Abstract
The purpose of the study was to evaluate the efficacy of an ophthalmic solution containing 0.1% fluorometholone (FML) and 0.1% sodium hyaluronate (HA) for the treatment of ocular dryness in Sjögren syndrome (SS) patients.
Forty SS patients were randomly assigned to the FML or cyclosporin A (CsA) treatment groups. The FML group was treated with 0.1% FML and 0.1% HA, and the CsA group was treated with 0.5% CsA and 0.1% HA. Primary outcomes were corneal fluorescein staining (CFS), the Ocular Surface Disease Index (OSDI) score, conjunctival goblet cell density, and the severity of conjunctival congestion. Patients were also evaluated based on tear film breakup time (TFBUT) and the Schirmer test.
After 8 weeks of treatment, the mean CFS scores were significantly lower in both the groups, compared with the baseline values, and the CFS score of the FML group at week 2 was significantly lower than that of the CsA group (P = 0.042). The OSDI scores improved significantly in both the groups throughout the study, and the OSDI score in the FML group at week 4 was significantly lower than that of the CsA group (P = 0.042). After 8 weeks of therapy, the conjunctival goblet cell density was significantly higher in both the groups (P < 0.001 for both) compared with the baseline values. Conjunctival congestion was reduced in both the groups throughout the study, and the severity in the FML group was significantly less at week 4 compared with that in the CsA group (P = 0.035). The TFBUT in the FML group at week 8 was significantly longer than in the CsA group (P = 0.04).
Treatment using topical 0.1% FML provided faster improvement in the symptoms of ocular dryness in SS patients compared with topical 0.5% CsA.
INTRODUCTION
Approximately 11% of dry eye (DE) patients suffer from Sjögren syndrome (SS), a severe systemic autoimmune exocrinopathy that can cause blindness.1 In SS, the lacrimal and salivary glands are affected by autoimmune processes, and approximately one-third of SS patients display extraglandular manifestations.2 Previous studies have reported that the prevalence of SS in the general population ranges from 0.1% to 4.8%.
The ocular dryness in SS is the result of lacrimal hyposecretion, which is caused by the inflammatory mediators present in the lacrimal gland, tears, and conjunctiva.3 Inflammation has been shown to be a major factor in the pathogenesis of DE,4,5 which is more severe in SS-DE patients,6,7 and typically topical anti-inflammatory medication has been used to treat SS-DE. A topical therapy for DE should aim to normalize the tear film through the routine use of artificial tears to protect the ocular surface and alleviate the discomfort caused by inflammation.8
Topical drugs used to treat ocular surface inflammation include cyclosporin A (CsA), corticosteroids, and nonsteroidal anti-inflammatory drugs. Previous studies of the use of topical CsA for DE patients with and without SS showed that CsA was effective for improving DE symptoms and the tear film stability.9–11 However, persistent burning after the application of ophthalmic solutions of CsA may reduce medication adherence. Topical corticosteroid therapies have been shown to improve the signs and symptoms of DE in clinical studies.12–14 However, these studies selected DE patients with different etiologies and studies that have compared the efficacies of topical corticosteroid and CsA treatments in SS-DE patients in China are limited. In this study, we performed a randomized, open, parallel-group analysis of topical applications of 0.1% sodium hyaluronate (HA) combined with 0.1% fluorometholone (FML) or 0.5% CsA for the treatment of DE in Chinese patients with SS.
PATIENTS AND METHODS
Patients
Between January 2013 and September 2013, 40 patients were recruited from the Eye, Ear, Nose, and Throat (EENT) Hospital of Fudan University, Shanghai, China, for participation in the study. We included patients aged ≥18 years who were diagnosed with primary or secondary SS, according to the criteria of the American–European Consensus Group.2 Diagnosis was based on a nonanesthetized Schirmer test result of ≤ 5 mm/min, a 1% fluorescein staining score of ≥3 out of 12, and the presence of at least one of the following autoantibodies in serum: antinuclear antibody, rheumatoid factor, anti-SS-A (Ro), or anti-SS-B (La). A diagnosis of DE required at least one of the following DE-related symptoms: dryness, foreign-body sensation, burning, asthenopia, redness, or discharge. Patients who had suffered an injury or infection to their eye had ocular inflammation unrelated to DE, had undergone ophthalmological surgery within the previous 6 months, had another uncontrolled illness, or were pregnant or lactating, were excluded from the study. Postmenopausal women receiving hormonal replacement therapy were also excluded. A total of 35 patients were included in our SS-DE study cohort.
Study Design
Our clinical trial used a randomized, open, parallel-group design (Figure 1). The study was registered with the ClinicalTrials.gov (Identifier: NCT02011776) on December 10, 2013, following the completion of the Chinese version of protocols. All ongoing and related trials have also been registered. Our study was conducted in compliance with the Declaration of Helsinki for research involving human participants and was approved by the Ethics Committee of the EENT Hospital of Fudan University. All of the participants provided written informed consent. The participants were randomly assigned to the FML or CsA group (n = 20 each) at a 1:1 ratio using the permuted block method. The participants were instructed to abstain from the use of topical ophthalmic medications for at least 2 weeks before the start date of the study and those who met the inclusion criteria at the end of the 2-week washout were selected to complete the 8-week treatment. The CsA group was treated with an ophthalmic solution containing 0.5% CsA (EENT Hospital) twice daily in both the eyes; 0.5 mL CsA was added to 4.5 mL 0.9% NaCl solution to make up 5 mL of solution. There was 5 mg CsA in 1 mL of this solution without antiseptic substances. The FML group was treated with an ophthalmic solution containing 0.1% FML (Santen Pharmaceutical, Osaka, Japan) 4 times per day in both the eyes. Both groups were also treated with an ophthalmic solution containing 0.1% HA (Santen Pharmaceutical) 4 times per day in both the eyes. Follow-up examinations were performed at 14 ± 2, 28 ± 2, and 56 ± 2 days after the first treatment. All of the patients underwent subjective symptom evaluation; slit-lamp microscopy examinations, including tear film breakup time (TFBUT); fluorescein staining of the cornea; conjunctival congestion evaluation; the Schirmer test without anesthesia; and a cytological examination. Visual acuity, intraocular pressure (IOP), and the fundus were also evaluated at each follow-up examination. Safety was evaluated based on the incidence of adverse events in each treatment group. Throughout the study period, the patients were asked to abstain from the concomitant use of any other ophthalmic drugs, topical corticosteroids, or lacrimal plugs.
