19,23–27,30,33,34,36–38 The pooled RR for ever versus never use was 1.09 (95% CI 0.96–1.23, I2 = 19.7%, PQ = 0.239) (Figure 6).
FIGURE 6.
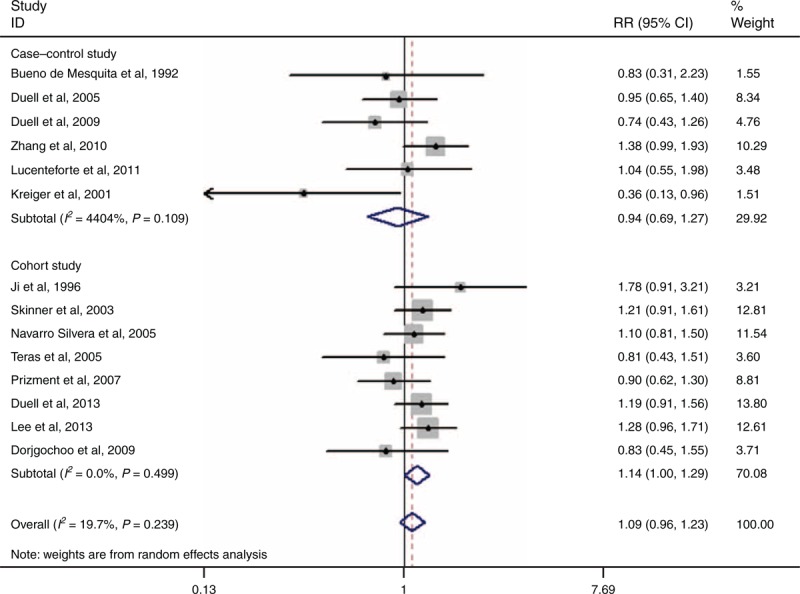
Forest plots of OC and PC risk. CI = confidence interval, RR = relative risk, OC = oral contraceptive, PC = pancreatic cancer.
Ever Versus Never HRT Use
Fifteen studies have addressed the correlation between HRT and PC risk.4,17,20,23–27,29–31,34,36–38 The combined RR for ever versus never use was 0.97 (95% 0.87–1.08, I2 = 37.50%, PQ = 0.071) (Figure 7). Seven of 15 studies examined the relation between the use of HRT and the incidence of PC in postmenopausal women.4,23–26,30,31 Limited to subjects who were postmenopausal women, the pooled RR was 0.97 (95% CI 0.85–1.12, I2 = 16.0%, PQ = 0.308). Five of 15 studies have assessed the components of HRT.23,25,27,34,38 Limited to subjects who only used the estrogen replacement therapy, the combined RR was 0.86 (95% CI 0.71–1.04, I2 = 41.5%, PQ = 0.145).
FIGURE 7.
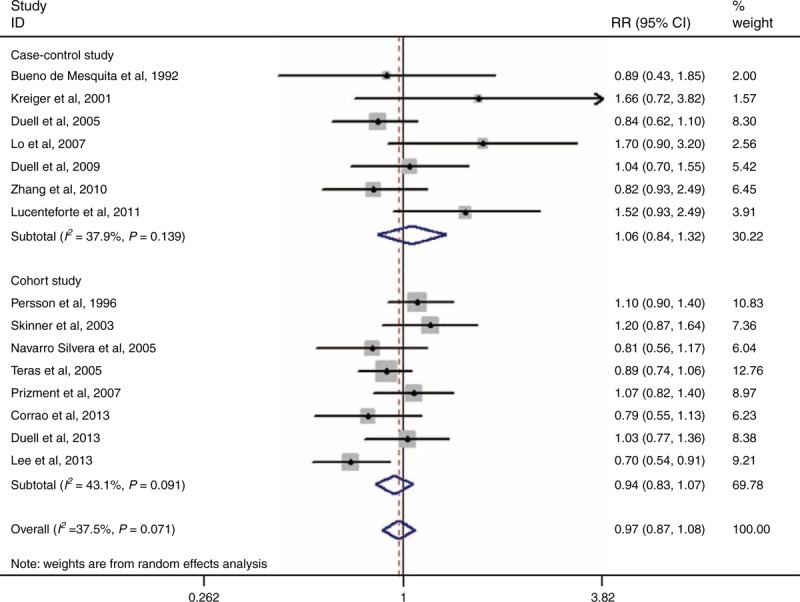
Forest plots of HRT and PC risk. CI = confidence interval, HRT = hormone replacement therapy, PC = pancreatic cancer, RR = relative risk.
