Supplemental Digital Content is available in the text
Abstract
We investigated the impact of heart failure (HF) etiology on the outcome of cardiac rehabilitation (CR) assessed by functional and clinical parameters.
Treatment of chronic HF requires multidisciplinary approaches with a recognized role for CR. INCARD is a French study aimed at evaluating the benefits of sustainable CR in coronary (C) and noncoronary patients (NC) treated and educated during a 24-month period of follow-up.
Prospective, monocentric patients with HF underwent inpatient physical training followed by a home-based program. Evaluations were performed at inclusion, discharge, 3 months after discharge, and subsequently every 6 months over the 24 months of outpatient rehabilitation.
A total of 147 HF patients with left ventricular ejection fraction (LVEF) <40 were admitted to the CR center, 63 accepted to join INCARD (29 C and 34 NC).
Although the C participants C having both an echocardiographic LVEF and an initially lower peak VO2, inpatient rehabilitation improved all functional parameters. Only NC showed an improved LVEF during the first 3 months of outpatient-follow-up. The main outcome of the outpatient rehabilitation was a trend toward stabilization of clinical and laboratory parameters with no significant difference between C and NC.
This study confirms the benefits of initial HF inpatient rehabilitation and encourages prolonged outpatient monitoring. The results on functional parameters suggest exercise training should be conducted regardless of the HF etiology.
INTRODUCTION
In 2013, heart disease was still the leading cause of death in the United States and according to the World Health Organization, cardiovascular disease is the number one cause of death globally.1 Chronic heart failure (HF) is a complex syndrome, clinically characterized by signs and symptoms secondary to abnormal cardiac function.2 HF can result from a variety of diseases and conditions that impair or overload the heart, notably heart attack, high blood pressure, a damaged heart valve, or cardiomyopathy. HF is a major public health issue with a current prevalence of over 5.8 million in the United States and over 23 million worldwide.3 From a community study in Worchester, MA, the 5-year mortality was >75% after the first hospitalization for HF.4 Treatment of HF requires a multidisciplinary approach.2 Cardiac rehabilitation (CR) has a recognized role in improving patients’ functional status and decreasing morbidity and mortality, which in turn leads to decreased rehospitalization rates and reduced overall costs of management of the disease.5,6
Physical exercise, a core component of CR, has beneficial effects on muscle fiber and mitochondrial apparatus,7–9 on the neuroendocrine system (in terms of decreasing sympathetic activity and renin angiotensin aldosterone activity10), and on cardiac function due to anti-remodeling8 and improvement of heart perfusion.11
Supervision of secondary prevention of patients with HF can be done through a coordinated network of primary and secondary care such as RESICARD12 or in specialized centers for HF monitoring.13 It was found that after several years of treatment in rehabilitation centers, participants had disparate improvements in functional status.10
Ischemia cardiomyopathy, the most common cause of HF, leads to regional changes in left ventricular wall motion, whereas nonischemic cardiomyopathy leads to large morphological changes, with dilated cardiomyopathy as the most common form.2 Therefore, benefits of CR were evaluated in coronary (C) and noncoronary (NC) patients. The INCARD study (French acronym for “INsuffisance CArdiaque en Réadaptation Durable”) was developed to evaluate the impact of etiology on the benefits of sustainable rehabilitation in HF patients treated optimally and educated during a follow-up period of 2 years.
METHODS
Type of Study
INCARD was a monocentric prospective cohort follow-up of rehabilitation of patients with HF.
The endpoints of the study were functional capacity, clinical and biological data.
Population
During 6 years, from February 8th, 2002 to May 2nd, 2008, 147 patients with systolic HF (left ventricular ejection fraction [LVEF] <40%) were admitted to the CR center of Centre Hospitalier Sud Francilien (Corbeil-Essonnes, France). Unstable patients or those with contraindications to exercise (unstable angina, myocarditis, outflow obstruction, mobile thrombus, recent decompensation of serious arrhythmias, major pulmonary arterial hypertension) were excluded (n = 30). Because of the constraints of the study, some patients decided not to participate (n = 54). After giving their consent, 63 patients with HF (43 %) were included in the follow-up program (Figure 1). These patients were admitted through intensive care units for coronary or acute cardiac care units. They were admitted to the rehabilitation center after stabilization (at least 3 weeks after the acute episode). An etiological screening was done to classify them into the C (n = 29) or NC group (n = 34) (supplementary S1, http://links.lww.com/MD/A208). All coronary patients had an acute coronary syndrome with complete revascularization by either angioplasty (24/29) or coronary artery bypass surgery (5/29). Of the 34 NC, 29 had dilated cardiomyopathy and 5 valvular heart disease (including 4 with aortic valve regurgitation and 1 with mitral valve regurgitation; these patients showed normal coronary and their valvular diseases had been repaired).
FIGURE 1.