FIGURE 1.
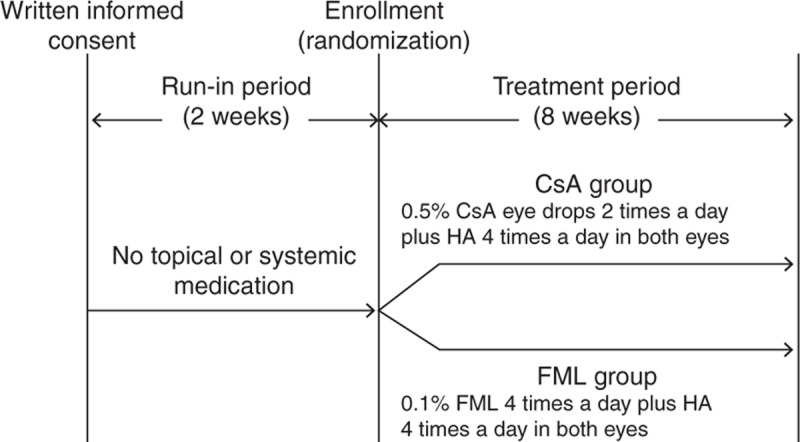
Study design. During the 2-week run-in period, the patients were instructed to abstain from the use of any topical ophthalmic medication. During the 8-week treatment period, both the groups were treated with an ophthalmic solution containing 0.1% HA 4 times per day. In addition, the CsA group was treated with an ophthalmic solution containing 0.5% CsA twice a day, and the FML group was treated with an ophthalmic solution containing 0.1% FML 4 times per day. Both the eyes of each patient were treated at all of the time points. CsA = cyclosporine, FML = fluorometholone, HA = sodium hyaluronate.
Fluorescence Staining
Differences between posttreatment and baseline corneal fluorescein staining (CFS) characteristics in each quarter of the corneal zone (Figure 2) were scored on a 4-point scale according to Macri et al,15 with slight modifications16 as follows: 0 = no staining, 1 = <50% staining, 2 = ≥50% staining, and 3 = 100% staining.
FIGURE 2.
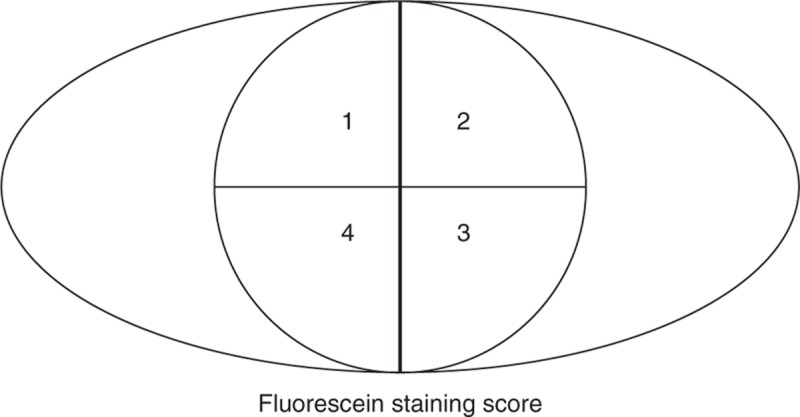
Fluorescein staining score. Fluorescein staining was graded in each quarter of the corneal zone based on the following 4-point scale: 0 (no staining), 1 (<50% staining), 2 (≥50% staining), and 3 (100% staining).
Subjective Symptom Assessment
Subjective symptoms were assessed at each examination using the Ocular Surface Disease Index (OSDI), which consisted of the bothersome symptoms, visual function, and environmental trigger subscales. The subjective symptoms were scored on a 5-point scale, with a score of 0 indicating least severe and a score of 4 indicating the most severe symptoms. A derived index score of ≤100 was calculated for each evaluation based on the total number of questions answered, as previously described.17
Conjunctival Examinations
Specimens were obtained for impression cytology from the eyes of all the patients, before treatment and at weeks 4 and 8, to determine the density of conjunctival goblet cells that tested positive in the periodic acid-Schiff (PAS) test. In the PAS test, Pierce nitrocellulose membranes (Thermo Fisher, Waltham, MA) were cut into 6-mm strips and pressed against the nasal bulbar conjunctiva for 10 seconds. The cells adhering to the membrane were fixed using a solution containing 37% formaldehyde, glacial acetic acid, and 70% ethanol (1:1:20), and were stained with PAS reagent. The number of PAS-positive conjunctival goblet cells present in 3 images of each specimen was recorded by 2 independent observers. The mean number of PAS-positive goblet cells/mm2 was compared between the FML and the CsA groups. Conjunctival congestion was also evaluated at each visit based on a 5-point scale as follows: 0 = absent, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe.
