Abstract
Administering diazepam intravenously or rectally in an adult with status epilepticus can be difficult and time consuming. The aim of this study was to examine whether intranasal diazepam is an effective alternative to intravenous diazepam when treating status epilepticus.
We undertook a retrospective cohort study based on the medical records of 19 stroke patients presenting with status epilepticus to our institution. We measured the time between arrival at the hospital, the intravenous or intranasal administration of diazepam, and the seizure termination.
Intranasal diazepam was administered about 9 times faster than intravenous diazepam (1 vs 9.5 minutes, P = 0.001), resulting in about 3-fold reduction in the time to termination of seizure activity after arrival at the hospital (3 minutes compared with 9.5 minutes in the intravenous group, P = 0.030). No adverse effects of intranasal diazepam were evident from the medical records.
Intranasal diazepam administration is safer, easier, and quicker than intravenous administration.
INTRODUCTION
Status epilepticus is defined as continuous or intermittent seizure activity for a duration of at least 30 minutes. It is recommended that medical interventions be made within 5 minutes,1 as status epilepticus may result in profound, permanent, complex, and widespread brain damage, leading to cognitive and behavioral deficits.2 Thus, it is essential to begin anticonvulsant treatment and terminate status epilepticus at the earliest.3
Intravenous benzodiazepines, phenytoin, and barbiturates are commonly used to treat status epilepticus, and benzodiazepines are preferred for acute management of all types of seizures.4 Benzodiazepines may also be administered rectally, orally, or intranasally to avoid treatment delays caused by difficulty in establishing an intravenous access. The rectal route has some advantages,5 but may still be challenging in an adult having a tonic-clonic seizure, and may occasionally result in allegations of sexual assault.6
The therapeutic benefits of buccal, intranasal, and intramuscular midazolam in adults have recently been examined,7,8 but there have been no reports regarding the effectiveness of intranasal diazepam for status epilepticus. We compared the time taken for seizure termination when using intranasal and intravenous diazepam in adults with status epilepticus.
MATERIALS AND METHODS
Study Design and Participants
We performed a longitudinal, retrospective cohort study based on the records of all consecutive emergency attendances at the University of Tokyo Hospital, Tokyo, Japan, between December 2007 and December 2012 of patients diagnosed with status epilepticus. Approval for the study was obtained from the Institutional Review Board at the University of Tokyo.
Study Setting
In Japan, emergency patients are transferred to a primary, secondary, or tertiary medical center according to the severity of their physical symptoms.9,10 As a tertiary center, our institution is the preferred destination for patients with persistent seizures. In Japan, paramedics are not permitted to administer anticonvulsants. Patients who had seizures for >30 minutes by the time of arrival at the emergency department and in whom it was anticipated that establishing intravenous access would be difficult were first administered intranasal midazolam. Occasionally, intranasal diazepam was inevitably administered at the emergency physician's discretion if time would have been lost preparing the midazolam, which was stored in a locked cupboard in our institution during the study period.
Eligibility Criteria
We analyzed the data from patients over 18 years of age, who attended our emergency department with seizures lasting >30 minutes, with a history of previous stroke, and a final diagnosis of epilepsy made by a neurologist and confirmed by electroencephalography during their admission. Exclusion criteria were incomplete patient record, seizures controlled by intranasal midazolam, and seizures occurring due to tumor, trauma, drug overdose, infection (eg, meningitis or other sepsis), arrhythmia, or alcohol withdrawal.
Study Drugs
The diazepam used in this study was commercially available parenteral diazepam (10 mg/2 mL, Takeda Pharmaceutical Company, Osaka, Japan). For intranasal administration, 10 mg diazepam was drawn up into a syringe and expelled directly into the nose. All patients were administered phenytoin after seizure termination.
Data Collection
The primary outcome measure was the time between arrival at the emergency department and termination of the seizure. We also recorded the time from diazepam administration to seizure termination, time from arrival at the hospital to medical intervention (placement of an intravenous catheter, administration of diazepam, and administration of phenytoin), and the total dose of diazepam delivered by each route. The following additional data were retrieved from the medical records for each patient: age, sex, details of epilepsy history, and prior chronic antiepileptic drug use.
Safety Evaluation
Intranasal diazepam is reported to be safe and effective when given as an anesthetic agent in healthy volunteers,11 but nonetheless, we noted adverse events recorded in the medical records, including arrhythmia, hemodynamic or respiratory compromise, nasal pain or hemorrhage, and nausea.
Statistical Analysis
Continuous variables are presented as medians with the interquartile range (IQR) and compared using the Wilcoxon–Mann–Whitney test. For categorical variables, the proportions of patients in each category were calculated and groups were compared using Pearson χ2 test. All analyses were undertaken using STATA software (version 13, Stata, College Station, TX). Statistical significance was defined as a 2-tailed P value < 0.05.