Results of Subgroup Analyses
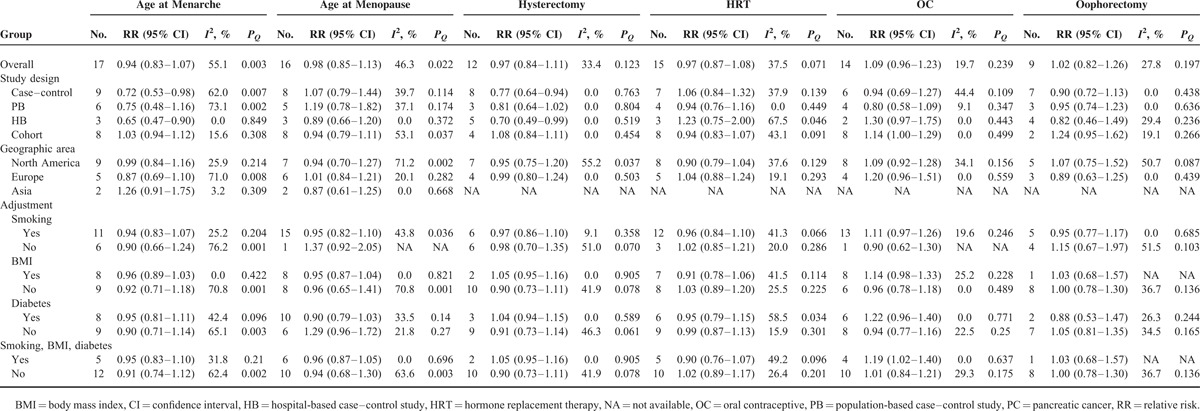
Subgroup analyses were performed according to study design (case–control vs cohort studies), source of control (population-based vs hospital-based case–control studies), and geographic regions (North America vs Europe vs Asia). For age at menopause, HRT, and oophorectomy, the overall results were not significantly influenced by geographic regions, study design, or source of control (Table 2). When subgroup analyses were conducted by study design, statistically significant associations between PC risk and age at menarche (RR = 0.72, 95% CI 0.53–0.98) and hysterectomy (RR = 0.77, 95% CI 0.64–0.94) were observed in case–control studies. However, these associations did not emerge in cohort studies (Table 2). In further analysis by source of control, a decreased risk of significance was observed only for subjects who were from hospital-based case–control studies (Table 2). For OC, a statistically marginal association was observed in cohort studies (RR = 1.14, 95% CI 1.00–1.29).
TABLE 2.
Summary Risk Estimates of the Association Between PC Risk and Hormonal and Menstrual Factors
Sensitivity Analysis
In the sensitivity analysis, one single study was excluded at a time to investigate the influence of individual study on the overall results. Sensitivity analysis demonstrated that the results of OC were not robust (Figure 8). When excluding the study conducted by Kreiger et al,23 an increased risk of borderline significance was found (RR = 1.12, 95% CI 1.00–1.24). For other exposures, the results were not meaningfully changed (data not shown). In addition, we performed an alternative sensitivity analysis to investigate whether the overall results were influenced by potential confounders or not. The results were shown in Table 2. All results were not significantly modified by smoking, body mass index (BMI), or diabetes except for OC. When we performed an analysis limited to those studies that provided risk estimates adjusted for smoking, BMI, and diabetes, a meaningful association between OC and PC risk was detected (RR = 1.19, 95% CI 1.02–1.40).