Tear Assessment
At each examination, patients underwent a Schirmer test without topical anesthesia to evaluate tear production and tear film stability. Sterile strips of filter paper (Jingming, Tianjing, China) were placed in the lateral canthus, away from the cornea, for 5 minutes, and the length of the strip that was moistened by the absorbed tears was recorded to evaluate tear production. Tear film stability was assessed based on the TFBUT. A fluorescein-impregnated strip (Jingming) made wet with saline solution without preservatives was placed in the lower conjunctival sac and the patient was asked to blink several times to ensure adequate mixing of the dye. The interval between the last complete blink and the appearance of the first black spot in the stained tear film on the cornea was defined as the TFBUT. The TFBUT was measured 3 times, and the mean TFBUT score was calculated.
Statistical Analysis
All of the statistical procedures were performed using SPSS version 18.0 statistical software (IBM, Armonk, NY). Normally distributed data are expressed as the mean ± standard deviation. Intergroup differences at various time points were examined using a Wilcoxon rank-sum test. Intragroup differences were evaluated using mixed models. The study eye was defined as the eye with the lowest baseline fluorescein-staining score. If both eyes had identical fluorescein-staining scores, the right eye was selected as the study eye. The results of comparisons, with P < 0.05, were considered to represent a statistically significant difference.
RESULTS
Patient Characteristics
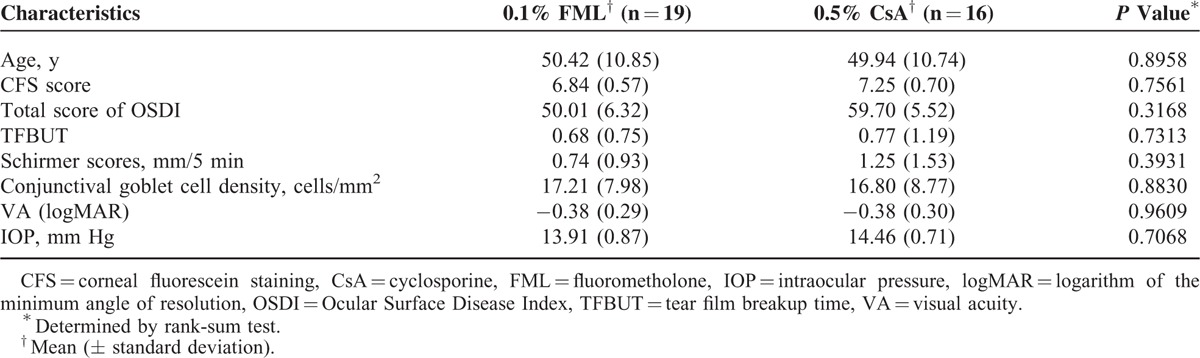
No significant differences in the baseline demographic and ocular characteristics were observed between the FML and the CsA groups (Table 1). Nineteen patients in the FML group and 16 patients in the CsA group completed the follow-up assessments because 1 and 4 patients, respectively, were lost to the follow-up.
TABLE 1.
Patient Baseline Characteristics
Corneal Fluorescein Staining
After 8 weeks of therapy, the mean CFS score in the FML group was reduced by 2.11 ± 1.56 (P < 0.001), compared with the baseline score, whereas the mean CFS score in the CsA group was reduced by 2.50 ± 1.71 (P < 0.001), compared with the baseline score (Figure 3). At week 2 (P = 0.042), the mean CFS score was significantly lower than that of the CsA group, but no significant intergroup difference in the CFS score was observed at week 4 or 8, indicating that the beneficial effect of the FML treatment was manifested more quickly at the cornea, compared with that of the CsA treatment.
FIGURE 3.
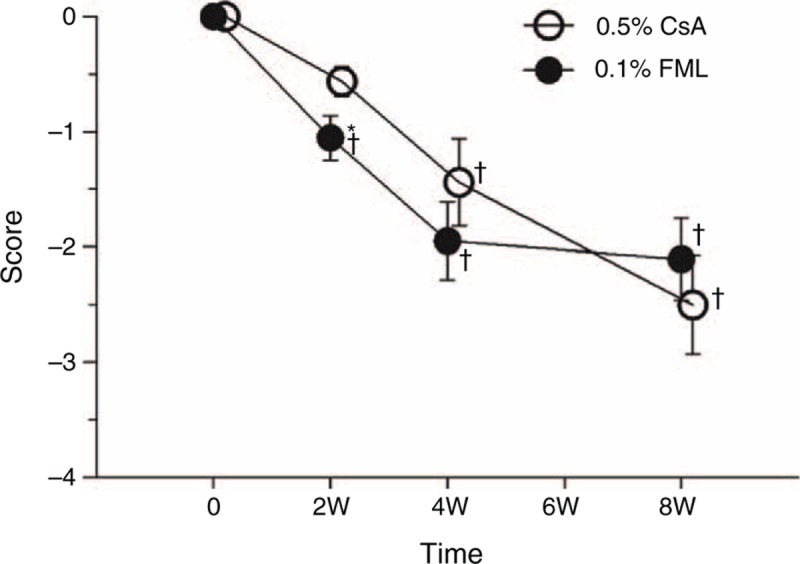
Changes of fluorescein staining. +Significantly different from the baseline value (FML group: P < 0.001 at weeks 2, 4, and 8; CsA group: P = 0.136 at week 2, P < 0.001 at week 4, and P < 0.001 at week 8). ∗Significant intergroup difference (P = 0.042 at week 2). CsA = cyclosporine, FML = fluorometholone, W = week.