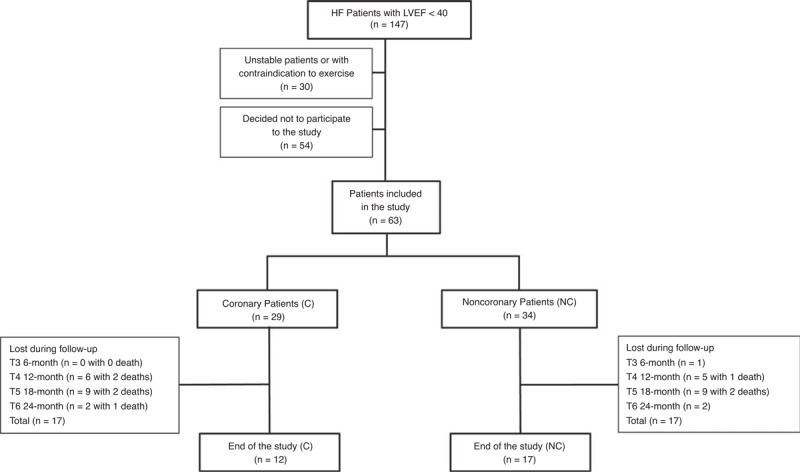
Flow-chart of the study. C = coronary patients, HF = heart failure, LVEF = left ventricular ejection fraction, NC = noncoronary patients.
This research protocol was registered in a clinical database (ClinicalTrials.gov NCT01683903) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
CR Program
CR programs were established independently from the patients’ HF etiology.
A logbook was given to the patient with reminders and guidelines for good management of his disease, weight control, and maintaining heart rate (HR). Patient was requested to comply with instructions reported in their logbook.
The inpatient physical training program was performed under the supervision of a cardiologist and a kinesiologist. The program consisted of 30 sessions, 2 hours per day scheduled over 2 periods. A first period of 10 sessions (5 sessions per week) with 1.5 hours on a KOCH bench10 focused on building up a small number of muscle groups simultaneously (the load for each patient was chosen according to the results of the exercise test and the measurement for maximum muscular strength determined for a particular muscle group) and then 30 min of physical exercises (consisting of 5–10 series; the kinesiologist directed the patient to follow a set of physical exercise that were reported as pictograms in his logbook). A second period of 20 sessions (3 sessions per week, 60 min each) entailed 30 minutes on a cycle ergometer set at the ventilatory threshold and 30 min of walking inside the center, alternating the order of the physical exercises. The physical training was optimized to safely achieve each patient maximum effort by taking into account data of the mid-term exercise test. There was no time gap between the first 10 sessions and the next 20 sessions. The rehabilitation program included behavior change and nutritional education (including therapeutic cooking classes), and optimization of treatment based on clinical status and drug tolerance.
The home program was based on a minimum of 1 hour of physical activity (PA) per day. Each day, patients did several minutes of stretching exercises, then performed 30 min of physical exercises and a 30 min of walking, without their HR exceeding the limit established as each patient's ventilatory threshold. This program was associated with dietary advice (use of sodium alternative, weekly weigh-ins). Time spent doing PA and walking, weight, and 3 classes of modalities for ankle swelling, dyspnea, and tiredness were self-recorded each day in a logbook.
Follow-Ups
Primary assessment of the patient was carried out at T0 before the rehabilitation program. At discharge (end of inpatient-follow-up), assessment T1 was performed. In agreement with the patient, the attending physician, and the cardiologist, follow-up evaluations were performed during a 1-day hospital stay. The first outpatient evaluation with detailed clinical investigations was conducted at 3 months (T2), during which patients were questioned about how well they had observed the recommendations they had received, and, if necessary were strongly encouraged to do better. The patients were reassessed at 6 (T3), 12 (T4), 18 (T5), and finally 24 months (T6) following discharge. All tests were made in the CR department with the exception of evaluation of LVEF by the isotopic method. Major events (hospitalization for cardiac events or death) during the study had to be declared by the attending physician and/or patients’relatives.
Parameters
Functional capacity was evaluated with peak VO2 (determined from exercise test obtained at the maximal capacity of PA on cycle ergometer with an incremental work load of 10 w/min) and a 6-minute walk test (6MWT). Quality of life assessment was determined by the Minnesota living with HF questionnaire (MLHFQ). HR, systolic arterial pressure (SAP), diastolic arterial pressure (DAP), body mass index (BMI), creatinine clearance (CrCl), and brain natriuretic peptide (BNP) were reported as clinical and biological parameters. LVEF was measured by transthoracic echocardiography using biplane Simpson method and/or and isotopic (ECG-gated SPECT) methods.
Statistics
Tracking software for the INCARD study was developed with a 4D database. Nonparametric tests were used in accordance with the analysis of a small population. The comparison of quantitative variables between C and NC was achieved either by a Mann–Whitney test when the Levene test for equality of variances indicated a P value <0.05 or by analysis of variance followed by a test post hoc Student–Newman–Keuls. The comparison of categorical variables was performed by analysis of the Chi-squared with the Fisher exact test. The Wilcoxon test was used for comparison of parameters measured during inpatient follow-up. Comparison of parameters over the outpatient follow-up was carried with the Friedman test (paired measurements) for the period T1 to T3 and because some participants were lost during follow-up (Figure 1) with the Kruskal–Wallis test for the period T3 to T6 followed when P < 0.05 by the post hoc test of Conover–Inman. Statistical analyses were performed using MedCalc, version 12.5 (MedCalc Software, Ostend, Belgium).