RESULTS
Participants
During the study period, 129 patients were diagnosed with status epilepticus. Fifty-seven were excluded as seizure activity had terminated spontaneously by the time of arrival, 20 as their records did not indicate the time of seizure termination, 20 as their records did not indicate the time of drug administration, 10 as they were treated successfully by intranasal midazolam, 1 due to cardiopulmonary arrest on arrival, 1 subsequently diagnosed with septic shock, and 1 whose seizure stopped spontaneously in the emergency department. The data of the remaining 19 patients were analyzed, of whom 9 received intranasal diazepam, and 10 received only intravenous diazepam.
Patient Characteristics
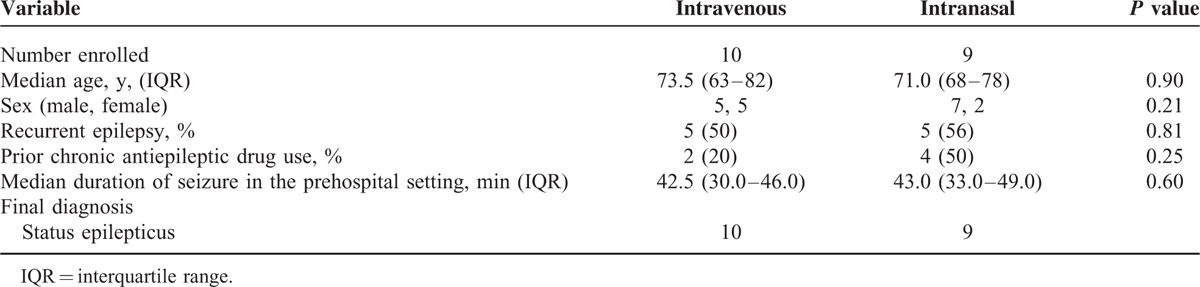
There were no differences in the baseline or clinical characteristics of patients treated with intravenous or intranasal diazepam (Table 1). Generally, patients presented with seizure episodes of 30 to 49 minutes, and were likely to have been diagnosed with recurrent epilepsy.
TABLE 1.
Patients’ Baseline Characteristics
Main Results
Table 2 presents the results of the treatments for status epilepticus. All patients responded to the initial treatment, and were administrated phenytoin after the seizure had terminated. Two patients required intravenous diazepam to control seizure activity after they had been administered intranasal diazepam.
TABLE 2.
Primary and Secondary Outcome Measures Taken at the Emergency Department
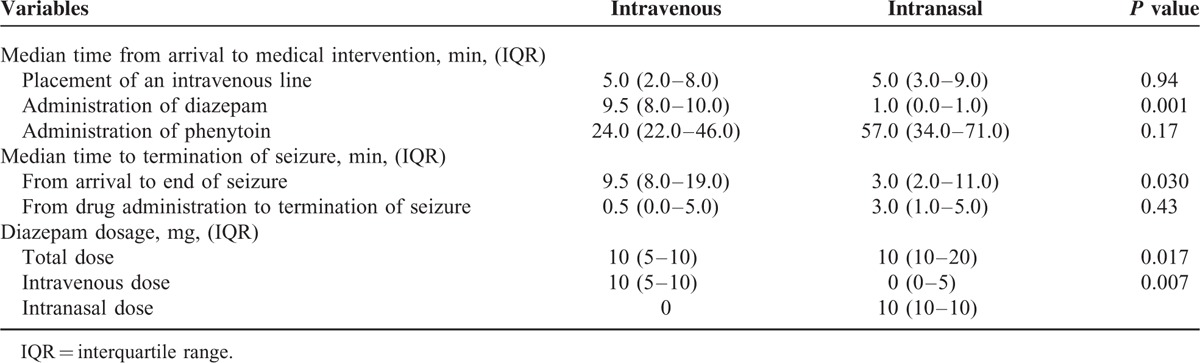
The delivery of intranasal diazepam was about 9 times faster than intravenous diazepam (P = 0.001). Additionally, termination of the seizure was significantly faster compared with the intravenous group, from the time of arrival at hospital (9.5 minutes in the intranasal group vs 3.0 minutes in the intravenous group, P = 0.003). Although the time from drug administration to seizure termination was 3 minutes in the intranasal diazepam group compared with 0.5 minute in the intravenous group, the difference was also not statistically significant (P = 0.43).
Safety Evaluation of Intranasal Diazepam
There were no incidents of respiratory or hemodynamic compromise, death within 72 hours, or other adverse events and side effects.
DISCUSSION
The rapid treatment of epileptic seizures remains challenging, as tonic-clonic convulsions impede health care professionals from administering drugs intravenously or rectally. We showed that the time from patient arrival to seizure termination was 3 times shorter in those who received intranasal diazepam, which was administered 9 times faster than the intravenous route. To the best of our knowledge, this is the first study to have compared the efficacy of intranasal and intravenous diazepam in treating patients with stroke.