Subjective Symptoms Analysis
The mean OSDI score in the FML group decreased significantly across all of the time points, compared with the baseline value (P < 0.001 for all) (Figure 4). However, the mean OSDI score in the FML group at week 4 did not differ significantly from that at week 8 (P = 0.573). The mean OSDI score in the CsA group was significantly lower at week 2 compared with the baseline value (P = 0.007). The mean OSDI score in the CsA group at week 8 was significantly lower that at week 4 (P < 0.001), whereas the mean OSDI score for the CsA group at week 2 did not differ significantly for that at week 4 (P = 0.164). At week 4, the mean OSDI score in the FML group was significantly lower than the CsA group (P = 0.042) (Figure 4). However, no significant difference in the mean OSDI score was observed between the 2 groups at weeks 2 and 8 (P = 0.116 and 0.456, respectively).
FIGURE 4.
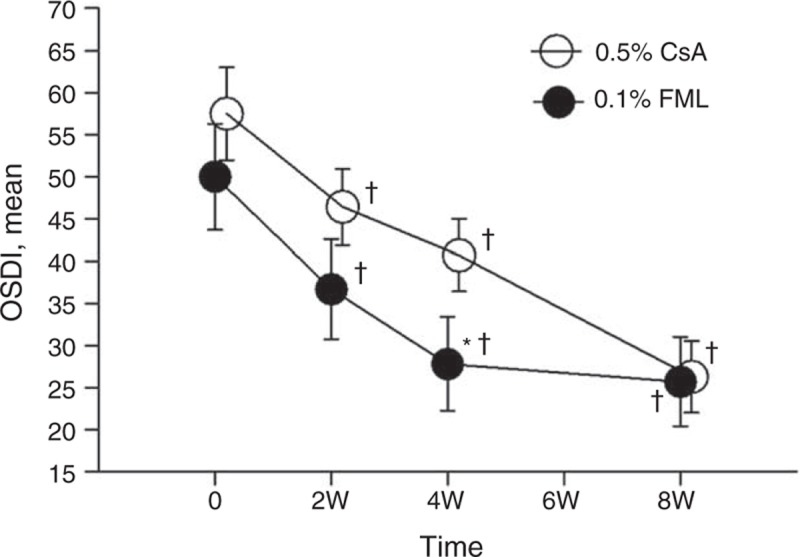
The mean OSDI during 8 weeks follow-up. +Significantly different from the baseline value (FML group: P < 0.001 at weeks 2, 4, and 8; CsA group: P = 0.007 at week 2, P < 0.001 at weeks 4 and 8). ∗Significant intergroup difference (P = 0.042 at week 4). CsA = cyclosporine, FML = fluorometholone, OSDI = Ocular Surface Disease Index, W = week.
Conjunctival Analyses
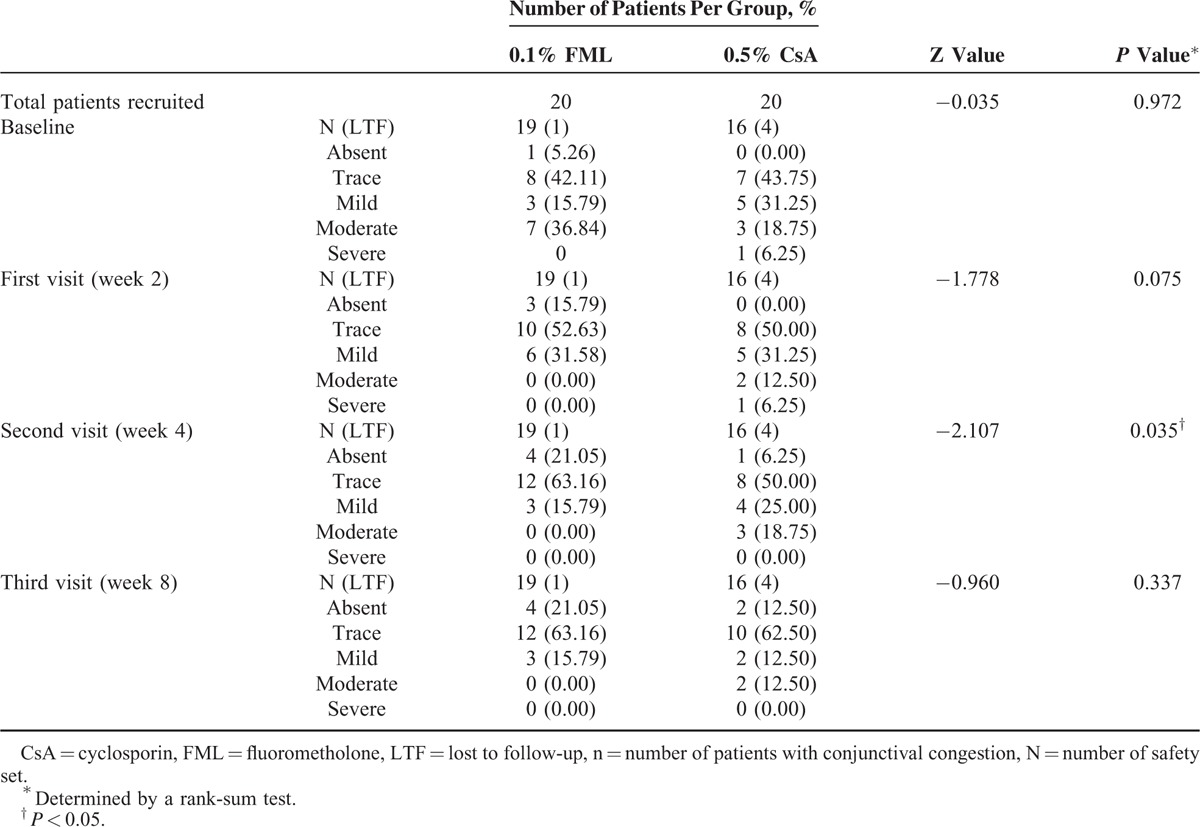
The cytology examinations revealed that a significantly higher number of PAS-positive cells were present in both the treatment groups at weeks 4 and 8, compared with the baseline values (Figure 5A). After 8 weeks of treatment, the conjunctival goblet cell density was also significantly higher in both the groups, increasing by 41.00 ± 37.79 cells/mm2 in the FML group (P < 0.001) and 39.4 ± 34 cells/mm2 in the CsA group (P < 0.001), compared with the baseline values (Figure 5B). However, no significant intergroup difference in conjunctival goblet cell density was observed at week 4 (P = 0.653) or week 8 (P = 0.934). The severity of conjunctival congestion was reduced in both the groups throughout the study period, compared with that at baseline (Table 2). Although no significant intergroup difference in the severity of conjunctival congestion was observed at weeks 2 and 8, the conjunctival congestion was significantly less severe in the FML group at week 4 (P = 0.035), compared with that in the CsA group, indicating that the beneficial effect of the FML treatment was manifested more quickly at the conjunctiva than that of the CsA treatment.