RESULTS
Demographic Data of the Population
The characteristics of the cohort stratified by coronary etiology at the inclusion (T0) are presented in Tables 1 and 3. The average age of the patients was 55 ± 13 years with no significant difference between C and NC. The proportion of women in C was significantly lower than in NC with 1 woman for 29 patients and 9 women for 34 patients, respectively. The values of BMI and blood pressure were similar in both groups. Biological parameters’ analysis revealed a higher CrCl and independent of sex a higher cholesterol in NC with 4.6 ± 1.3 (n = 25) and 3.7 ± 1.3 (n = 28) mmol/L for men in NC and C (P < 0.01). The LVEF measured with the isotopic method was higher in NC. BNP concentration was higher in C.
TABLE 1.
Clinical Characteristics of the Patients at Inclusion
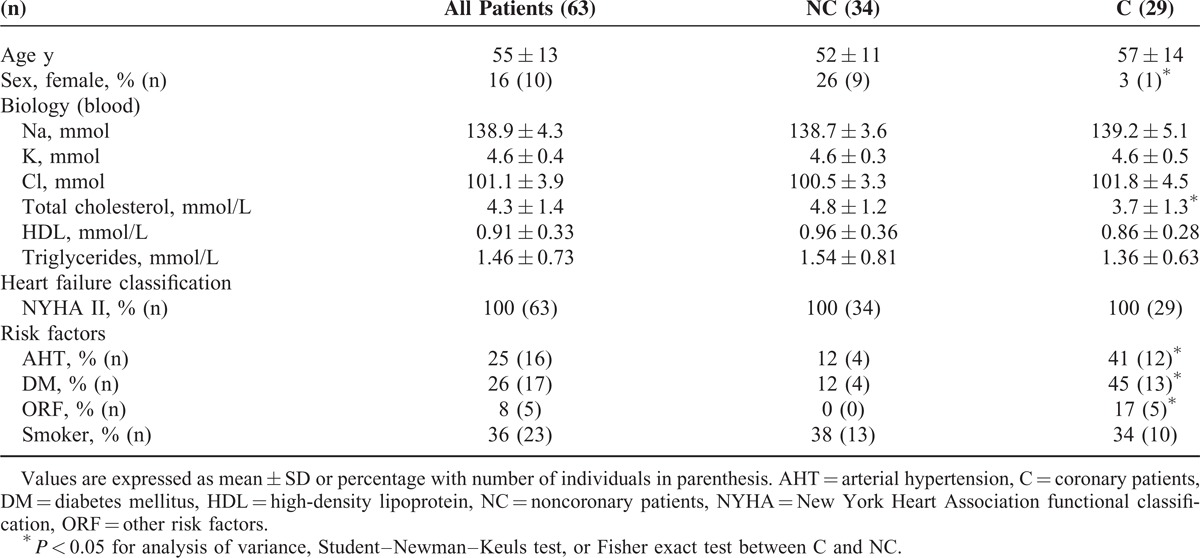
TABLE 3.
Follow-up of Clinical and Paraclinical Parameters
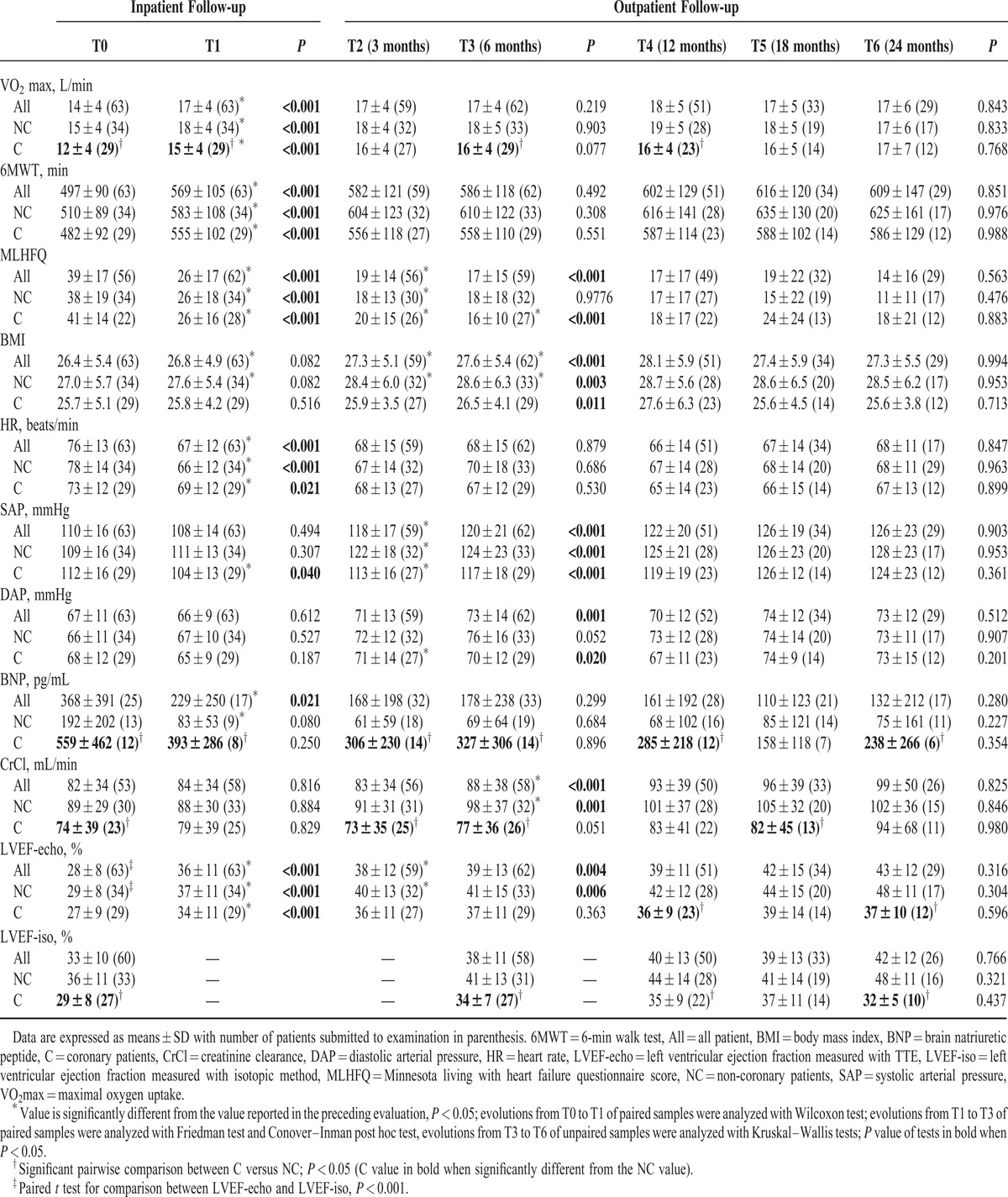
The peak VO2 was higher in NC, whereas the 6MWT and MLHFQ scores were similar in C and NC.
Regarding risk factors, C displayed a greater proportion of diabetics and hypertensives.
Mean Duration of Follow-ups
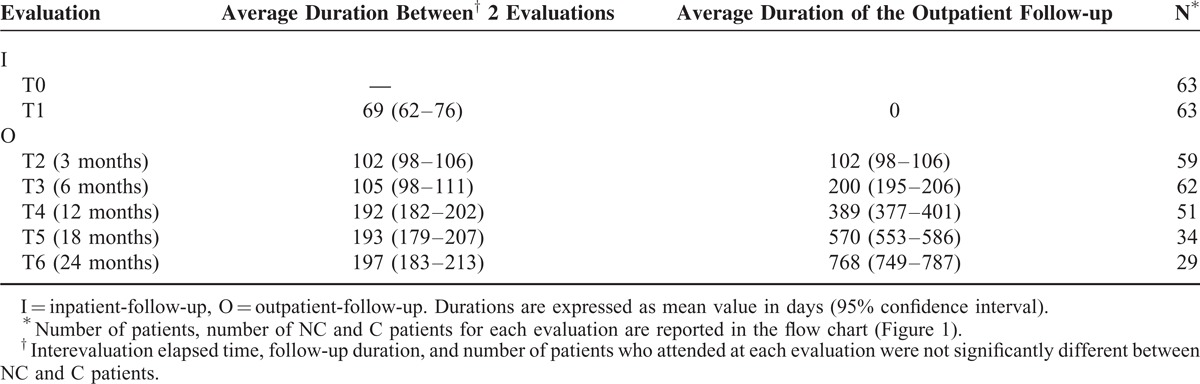
The average duration for inpatient rehabilitation was 69 days (inpatient-follow-up) (Table 2). Periodicities of the successive outpatient evaluations (outpatient-follow-up) with number of presentations are reported in Table 2. The latest assessment was made on average 768 days after hospital discharge. The number of follow-up visits decreased significantly at T4 and reached 46% of the initial rate at T6. Patients lost during follow-up for each group at each evaluation period are reported in Figure 1. There were similar rates of attendances in both groups.
TABLE 2.
Follow-up Timing
Drug Treatment
Drug treatment was conducted according to the recommendations of the European Society of Cardiology. Optimization of beta-blocker treatment occurred during the study started at 63% and reached 86% of all patients at the end of inpatient follow-up (Figure 2). There was no difference between treatment coverage of C and NC until the T3 evaluation. The coverage of anti-aldosterone drugs at T3 was 17% and 45% for C and NC, respectively (P < 0.05), and the coverage rate at T5 of the angiotensin II receptor blockers was 43% and 0% (P < 0.01), whereas that of angiotensin-converting-enzyme inhibitors was 57% and 95% (P < 0.05) for C and NC, respectively.
FIGURE 2.