FIGURE 8.
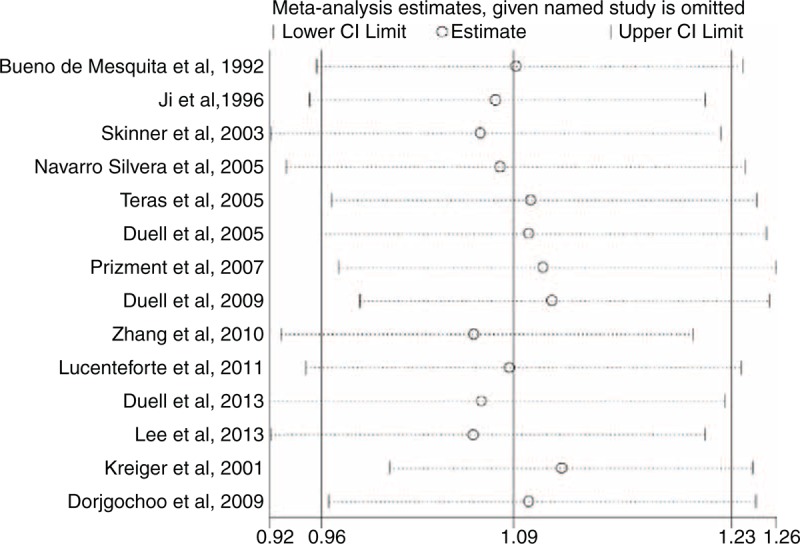
Sensitivity analyses for OC. CI = confidence interval, OC = oral contraceptive.
Publication Bias
The results of Egger test that showed no evidence of publication bias was observed for age at menarche (P = 0.235), age at menopause (P = 0.931), hysterectomy (P = 0.160), HRT (P = 0.176), and oophorectomy (P = 0.504). However, a publication bias was identified for OC (P = 0.031). Then, the Duval and Tweedie44 nonparametric trim-and-fill procedure was undertaken to assess the effect of the possible missing studies on our results. However, we did not find any possible missing studies, and the corresponding results were not changed, suggesting that the results about OC were stable.
DISCUSSION
To our knowledge, this study is the first meta-analysis assessing the relationship between PC risk and hormonal and menstrual factors, and this meta-analysis included 14 case–control and 13 cohort studies involving >2,300,000 subjects. Findings from our meta-analysis revealed no significant associations between the risk of PC and age at menarche, age at menopause, hysterectomy, oophorectomy, HRT, and OC.
Hysterectomy is one of the most frequent gynecological procedures for women in developed countries. It has been estimated that approximately a third of women will have a hysterectomy in their lifetime.57 Having a good understanding of the consequences of this procedure is very important. In our meta-analysis, we found that 12 studies have addressed the relationship between hysterectomy and the risk of PC. The pooled results showed that hysterectomy was not correlated with the risk of PC. When subgroup analyses were performed according to study design, hysterectomy was inversely associated with PC risk in case–control studies; however, in cohort studies, this association did not emerge. In further analyses by source of control, the inverse association was observed in hospital-based case–control studies but not in population-based case–control studies. This discrepancy may be related with the limitations of hospital-based case–control studies. In hospital-based case–control studies, the likelihood of bias may be greater. Moreover, residual confounding was possible as 4/5 risk estimates in hospital-based case–control studies were not adjusted for any confounders.13–16 Therefore, the true relationship between hysterectomy and PC risk may be overestimated or underestimated, and the results of case–control studies should be interpreted with caution. Assessment of partial versus total hysterectomy has been performed in a Finland cohort with 25,382 women.21 Luoto et al21 found a decreased risk for women with partial hysterectomy but an increased risk for women with total hysterectomy; however, these associations were not statistically significant. The significance of these findings is unclear. Therefore, the correlation between hysterectomy and the risk of PC requires further discussion.