FIGURE 5.
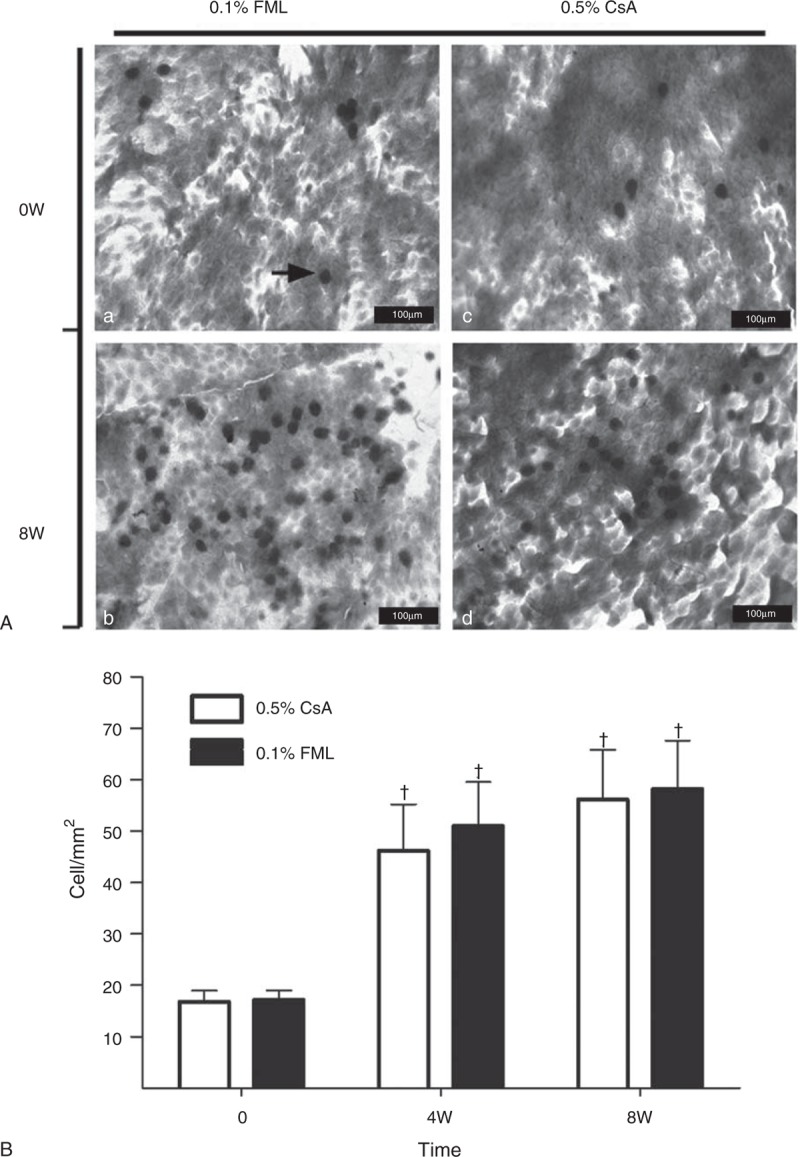
Number of conjunctival goblet cells in the CsA and FML patients at baseline and after 8 weeks of treatment. (A) Conjunctival goblet cells were stained with periodic acid-Schiff (PAS) stain. The arrow indicates a PAS-positive cell (bar = 100 μm). a: Conjunctival goblet cells in the FML group before treatment. b: Conjunctival goblet cells after 8 weeks of FML treatment. c: Conjunctival goblet cells in the CsA group before treatment. d: Conjunctival goblet cells after 8 weeks of CsA treatment. (B) Density of PAS-positive conjunctival goblet cells. +Significantly different from the baseline value (FML group: P < 0.001 at weeks 4 and 8; CsA group: P < 0.001 at weeks 4 and 8). No significant intergroup difference was observed at any time point (P = 0.653 at week 4; P = 0.934 at week 8). CsA = cyclosporine, FML = fluorometholone, W = week.
TABLE 2.