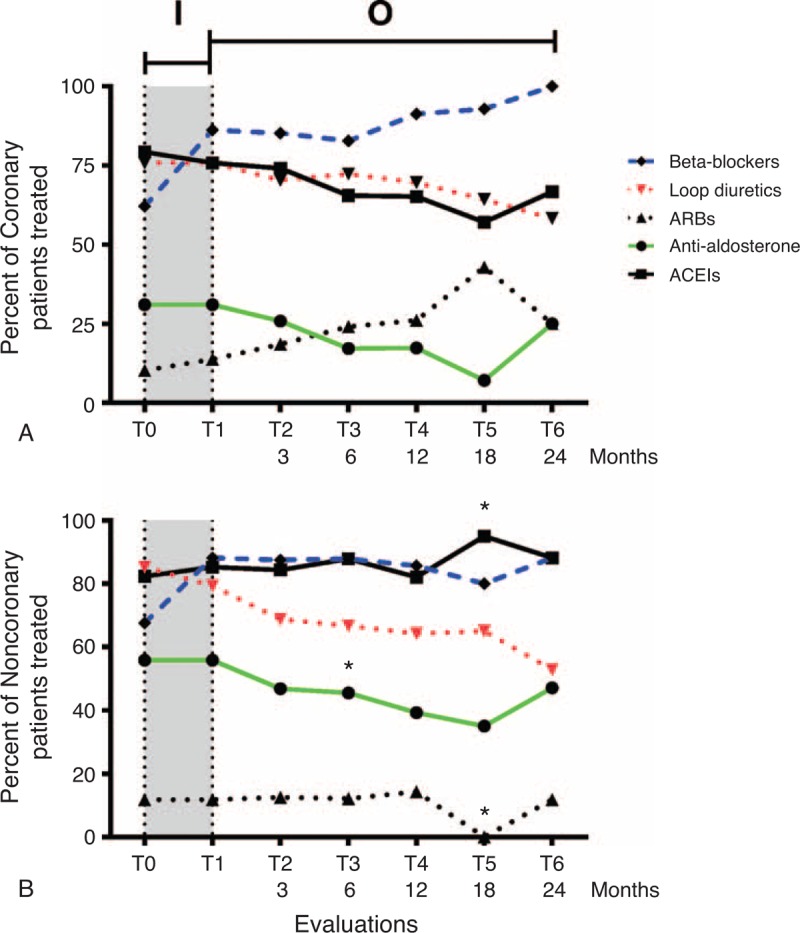
Medication management during the follow-up. Percentages of coronary (A) and noncoronary (B) patients treated with beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs), loop diuretics, anti-aldosterone, or angiotensin II receptor blockers (ARBs) are indicated for each evaluation during the inpatient follow-up (I) period and outpatient follow-up (O) period. ∗% treated patients significantly different between C and NC, P < 0.05.
Evolution of Functional Parameters
The peak VO2 was higher in NC up to 1 year after the beginning of outpatient follow-up (Table 3). For NC patients, the peak VO2 recovery was mainly achieved during the hospitalization period, whereas for C, the recovery was slower. At T4, 12 months after discharge, peak VO2 was similar in the 2 groups.
Scores of the 6MWT increased during the inpatient follow-up and then remained unchanged during the outpatient-follow-up. Although scores were not significantly different between the groups, overall scores measured in NC during the outpatient follow-up were consistently higher (P = 0.02).
The main improvement of the MLHFQ score occurred during the inpatient follow-up and extended moderately up to 6 months (T3) after discharge. MLHFQ scores measured in C and NC were similar.
LVEF increased similarly in C and NC during inpatient follow-up with 28% (P < 0.001, n = 63) increasing the ratio (Table 3). Only NC showed an improved LVEF during the outpatient follow-up with a significant increase at 3 months (T2). LVEF measured in C was significantly lower than in NC during outpatient follow-up reaching a net difference of 11% at 24 months (T6). Measurements of LVEF by ultrasound and isotopic method revealed good correlation of all measurements (r = 0.71, P < 0.0001, n = 147); however, a significant difference in the measurement was recorded in the first assessment (paired sample t-test, P < 0.001, n = 60) with a 5% higher LVEF value estimated by the isotopic method.
NC had a significant decrease of average HR of 12 beats/min without change of arterial pressures during inpatient follow-up (Table 3). Then, HR remained stable and similar in C and NC during outpatient follow-up. There was a slight increase in SAP during the first 3 months (T2) after discharge and then SAP remained stable and similar in the 2 groups. BMI increased significantly until 6 months following discharge and then remained stable in the 2 groups.
Evolution of CrCl and BNP
There was improvement in renal function in patients in the NC group over 1 year (T4) of outpatient follow-up, whereas the CrCl in C group remained lower and did not significantly improve during CR (Table 3). Plasma BNP concentration decreased in NC during inpatient follow-up. Afterwards, no significant change was measured, but it was noteworthy that the plasma BNP concentration was higher in C in all the follow-ups.
Major Events and Death
No death occurred before discharge, whereas a total of 8 deaths out of 63 patients (13%) were registered during outpatient follow-up (Figure 1). Mortality was independent of sex with 2 women and 6 men deceased out of 9 and 54, respectively (P = 0.60). Mortality between C and NC was similar (P = 0.45), with 5 C and 3 NC out of 29 and 34 respectively. No hospitalization for cardiac events occurred during the outpatient follow-up.