With regard to oophorectomy, 3 case–control and 2 cohort studies were included in our analysis. The pooled results demonstrated no significant correlation between oophorectomy and the risk of PC. In further analyses that account for the type of oophorectomy (ie, bilateral vs unilateral procedures), similar trends were detected in the 2 subgroups.
Earlier age at menarche and older age at menopause imply that a higher number of menstrual cycles occurred between menarche and menopause, thereby providing a longer cumulative time of female hormone exposure. However, no significant associations between the risk of PC and age at menarche or menopause were observed. In a subgroup analysis by study design, a 38% decreased risk was observed among women who were older at menarche in case–control studies, but this association did not emerge for population-based case–control and cohort studies. Concerning the limitations listed above, this discrepancy is plausible.
Concerning the use of exogenous hormones, we found that HRTs were not significantly associated with the risk of PC, and that the association between HRT and PC risk was not significantly modified by study design, source of control, or the potential confounders (smoking, BMI, and diabetes). When an analysis was limited to subjects who were postmenopausal women, similar trend was detected. With regard to the OC use, the overall results showed no significant association between OC use and PC risk. However, in subgroup analysis by study design, an increased risk of borderline significance was associated with OC use, and the results from the previous 2 methods of sensitivity analysis showed that OC use may be correlated with PC risk.
Five studies have assessed the association between the formulation of HRT with PC risk.23,25,27,34,38 In 4/5 studies, nonsignificant increase or decrease in PC risk emerged23,25,27,34 However, in a cohort of 118,164 female public school professionals, Lee et al38 found that ever use of estrogen replacement therapy (but not estrogen-plus-progestin therapy or progestin-only therapy) was statistically significantly correlated with a decreased risk of PC. In our meta-analysis, we summarized the evidence of 5 studies and found that estrogen-only therapy was not statistically significantly associated with PC risk. With respect to OC, the components have changed. During the 1980s, OCs markedly differed from the ones used later on (low estrogen, triphasic).58 In our study, none of the studies evaluated the formulation of OC with PC risk. Therefore, further studies of OC and HRT should pay more attention to the formulation of female hormones.
The relationship between reproductive factors and PC risk has been investigated by 2 meta-analyses.3,11 The meta-analysis of 11 prospective and 11 case–control studies that reported the summary RR for PC comparing the highest versus lowest parity was 0.86 (95% CI 0.73–1.02).3 However, significant inverse associations were observed in the studies that adjusted for cigarette smoking, BMI, and DM.3 In the latter meta-analysis of 10 cohort studies and 10 case–control studies with 8205 cases, Zhu et al11 distinguished the number of pregnancy from the number of parity and found ever-parous women were associated with a decreased risk of PC; there was no linear relationship between number of parity and risk of PC.
Several limitations of the current study should be addressed. First, residual confounders are often of concern in a meta-analysis of observational studies. The overwhelming majority of risk estimates were drawn from multivariable models, but there was lack of uniformity in the variables for which adjustment was made in each study. Therefore, we still cannot entirely exclude the possibility that our findings were confounded by other risk factors. Second, observational studies are susceptible to various biases (ie, select bias, recall bias). Third, misclassification or measurement errors for exogenous hormone use and menstrual factors may have distorted the association because our analyses were based on the data that were collected at baseline, and changes in the exposures of interest were not evaluated during the follow-up. Fourth, the exclusion of non-English language citations may yield language bias. During the search, no language limitation was imposed. Moreover, after carefully reviewing the articles identified from databases, non-English articles about this topic definitively were not found. Therefore, the exclusion of non-English articles did not yield noticeable harm. Fifth, we did not evaluate the quality of included studies because there are no well-established standard assessment methods for observational studies.59 Nevertheless, we investigated the influence of study design, source of control, geographic regions, and potential confounders (smoking, BMI, and diabetes) on the relationship between hormonal and menstrual factors and PC risk. Finally, publication bias is always a subject of major concern in a meta-analysis. Although the results of Egger test showed no evidence of publication bias for all exposures (except for OC), the potential publication bias may mask the true association.