Summary of Assessment of the Severity of Conjunctival Congestion
Tear Analyses
No significant change in the mean Schirmer test score was observed in either group (data not shown). However, the mean TFBUT in both the groups was longer at week 8, compared with the baseline value for each group. In addition, at week 8, the mean TFBUT in the FML group was significantly longer than that in the CsA group (P = 0.04) (Figure 6).
FIGURE 6.
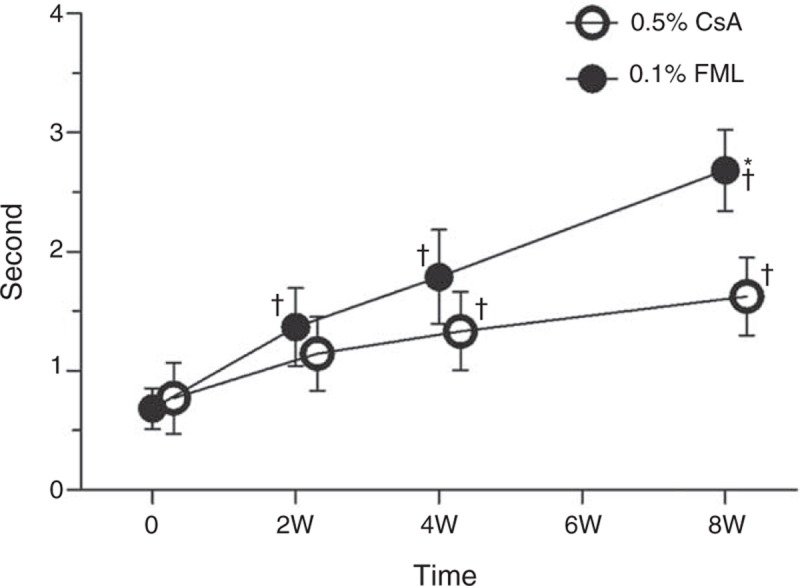
Tear film breakup time (TFBUT). +Significantly different from the baseline value (FML group: P = 0.005 at week 2, P < 0.001 at weeks 4 and 8; CsA group: P = 0.01 at week 2, P = 0.034 at week 4, and P = 0.001 at week 8). ∗Significant intergroup difference (P = 0.040 at week 8). CsA = cyclosporine, FML = fluorometholone, W = week.
Adverse Events and Tolerability
All of the 35 patients who completed the study displayed good compliance. No serious or severe adverse effects occurred. At week 8, the mean IOP had increased by 0.40 mm Hg in the FML group and decreased by 1.15 mm Hg in the CsA group (P = 0.389). Neither treatment resulted in an increase in IOP of >24 mm Hg during the 8-week treatment period, compared with the baseline values (Figure 7). To assess tolerability, participants were questioned regarding side effects associated with FML and CsA treatments. A moderate-to-severe transient burning sensation (grade 2 or 3) upon instillation of the CsA solution was reported by 31.25% (5/16), 12.50% (2/16), and 12.50% (2/16) of the patients in the CsA group at weeks 2, 4, and 8, respectively. No such side effects were reported in the FML group.
FIGURE 7.
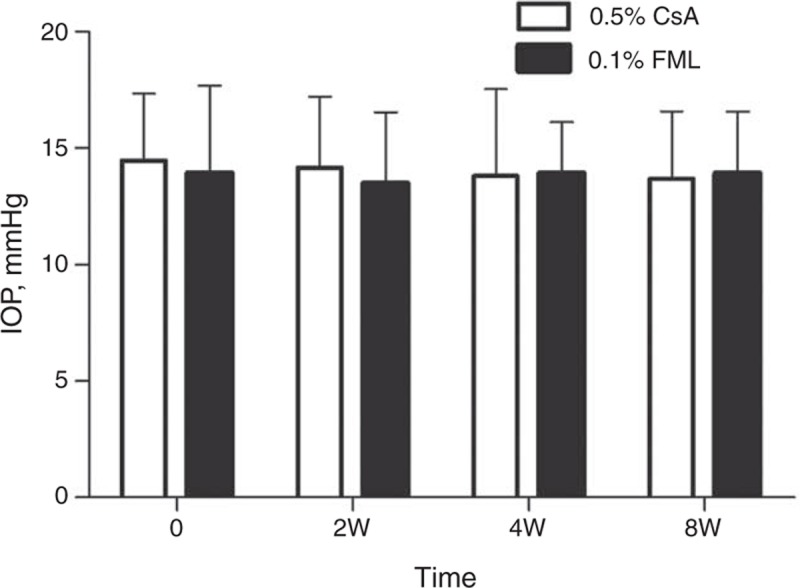
Intraocular pressure (IOP). The intraocular pressure had increased by 0.4 mm Hg in the FML group and decreased by 1.15 mm Hg in the CsA group at week 8 (P = 0.389). CsA = cyclosporine, FML = fluorometholone, W = week.
DISCUSSION
The significantly different values of fluorescence staining at week 2, OSDI and congestion at week 4, as well as TFBUT at week 8 showed that the beneficial effects of FML manifested faster than those of CsA. We propose that SS-DE patients should initially be treated with FML to rapidly control inflammation and that CsA should be used afterward as a consolidation therapy.