DISCUSSION
Several trials have revealed the benefit of training and/or outpatient monitoring; however, few identify the etiological subgroups that take advantages of these programs.14 Few studies have reached an exercise-based outpatient rehabilitation follow-up as long as 24 months. In the meta-analysis of 19 trials devoted to rehabilitation from 2001 to 2008, only 4 studies were included because they reached a follow-up of at least 1 year.15 In a recent randomized multicentric study16 whose objective was the monitoring of HF patients during the outpatient exercise-based rehabilitation for an average period of 4 years, the median follow-up was 2.5 years. The duration record was 10 years of exercise training with assessment of clinical and functional parameters.14
The ExTraMATCH meta-analysis study has already established clear evidence of mortality reduction (HR = 0.6 and 22% of mortality after 709 days of CR).17 In our study, we also observed a weak mortality rate of 13% during the outpatient follow-up with no death occurring during inpatient-follow-up.
Recent meta-analysis of 33 trials involving 4740 patients confirmed the impact of long-term rehabilitation of HF patients, which reduces HF-related hospital admission and improves patient quality of life.15
Individuals with HF have reported difficulty in following exercise recommendations.18 In the present study, the adherence to the CR program was 76 % after 1 year, much higher than the 24% to 50% adherence rates reported in other studies.19 The high adherence rate could be explained by the fact that the patient was encouraged to participate in this study. However, it could also be overestimated because the outpatient PA was not supervised and its assessment was only declarative.
Although at the moment of inclusion the isotopic method provided a 5% higher estimation assessment of LVEF, during the outpatient follow-up, there was no significant difference between LVEF determined with echocardiography and isotopic methods. A similar observation was done in the ancillary study of HF-ACTION20 regarding the initial value of resting LVEF measured with the isotopic method. The echocardiographic assessment of LVEF, which is more patient-friendly, seems satisfactory for patient follow-up over a long period.
Inpatient Follow-up
At the moment of inclusion, renal function in NC and C differed, with more impaired CrCl in C, in line with the more impaired LVEF in this group. The reduced cholesterolemia observed in C sheds a positive light on the aggressive therapy for this risk factor.
The improvement of heart function with increase of LVEF was not observed in all the previous studies. Thus, one study observed no change in LVEF after 2 months of training of HF patients,21 whereas another did not even observe decrease in LVEF after training for 12 weeks (down from 43%–30% in LVEF).22 In our study, which was not randomized for PA, the combination of treatment optimization and PA per se likely contributed to LVEF improvement during inpatient follow-up.
In line with numerous studies, functional capacity (peak VO2) and quality of life (MLHFQ) of patients improved in the first months of CR and stabilized after 1 year.14,23–27 Peak VO2 increased modestly (ie, ∼20%) as seen in a previous study,21 but it was more elevated than observed in EXERT28 or in HF-ACTION29 with increases of 10% and 4%, respectively.
In conjunction with the improvement of functional parameters, BNP decreased during inpatient follow-up. However, C maintained an elevated plasma level of BNP. This observation is likely indicative of persistent cardiac stress. C and NC had few differences regarding clinical parameters. A more pronounced decrease in HR was observed in NC (mean difference of -6 beats/min, P = 0.04) concomitantly with an increase in beta-blocker prescription rate. This indicates that there is still a space to increase beta-blocker treatment in C. The decrease in HR was likely related to the consequences of rehabilitation30,31 and beta-blocker medications, which were optimized in accordance to the guidelines.2 It is now well documented that exercise training leads to decrease in HR at rest and to increases in both the chronotropic reserve and HR recovery, via a beneficial effect on the sympathetic nervous system, even in patients receiving a beta-blocker.32,33 Effects of exercise on cardiovascular catecholamine responsiveness have been extensively studied, pointing out the role β adrenergic receptors (β AR) have in particular with the amelioration of β AR responsiveness which contributes to clinical improvement.34
Thus, inpatient rehabilitation improved all the functional parameters and LVEF for C and NC, notwithstanding the fact that the latter group had better performance.
Outpatient Follow-up
In the HF-ACTION study, no difference in all-cause mortality was found between the retrained group and the control group with 51% coronary patients, which is similar to the 46% in the INCARD cohort. In a post hoc analysis of the HF-ACTION cohort, no interaction of the etiology on all-cause mortality or hospitalization for cardiovascular causes was found. It was concluded that aerobic exercise training should be performed regardless of the etiology and severity of HF.35
Only NC patients improved their LVEF at the 3 month outpatient follow-up in agreement with a previous study reporting an improvement of LVEF during both the hospital exercise-rehabilitation period and within 6 months of outpatient exercise rehabilitation.36 Few studies have evaluated the change of LVEF for long-term outpatient exercise rehabilitation. Although no significant improvement in LVEF was measured over several years,14,21,37 after 5 years, trained patients were found to have higher LVEFs than controls.14,37 LVEF measured in C patients remained lower than in NC patients. Inversely, BNP remained higher in C patients in agreement with the relationship observed between plasma BNP levels and the left ventricular systolic function.38,39 BNP concentrations measured in plasma of coronary patients remained significantly higher than those of NC patients. BNP concentrations of C patients were <400 pg/mL and remained in the range measured in similar patients.40
In a 10-year evaluation, the improvement of peak VO2 for trained patients occurred during the first year and stabilized thereafter.14 A similar observation was carried out for the INCARD study: peak VO2 increased for the first 6 months of outpatient follow-up and thereafter stabilized in both groups. Modifications of CrCl during the outpatient period are likely to be a consequence of appropriate medication rather than a consequence of CR. The slight increase of SAP observed during the first 3 months, in contrast to the well-known effect of physical exercise in decreasing the resting SAP value, could be related to the decrease of diuretic prescription and to hypertensive status of our enrolled population (25% AHT).