The intranasal delivery of diazepam was faster than intravenously administration, likely owing to the difficulty of immobilizing the patient for the placement of a peripheral intravenous catheter. This consequently led to a more rapid seizure termination; after administration, the time to seizure control was comparable for the 2 delivery routes. Since the same dose was administered intranasally and intravenously, these data suggest equivalent bioavailability.12 Thus, intranasal diazepam is easy to administer, and may have similar advantages over the rectal route.
Two mechanisms support the effectiveness of intranasal diazepam. First, the nasal mucosa provides a large, highly vascular absorptive surface adjacent to the brain. This vascular plexus and the adjacent olfactory mucosa provide direct routes for benzodiazepine absorption into the blood and the cerebrospinal fluid. Studies conducted with healthy subjects showed that plasma diazepam concentrations increase rapidly after intranasal administration.11 Second, the drug may also be rapidly absorbed directly into the cerebrospinal fluid and into the brain via the olfactory route.13
No adverse events were reported when diazepam was given intranasally in our study, concurring with the finding that intranasal diazepam caused no clinically significant changes in the vital signs or electrocardiograms of healthy volunteers.12 Furthermore, the risk of needle stick injury to health care professionals would likely be reduced, and subsequent intravenous access and drug administration would be achieved more quickly.
Our study had some limitations. First, the termination of the seizure was diagnosed clinically rather than by electroencephalography. However, this reflects real-world clinical practice, and establishing electroencephalographic monitoring, such as obtaining intravenous access or undertaking endotracheal intubation, would have delayed other treatments. Second, our cohort comprised of elderly people with previous stroke; therefore, the effect of intranasal diazepam in other groups of patients with epilepsy, including idiopathic epilepsy, will require further studies in larger clinical trials. Third, this study was retrospective and not blinded, so there may have been sampling bias.
CONCLUSION
It is substantially faster to administer diazepam intranasally to patients with status epilepticus than intravenously, leading to faster seizure termination. Intranasal diazepam appears to be a safe and effective means of terminating status epilepticus that could be employed by health care providers outside hospitals.
Acknowledgment
We would like to Thank Mrs Takako Sakamaki for her assistance.
Footnotes
Abbreviation: IQR = interquartile range.
RI and NO-F contributed equally to this work.
NO and MG collected the data. RI and NO-F drafted the initial manuscript. KN, YK, and SN contributed to writing the manuscript. RI performed the statistical analyses. YW and NY critically reviewed the manuscript. All authors have provided written consent for publication.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Nair PP, Kalita J, Misra UK. Status epilepticus: why, what, and how. J Postgrad Med 2011; 57:242–252. [DOI] [PubMed] [Google Scholar]
- 2.de Araujo Furtado M, Rossetti F, Chanda S, et al. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology 2012; 33:1476–1490. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch LJ, Donner EJ, So EL, et al. Abbreviated report of the NIH/NINDS workshop on sudden unexpected death in epilepsy. Neurology 2011; 76:1932–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shorvon S. The historical evolution of, and the paradigms shifts in, the therapy of convulsive status epilepticus over the past 150 years. Epilepsia 2013; 54:64–67. [DOI] [PubMed] [Google Scholar]
- 5.Riss J, Cloyd J, Gates J, et al. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand 2008; 118:69–86. [DOI] [PubMed] [Google Scholar]
- 6.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet 1999; 353:623–626. [DOI] [PubMed] [Google Scholar]
- 7.Humphries LK, Eiland LS. Treatment of acute seizures: is intranasal midazolam a viable option? J Pediatr Pharmacol Ther 2013; 18:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad S, Ellis JC, Kamwendo H, et al. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet 2006; 367:1591–1597. [DOI] [PubMed] [Google Scholar]
- 9.Inokuchi R, Sato H, Nakajima S, et al. Development of information systems and clinical decision support systems for emergency departments: a long road ahead for Japan. Emerg Med J 2013; 30:914–917. [DOI] [PubMed] [Google Scholar]
- 10.Inokuchi R, Sato H, Nakamura K, et al. Motivations and barriers to implementing electronic health records and ED information systems in Japan. Am J Emerg Med 2014; 32:725–730. [DOI] [PubMed] [Google Scholar]
- 11.Ivaturi VD, Riss JR, Kriel RL, et al. Pharmacokinetics and tolerability of intranasal diazepam and midazolam in healthy adult volunteers. Acta Neurol Scand 2009; 120:353–357. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal SK, Kriel RL, Brundage RC, et al. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res 2013; 105:362–367. [DOI] [PubMed] [Google Scholar]
- 13.Wermeling DP. Intranasal delivery of antiepileptic medications for treatment of seizures. Neurotherapeutics 2009; 6:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]