Topical corticosteroids have been successfully used to treat SS-DE, with patients reporting symptomatic relief months after treatment had been stopped.18 The effect of corticosteroids is attributed to reduced levels of cytokines, such as interleukin (IL)-1 and IL-8,19,20 and the reduced activity of matrix metalloproteinases (MMPs).21 CsA inhibits cytokine (primarily IL-6) production in T cells that exacerbates the inflammation associated with DE.22,23 However, CsA does not affect all of the inflammatory components of DE.24 The Management and Therapy Subcommittee of the International Dry Eye Workshop25 has made recommendations for treating DE based on a modification of the International Task Force (ITF) severity grading scheme.26 Anti-inflammatory therapy is recommended when the severity of DE reaches level 2 or higher on the ITF scale.25 Topical corticosteroids, which exert rapid and intense anti-inflammatory effects, are potentially beneficial for SS-DE treatments, which is consistent with the results of this study. We did not stratify our analysis based on sex because all of the 35 patients who completed the study were women. This finding reflects the sex-biased incidence of SS, which exhibits a female-to-male ratio of 9:1 and mainly affects middle-aged women.27
Studies of SS using fluorescein staining and impression cytology have shown that both basic and reflex tearing are diminished as a result of damage to the lacrimal gland caused by infiltrating lymphocytes, which is considered a clinical hallmark of SS-DE pathology.6,7 In this study, the fluorescein staining scores were significantly higher in the FML group at week 2 than that in the CsA group. The severity of conjunctival congestion was reduced in both the groups over the course of treatment relative to that in each group at baseline, indicating that inflammation was reduced at the ocular surface. However, the percentage of patients with obvious conjunctival congestion in the FML at week 4 was significantly lower than that in the CsA group. In addition, the FML group exhibited a faster improvement in subjective symptoms (Figure 4). Although the total OSDI scores were significantly lower in both the groups at all of the time points, compared with the baseline values for each group, the mean OSDI score in the FML group at week 4 was significantly lower than that of the CsA group.
The release of inflammatory cytokines and the activation of MMPs can cause apoptosis in various types of surface epithelial cells, including corneal epithelial cells and conjunctival goblet cells.28 Therefore, the diminished function of the corneal epithelial barrier and the loss of conjunctival goblet cells in SS-DE might be directly related to the effects of chronic inflammation.29,30 Although SS is an autoimmune exocrinopathy, previous studies have shown that local anti-inflammatory therapies significantly improved corneal epithelial barrier function and conjunctival goblet cell density,31–34 which is consistent with the findings in this study, as evidenced by the significantly higher number of PAS-positive conjunctival goblet cells in both the groups at weeks 4 and 8 (Figure 5A and B).
The mean Schirmer test scores did not change significantly in either group in our study. Long-term chronic inflammation of the lacrimal gland can cause impaired tear production and apoptosis of the acinar and ductal cells,35 which leads to hypolacrimation, especially in SS-DE patients. Although other investigators noted that topical anti-inflammatory treatments including CsA and corticosteroids can increase the Schirmer score in patients with moderate–severe dry eyes, patients with SS in our study might have insufficient residual functional capacity in the lacrimal glands to increase their tear production in response to anti-inflammatory treatments. This hypothesis is reflected in the baseline Schirmer scores of 0 for both the eyes in 50% of the patients at initial presentation and baseline Schirmer scores of 0.74 in the FML and 1.25 in the CsA group (Table 1). Furthermore, the studies from Jain et al36 and Hyon et al37 did not find evidence that anti-inflammatory treatments improved tear production in SS patients, which is in accordance with the findings in our study.
In this study, TFBUT increased in both the FML and the CsA groups compared with the baseline values, and the TFBUT in the FML group was significantly longer at week 8 than that of the CsA group. Our findings collectively indicate that although the efficacies of topical FML and CsA were approximately equivalent for the treatment of SS-DE at week 8, the FML treatment provided beneficial effects more rapidly than CsA treatment.
Systemic immunosuppressive or anti-inflammatory agents may affect clinical ocular parameters in SS. However, no serious or severe adverse events occurred in our SS-DE cohort and no clinically problematic changes were detected in clinical laboratory examinations. The inability to eliminate corticosteroid-induced ocular hypertension has restricted the use of topical corticosteroids in ophthalmic practice. No significant increase in IOP was observed during the 8-week treatment period in this study. Thus, the topical FML regimen used in our study demonstrated a high level of safety. Compared with methylprednisolone and dexamethasone, FML is a relatively weak corticosteroid; previous studies have shown that FML-related increases in IOP occur less frequently, compared to that observed with other corticosteroids.38 Nevertheless, the long-term ophthalmic use of FML in SS-DE patients should be monitored closely to avoid ocular hypertension, cataract development, and opportunistic infections. Given these risks, we propose that SS-DE patients can be treated using topical corticosteroids to provide rapid improvement in symptoms during the first 4 weeks of treatment, followed by a topical CsA treatment to maintain clinical improvements with a lower risk of adverse events.
The limitations of this study include the lack of an additional rose bengal or lissamine green conjunctival dying to the CFS, the small sample size, and the open-label design in which both the investigator and the patients were aware of the treatment. Future large, population-based studies of the efficacy of topical 0.1% FML for the treatment of SS-DE are warranted to confirm our findings.
CONCLUSIONS
Topical 0.1% FML is safe and efficacious for the ophthalmic treatment of SS-DE patients. Although treatments using ophthalmic solutions of 0.5% CsA or 0.1% FML provide comparable improvements in SS-DE-related symptoms during an 8-week period, the beneficial effects of topical FML are manifested significantly faster than those of CsA.
Acknowledgments
The authors would like to thank Naiqing Zhao, Department of Biostatistics, School of Public Health, Fudan University, Shanghai, China, for assistance with the statistical analyses in the study.