There was a slight improvement in the quality of life up to 6 months following the beginning of the outpatient follow-up for C. Such improvements were noted in most studies23,41 and scarcely increased beyond 6-month time-point, though it must be noted that the quality of life was not scored using the MLHFQ. Indeed, the benefits of quality of life were obtained until the 3rd month as observed in the HF-ACTION16 study and preserved during the outpatient follow-up.14
STUDY LIMITATION
The present (INCARD) study had some limitations. INCARD as other previously reported studies21,24,42 was monocentric. Furthermore, since this study was set-up, new data26 and recommendations6,43 have been published in favor of higher intensity exercise training. Although this new mode of training, compared with moderate continuous exercise was shown to improve peak VO2, its improvement on LVEF at rest was not established.44 The INCARD cohort was small, with 63 patients followed. This number was mostly due to the difficulties in recruitment and monitoring of these fragile patients. Unfortunately, despite its benefits, CR is underexploited, mainly because it is not commonly required and a low percentage of patients participate.45 An advisory committee of the AHA has recently established a list of recommendations to improve the quality and participation of CR programs.5
CONCLUSION
Stabilization of various clinical and laboratory parameters with no significant difference between C and NC patients was the main finding resulting from analysis of the 2-year outpatient follow-up. Clearly, optimization of treatment and patient education had a positive impact on these patients, enabling them to maintain a lower HR without alteration of renal function while keeping their weight stable. Monitoring of LVEF exclusively through echocardiography is seen as reliable in this study and may obviate the absolute or concurrent need for the isotopic method. Thus, the INCARD study confirms the benefits of initial rehabilitation of HF patients at the hospital and encourages prolonged outpatient monitoring. In agreement with a previous study,35 exercise training should be done, regardless of the cause of HF. Regarding our recruitment method and differences in HF etiology and drug treatments that could introduce bias, additional multicentric studies with outpatient follow-up would be welcomed to compare the full benefits of sustained training exercise between C and NC patients.
Footnotes
Abbreviations: 6MWT = 6-minute walk test, BMI = body mass index, BNP = brain natriuretic peptide, C = coronary patients, CR = cardiac rehabilitation, CrCl = creatinine clearance, DAP = diastolic arterial pressure, HF = Heart failure, HR = Heart rate, INCARD = French acronym for «INsuffisance Cardiaque en Réadaptation Durable» (Sustainable Rehabilitation in Heart Failure Patients), LVEF = left ventricular ejection fraction, MLHFQ = Minnesota living with heart failure questionnaire, NC = non-coronary patients, PA = physical activity, SAP = systolic arterial pressure.
FK and FD contributed equally to this work.
Clinical trial registration, ClinicalTrials.gov: NCT01683903.
The authors have no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. J Cardiovasc Dis 2013; 1:1–2. [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010; 121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RJ, Ciampa J, Lessard D, et al. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med 2007; 167:490–496. [DOI] [PubMed] [Google Scholar]
- 5.Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 2011; 124:2951–2960. [DOI] [PubMed] [Google Scholar]
- 6.Piepoli MF, Conraads V, Corra U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011; 13:347–357. [DOI] [PubMed] [Google Scholar]
- 7.Kavazis AN, Alvarez S, Talbert E, et al. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol 2009; 297:H144–H152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos JC, Queliconi BB, Dourado PM, et al. Exercise training restores cardiac protein quality control in heart failure. PLoS One 2012; 7:e52764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang HK, Wang YH, Sun L, et al. Aerobic interval training attenuates mitochondrial dysfunction in rats post-myocardial infarction: roles of mitochondrial network dynamics. Int J Mol Sci 2014; 15:5304–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 2014; 126:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendziorra K, Walther C, Foerster M, et al. Changes in myocardial perfusion due to physical exercise in patients with stable coronary artery disease. Eur J Nucl Med Mol Imaging 2005; 32:813–819. [DOI] [PubMed] [Google Scholar]
- 12.Assyag P, Renaud T, Cohen-Solal A, et al. RESICARD: East Paris network for the management of heart failure: absence of effect on mortality and rehospitalization in patients with severe heart failure admitted following severe decompensation. Arch Cardiovasc Dis 2009; 102:29–41. [DOI] [PubMed] [Google Scholar]
- 13.Erhardt LR, Cline CM. Organisation of the care of patients with heart failure. Lancet 1998; 352 Suppl 1:SI15–SI18. [DOI] [PubMed] [Google Scholar]
- 14.Belardinelli R, Georgiou D, Cianci G, et al. 10-year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol 2012; 60:1521–1528. [DOI] [PubMed] [Google Scholar]