In conclusion, our meta-analysis suggests that HRT, OC, age at menarche, age at menopause, hysterectomy, and oophorectomy are not significantly associated with the risk of PC. However, available prospective data are still sparse. Therefore, our findings need to be confirmed in further studies with a good design.
Footnotes
Abbreviations: BMI = body mass index, HRT = hormone replacement therapy, OC = oral contraceptive, PC = pancreatic cancer.
BT and JL contributed equally to this article.
This research was supported in part by the National Natural Science Foundation of China (No. 81160066 and No. 30870719), Scientific Research Foundation for Returned Scholars, and Ministry of Education of China (jyb2010–01).
The funders had no role in study design, data collection and analysis, decision to publish, and preparation of the manuscript. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wörmann SM, Algül H. Risk factors and therapeutic targets in pancreatic cancer. Front Oncol 2013; 3:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case–control study. Am J Gastroenterol 2007; 102:2696–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan HB, Wu L, Wu QJ, et al. Parity and pancreatic cancer risk: a dose-response meta-analysis of epidemiologic studies. PLoS ONE 2014; 9:e92738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duell EJ, Travier N, Lujan-Barroso L, et al. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer 2013; 132:2164–2175. [DOI] [PubMed] [Google Scholar]
- 5.Robles-Diaz G, Duarte-Rojo A. Pancreas: a sex steroid-dependent tissue. Isr Med Assoc J 2001; 3:364–368. [PubMed] [Google Scholar]
- 6.Greenway B, Iqbal MJ, Johnson PJ, et al. Oestrogen receptor proteins in malignant and fetal pancreas. Br Med J (Clin Res Ed) 1981; 283:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Baker PR, Poulsom R, et al. Expression of oestrogen receptor and oestrogen-inducible genes in pancreatic cancer. Br J Surg 1997; 84:1085–1089. [PubMed] [Google Scholar]
- 8.Sumi C, Longnecker DS, Roebuck BD, et al. Inhibitory effects of estrogen and castration on the early stage of pancreatic carcinogenesis in Fischer rats treated with azaserine. Cancer Res 1989; 49:2332–2336. [PubMed] [Google Scholar]
- 9.Sumi C, Brinck-Johnsen T, Longnecker DS. Inhibition of a transplantable pancreatic carcinoma by castration and estradiol administration in rats. Cancer Res 1989; 49:6687–6692. [PubMed] [Google Scholar]
- 10.Andrén-Sandberg A, Hoem D, Bäckman PL. Other risk factors for pancreatic cancer: hormonal aspects. Ann Oncol 1999; 10:131–135. [PubMed] [Google Scholar]
- 11.Zhu B, Zou L, Han J, et al. Parity and pancreatic cancer risk: evidence from a meta-analysis of twenty epidemiologic studies. Sci Rep 2014; 4:5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr 1987; 45:277–282. [DOI] [PubMed] [Google Scholar]
- 13.Soloway HB, Sommers SC. Endocrinopathy associated with pancreatic carcinomas. Review of host factors including hyperplasia and gonadotropic activity. Ann Surg 1966; 164:300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynder EL, Mabuchi K, Maruchi N, et al. A case control study of cancer of the pancreas. Cancer 1973; 31:641–648. [DOI] [PubMed] [Google Scholar]
- 15.Haines AP, Moss AR, Whittemore A, et al. A case–control study of pancreatic carcinoma. J Cancer Res Clin Oncol 1982; 103:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack TM, Yu MC, Hanisch R, et al. Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst 1986; 76:49–60. [PubMed] [Google Scholar]