Footnotes
Abbreviations: CFS = corneal fluorescein staining, CsA = cyclosporin A, DE = dry eye, FML = fluorometholone, HA = sodium hyaluronate, IOP = intraocular pressure, MMPs = matrix metalloproteinases, OSDI = Ocular Surface Disease Index, PAS = periodic acid-Schiff, SS = Sjögren syndrome, TFBUT = tear film breakup time.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Bjerrum KB. Keratoconjunctivitis sicca and primary Sjogren's syndrome in a Danish population aged 30–60 years. Acta Ophthalmol Scand 1997; 75:281–286. [DOI] [PubMed] [Google Scholar]
- 2.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones DT, Monroy D, Ji Z, et al. Sjogren's syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci 1994; 35:3493–3504. [PubMed] [Google Scholar]
- 4.Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol 2009; 2:375–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng X, de Paiva CS, Li DQ, et al. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci 2010; 51:3083–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 1998; 17:38–56. [DOI] [PubMed] [Google Scholar]
- 7.Rivas L, Murube J, Shalaby O, et al. Impression cytology contribution to differential diagnosis of Sjogren syndrome in the ophthalmological clinic [in Spanish]. Arch Soc Espanola Oftalmol 2002; 77:63–72. [PubMed] [Google Scholar]
- 8.Tincani A, Andreoli L, Cavazzana I, et al. Novel aspects of Sjogren's syndrome in 2012. BMC Med 2013; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry HD, Solomon R, Donnenfeld ED, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol 2008; 126:1046–1050. [DOI] [PubMed] [Google Scholar]
- 10.Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000; 107:631–639. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Ophthalmology 2000; 107:967–974. [DOI] [PubMed] [Google Scholar]
- 12.Sainz De La Maza Serra M, Simon Castellvi C, Kabbani O. Nonpreserved topical steroids and lacrimal punctal occlusion for severe keratoconjunctivitis sicca [in Spanish]. Arch Soc Espanola Oftalmol 2000; 75:751–756. [PubMed] [Google Scholar]
- 13.Avunduk AM, Avunduk MC, Varnell ED, et al. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol 2003; 136:593–602. [DOI] [PubMed] [Google Scholar]
- 14.Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol 2004; 138:444–457. [DOI] [PubMed] [Google Scholar]
- 15.Macri A, Rolando M, Pflugfelder S. A standardized visual scale for evaluation of tear fluorescein clearance. Ophthalmology 2000; 107:1338–1343. [DOI] [PubMed] [Google Scholar]
- 16.Qiu X, Gong L, Sun X, et al. Age-related variations of human tear meniscus and diagnosis of dry eye with Fourier-domain anterior segment optical coherence tomography. Cornea 2011; 30:543–549. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000; 118:615–621. [DOI] [PubMed] [Google Scholar]
- 18.Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology 1999; 106:811–816. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Min K, Kim SK, et al. Inflammatory cytokine and osmolarity changes in the tears of dry eye patients treated with topical 1% methylprednisolone. Yonsei Med J 2014; 55:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon A, Rosenblatt M, Li DQ, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest Ophthalmol Vis Sci 2000; 41:2544–2557. [PubMed] [Google Scholar]
- 21.Dursun D, Kim MC, Solomon A, et al. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol 2001; 132:8–13. [DOI] [PubMed] [Google Scholar]
- 22.2000; Turner K, Pflugfelder SC, Ji Z, et al. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. 19:492–496. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today 1992; 13:136–142. [DOI] [PubMed] [Google Scholar]
- 24.Djalilian AR, Nagineni CN, Mahesh SP, et al. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea 2006; 25:709–714. [DOI] [PubMed] [Google Scholar]
- 25.Pflugfelder SC. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Ocular Surface 2007; 5:163–178. [DOI] [PubMed] [Google Scholar]
- 26.Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea 2006; 25:900–907. [DOI] [PubMed] [Google Scholar]
- 27.Mavragani CP, Moutsopoulos HM. The geoepidemiology of Sjogren's syndrome. Autoimmun Rev 2010; 9:A305–A310. [DOI] [PubMed] [Google Scholar]
- 28.Yeh S, Song XJ, Farley W, et al. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci 2003; 44:124–129. [DOI] [PubMed] [Google Scholar]
- 29.Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci 2000; 41:1356–1363. [PubMed] [Google Scholar]
- 30.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol 2002; 120:330–337. [DOI] [PubMed] [Google Scholar]
- 31.De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci 2006; 47:2847–2856. [DOI] [PubMed] [Google Scholar]
- 32.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res 2006; 83:526–535. [DOI] [PubMed] [Google Scholar]
- 33.Ecoiffier T, El Annan J, Rashid S, et al. Modulation of integrin alpha4beta1 (VLA-4) in dry eye disease. Arch Ophthalmol 2008; 126:1695–1699. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Chen W, De Paiva CS, et al. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci 2011; 52:6279–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peri Y, Agmon-Levin N, Theodor E, et al. Sjogren's syndrome, the old and the new. Best Pract Res Clin Rheumatol 2012; 26:105–117. [DOI] [PubMed] [Google Scholar]
- 36.Jain AK, Sukhija J, Dwedi S, et al. Effect of topical cyclosporine on tear functions in tear-deficient dry eyes. Ann Ophthalmol 2007; 39:19–25. [DOI] [PubMed] [Google Scholar]
- 37.Hyon JY, Lee YJ, Yun PY. Management of ocular surface inflammation in Sjogren syndrome. Cornea 2007; 26 suppl 1:S13–S15. [DOI] [PubMed] [Google Scholar]
- 38.Akingbehin AO. Comparative study of the intraocular pressure effects of fluorometholone 0.1% versus dexamethasone 0.1%. Br J Ophthalmol 1983; 67:661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]