- 15.Davies EJ, Moxham T, Rees K, et al. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 2010; CD003331. [DOI] [PubMed] [Google Scholar]
- 16.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piepoli MF, Davos C, Francis DP, et al. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004; 328:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbour KA, Miller NH. Adherence to exercise training in heart failure: a review. Heart Fail Rev 2008; 13:81–89. [DOI] [PubMed] [Google Scholar]
- 19.Turk-Adawi KI, Oldridge NB, Tarima SS, et al. Cardiac rehabilitation patient and organizational factors: what keeps patients in programs? J Am Heart Assoc 2013; 2:e000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atchley AE, Kitzman DW, Whellan DJ, et al. Myocardial perfusion, function, and dyssynchrony in patients with heart failure: baseline results from the single-photon emission computed tomography imaging ancillary study of the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Trial. Am Heart J 2009; 158:S53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999; 99:1173–1182. [DOI] [PubMed] [Google Scholar]
- 22.Jugdutt BI, Michorowski BL, Kappagoda CT. Exercise training after anterior Q wave myocardial infarction: importance of regional left ventricular function and topography. J Am Coll Cardiol 1988; 12:362–372. [DOI] [PubMed] [Google Scholar]
- 23.Austin J, Williams R, Ross L, et al. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail 2005; 7:411–417. [DOI] [PubMed] [Google Scholar]
- 24.Wilson JR, Groves J, Rayos G. Circulatory status and response to cardiac rehabilitation in patients with heart failure. Circulation 1996; 94:1567–1572. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation 1988; 78:506–515. [DOI] [PubMed] [Google Scholar]
- 26.Arvan S. Exercise performance of the high risk acute myocardial infarction patient after cardiac rehabilitation. Am J Cardiol 1988; 62:197–201. [DOI] [PubMed] [Google Scholar]
- 27.Freyssin C, Verkindt C, Prieur F, et al. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Phys Med Rehabil 2012; 93:1359–1364. [DOI] [PubMed] [Google Scholar]
- 28.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT). Am Heart J 2002; 144:23–30. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 1992; 85:2119–2131. [DOI] [PubMed] [Google Scholar]
- 31.Streuber SD, Amsterdam EA, Stebbins CL. Heart rate recovery in heart failure patients after a 12-week cardiac rehabilitation program. Am J Cardiol 2006; 97:694–698. [DOI] [PubMed] [Google Scholar]
- 32.Tabet JY, Meurin P, Driss AB, et al. Benefits of exercise training in chronic heart failure. Arch Cardiovasc Dis 2009; 102:721–730. [DOI] [PubMed] [Google Scholar]
- 33.Passino C, Severino S, Poletti R, et al. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol 2006; 47:1835–1839. [DOI] [PubMed] [Google Scholar]
- 34.Santulli G, Ciccarelli M, Trimarco B, et al. Physical activity ameliorates cardiovascular health in elderly subjects: the functional role of the beta adrenergic system. Front Physiol 2013; 4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whellan DJ, Nigam A, Arnold M, et al. Benefit of exercise therapy for systolic heart failure in relation to disease severity and etiology-findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training study. Am Heart J 2011; 162:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 2000; 283:3095–3101. [DOI] [PubMed] [Google Scholar]
- 37.Muller L, Myers J, Kottman W, et al. Long-term myocardial adaptations after cardiac rehabilitation in heart failure: a randomized six-year evaluation using magnetic resonance imaging. Clin Rehabil 2009; 23:986–994. [DOI] [PubMed] [Google Scholar]
- 38.Salustri A, Cerquetani E, Piccoli M, et al. Relationship between B-type natriuretic peptide levels and echocardiographic indices of left ventricular filling pressures in post-cardiac surgery patients. Cardiovasc Ultrasound 2009; 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muders F, Kromer EP, Griese DP, et al. Evaluation of plasma natriuretic peptides as markers for left ventricular dysfunction. Am Heart J 1997; 134:442–449. [DOI] [PubMed] [Google Scholar]
- 40.Bethell HJN, Glover JD, Evans JA, et al. The relationship between BNP and risk assessment in cardiac rehabilitation patients. Br J Cardiol 2008; 15:161–165. [Google Scholar]
- 41.Giordano A, Zanelli E, Scalvini S. Home-based telemanagement in chronic heart failure: an 8-year single-site experience. J Telemed Telecare 2011; 17:382–386. [DOI] [PubMed] [Google Scholar]
- 42.Kulcu DG, Kurtais Y, Tur BS, et al. The effect of cardiac rehabilitation on quality of life, anxiety and depression in patients with congestive heart failure. A randomized controlled trial, short-term results. Eura Medicophys 2007; 43:489–497. [PubMed] [Google Scholar]
- 43.Moholdt T, Aamot IL, Granoien I, et al. Long-term follow-up after cardiac rehabilitation: a randomized study of usual care exercise training versus aerobic interval training after myocardial infarction. Int J Cardiol 2011; 152:388–390. [DOI] [PubMed] [Google Scholar]
- 44.Haykowsky MJ, Timmons MP, Kruger C, et al. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol 2013; 111:1466–1469. [DOI] [PubMed] [Google Scholar]
- 45.Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