- 17.Bueno DMH, Maisonneuve P, Moerman CJ, et al. Anthropometric and reproductive variables and exocrine carcinoma of the pancreas: a population-based case–control study in The Netherlands. Int J Cancer 1992; 52:24–29. [DOI] [PubMed] [Google Scholar]
- 18.Kalapothaki V, Tzonou A, Hsieh CC, et al. Tobacco, ethanol, coffee, pancreatitis, diabetes mellitus, and cholelithiasis as risk factors for pancreatic carcinoma. Cancer Causes Control 1993; 4:375–382. [DOI] [PubMed] [Google Scholar]
- 19.Ji BT, Hatch MC, Chow WH, et al. Anthropometric and reproductive factors and the risk of pancreatic cancer: a case–control study in Shanghai, China. Int J Cancer 1996; 66:432–437. [DOI] [PubMed] [Google Scholar]
- 20.Persson I, Yuen J, Bergkvist L, et al. Cancer incidence and mortality in women receiving estrogen and estrogen–progestin replacement therapy—long-term follow-up of a Swedish cohort. Int J Cancer 1996; 67:327–332. [DOI] [PubMed] [Google Scholar]
- 21.Luoto R, Auvinen A, Pukkala E, et al. Hysterectomy and subsequent risk of cancer. Int J Epidemiol 1997; 26:476–483. [DOI] [PubMed] [Google Scholar]
- 22.Hanley AJ, Johnson KC, Villeneuve PJ, et al. Canadian Cancer Registries Epidemiology Research Group. Physical activity, anthropometric factors and risk of pancreatic cancer: results from the Canadian enhanced cancer surveillance system. Int J Cancer 200; 94:140–147. [DOI] [PubMed] [Google Scholar]
- 23.Kreiger N, Lacroix J, Sloan M. Hormonal factors and pancreatic cancer in women. Ann Epidemiol 2001; 11:563–567. [DOI] [PubMed] [Google Scholar]
- 24.Skinner HG, Michaud DS, Colditz GA, et al. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev 2003; 12:433–438. [PubMed] [Google Scholar]
- 25.Duell EJ, Holly EA. Reproductive and menstrual risk factors for pancreatic cancer: a population-based study of San Francisco Bay Area women. Am J Epidemiol 2005; 161:741–747. [DOI] [PubMed] [Google Scholar]
- 26.Navarro SS, Miller AB, Rohan TE. Hormonal and reproductive factors and pancreatic cancer risk: a prospective cohort study. Pancreas 2005; 30:369–374. [DOI] [PubMed] [Google Scholar]
- 27.Teras LR, Patel AV, Rodriguez C, et al. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. women (United States). Cancer Causes Control 2005; 16:1035–1040. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y, Kikuchi S, Tamakoshi A, et al. Association of menstrual and reproductive factors with pancreatic cancer risk in women: findings of the Japan Collaborative Cohort Study for Evaluation of Cancer Risk. J Gastroenterol 2006; 41:878–883. [DOI] [PubMed] [Google Scholar]
- 29.Lo AC, Soliman AS, El-Ghawalby N, et al. Lifestyle, occupational, and reproductive factors in relation to pancreatic cancer risk. Pancreas 2007; 35:120–129. [DOI] [PubMed] [Google Scholar]
- 30.Prizment AE, Anderson KE, Hong CP, et al. Pancreatic cancer incidence in relation to female reproductive factors: Iowa Women's Health Study. JOP 2007; 8:16–27. [PubMed] [Google Scholar]
- 31.Corrao G, Zambon A, Conti V, et al. Menopause hormone replacement therapy and cancer risk: an Italian record linkage investigation. Ann Oncol 2008; 19:150–155. [DOI] [PubMed] [Google Scholar]
- 32.Heuch I, Jacobsen BK, Albrektsen G, et al. Reproductive factors and pancreatic cancer risk: a Norwegian cohort study. Br J Cancer 2008; 98:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorjgochoo T, Shu XO, Li HL, et al. Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer 2009; 124:2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duell EJ, Maisonneuve P, Baghurst PA, et al. Menstrual and reproductive factors and pancreatic cancer in the SEARCH program of the IARC. Cancer Causes Control 2009; 20:1757–1762. [DOI] [PubMed] [Google Scholar]
- 35.Stevens RJ, Roddam AW, Green J, et al. Reproductive history and pancreatic cancer incidence and mortality in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev 2009; 18:1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Coogan PF, Palmer JR, et al. A case–control study of reproductive factors, female hormone use, and risk of pancreatic cancer. Cancer Causes Control 2010; 21:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucenteforte E, Zucchetto A, Bosetti C, et al. Reproductive and hormonal factors and pancreatic cancer risk in women. Pancreas 2011; 40:460–463. [DOI] [PubMed] [Google Scholar]
- 38.Lee E, Horn-Ross PL, Rull RP, et al. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol 2013; 178:1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9:1–30. [DOI] [PubMed] [Google Scholar]
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higginz JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 43.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 45.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27:954–970. [DOI] [PubMed] [Google Scholar]
- 46.Cuzick J, Babiker AG. Pancreatic cancer, alcohol, diabetes mellitus and gall-bladder disease. Int J Cancer 1989; 43:415–421. [DOI] [PubMed] [Google Scholar]
- 47.Andrén-Sandberg A, Bäckman PL. Sex hormones and pancreatic cancer. Baillieres Clin Gastroenterol 1990; 4:941–952. [DOI] [PubMed] [Google Scholar]
- 48.Wahi MM, Shah N, Schrock CE, et al. Reproductive factors and risk of pancreatic cancer in women: a review of the literature. Ann Epidemiol 2009; 19:103–111. [DOI] [PubMed] [Google Scholar]
- 49.Karlson BM, Wuu J, Hsieh CC, et al. Parity and the risk of pancreatic cancer: a nested case–control study. Int J Cancer 1998; 77:224–227. [DOI] [PubMed] [Google Scholar]
- 50.Chang CC, Chiu HF, Yang CY. Parity, age at first birth, and risk of death from pancreatic cancer: evidence from a cohort in Taiwan. Pancreas 2010; 39:567–571. [DOI] [PubMed] [Google Scholar]
- 51.Kvale G, Heuch I, Nilssen S. Parity in relation to mortality and cancer incidence: a prospective study of Norwegian women. Int J Epidemiol 1994; 23:691–699. [DOI] [PubMed] [Google Scholar]
- 52.Zhou GZ, Li ZS. Clinical epidemiological research on pancreatic cancer: an analysis of 1027 cases. World Chin J Digestol 2005; 13:55–60. [Google Scholar]
- 53.Cantor KP, Lynch CF, Johnson D. Reproductive factors and risk of brain, colon, and other malignancies in Iowa (United States). Cancer Causes Control 1993; 4:505–511. [DOI] [PubMed] [Google Scholar]
- 54.Adami HO, Persson I, Hoover R, et al. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer 1989; 44:833–839. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez E, La Vecchia C, D’Avanzo B, et al. Menstrual and reproductive factors and pancreatic cancer risk in women. Int J Cancer 1995; 62:11–14. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez E, Gallus S, Bosetti C, et al. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case–control studies. Int J Cancer 2003; 105:408–412. [DOI] [PubMed] [Google Scholar]
- 57.Jordan SJ, Nagle CM, Coory MD, et al. Has the association between hysterectomy and ovarian cancer changed over time? A systematic review and meta-analysis. Eur J Cancer 2014; 49:3638–3647. [DOI] [PubMed] [Google Scholar]
- 58.Gandini S, Iodice S, Koomen E, et al. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer 2011; 47:2607–2617. [DOI] [PubMed] [Google Scholar]
- 59.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007; 36:666–676. [DOI] [PubMed] [Google Scholar]


