Abstract
Pilocytic astrocytomas (PAs) are increasingly tested for KIAA1549-BRAF fusions. We used reverse transcription polymerase chain reaction for the 3 most common KIAA1549-BRAF fusions, together with BRAF V600E and histone H3.3 K27M analyses to identify relationships of these molecular characteristics with clinical features in a cohort of 32 PA patients. In this group, the overall BRAF fusion detection rate was 24 (75%). Ten (42%) of the 24 had the 16-9 fusion, 8 (33%) had only the 15-9 fusion, and 1 (4%) of the patients had only the 16-11 fusion. In the PAs with only the 15-9 fusion, 1 PA was in the cerebellum and 7 were centered in the midline outside of the cerebellum, that is, in the hypothalamus (n = 4), optic pathways (n = 2), and brainstem (n = 1). Tumors within the cerebellum were negatively associated with fusion 15-9. Seven (22%) of the 32 patients had tumor-related deaths and 25 of the patients (78%) were alive between 2 and 14 years after initial biopsy. Age, sex, tumor location, 16-9 fusion, and 15-9 fusion were not associated with overall survival. Thus, in this small cohort, 15-9 KIAA1549-BRAF fusion was associated with midline PAs located outside of the cerebellum; these tumors, which are generally difficult to resect, are prone to recurrence.
Key Words: BRAF, Gene fusion, KIAA1549-BRAF, Pilocytic astrocytoma, RT-PCR
INTRODUCTION
Pediatric low-grade gliomas are heterogeneous and include the entities such as pilocytic astrocytoma (PA), pilomyxoid astrocytoma, and diffuse fibrillary astrocytoma. Pilocytic astrocytomas are the most prevalent, accounting for 23.5% of childhood central nervous system tumors (1). Survivals are also variable, with the 5-year survival in PAs (World Health Organization [WHO] grade I) reported as high as 100% when the tumor can be completely resected compared with 45% in diffuse fibrillary astrocytoma (WHO grade II) (2). Pilocytic astrocytomas are slow growing and, although many are cured by gross total resection, approximately 20% are located at unresectable sites such as the optic tract and hypothalamus and therefore tend to recur (1).
Jones et al (3) described a novel fusion oncogene comprising KIAA1549 and BRAF formed through the tandem duplication at the 7q34 locus in 66% of PAs but not in high-grade gliomas. This study found 3 fusion variants, which in total account for 96% of fusion variants in the literature (3–6). The most common fusion is between exon 16 of KIAA1549 and exon 9 of BRAF (63%), with less common fusion variants including exon 15-exon 9 (23%) and exon 16-exon 11 (10%) (3–6). All fusions were found to have constitutive BRAF kinase activity and transforming ability in NIH5T3 cell lines (3). The constitutive kinase activity of KIAA1549-BRAF fusion oncoprotein is caused by the loss of the BRAF autoregulatory N-terminal domain while the C-terminal kinase domain is retained (7). Forshew et al (8) found KIAA1549-BRAF fusion variants in both diffuse fibrillary astrocytomas and pilomyxoid astrocytomas, albeit at lower frequency than that observed in PAs. The evidence for the specificity of BRAF rearrangements for PAs is divided, with several reports suggesting no cases of BRAF fusion proteins in a spectrum of low-grade gliomas, including ganglioglioma, desmoplastic infantile low-grade glioma, dysembryoplastic neuroepithelial tumor, pilomyxoid astrocytoma, and pleomorphic xanthoastrocytoma (5, 9–12), and yet in other cohorts, BRAF rearrangements have been reported in up to 15% of nonpilocytic low-grade gliomas (13–15).
Subsequent research has unveiled several other rare novel BRAF fusion genes and fusion genes involving RAF1, another RAF kinase involved in the mitogen-activated protein kinase (MAPK) pathway, accounting for approximately 4% of all reported gene fusions in PAs (5, 6, 16–19). For example, Cin et al (6) identified the known KIAA1549-BRAF fusions, the SRGAP3-RAF1 fusion, and described a novel fusion between FAM131B and BRAF in a large cohort of PAs. Constitutive activation of BRAF in PAs can also occur through a point mutation in the BRAF kinase domain, c.1799T>A p.Val600Glu (commonly referred to as V600E). This mutation is common in a wide variety of tumor types and has been reported in 6.2% of PAs (15).
The KIAA1549-BRAF fusion is a useful putative diagnostic marker particularly for PAs, which can show the neuropathologic features of necrosis and microvascular proliferation, which are also seen in high-grade gliomas (1). It was originally reported that there was no significant difference in survival at follow-up of fusion-positive versus fusion-negative PAs (3). By exploring the significance of the fusion in a clinically relevant cohort of subtotally resected tumors outside the cerebellum, Hawkins et al (20) subsequently found that the KIAA1549-BRAF fusion was an independent prognostic marker for significantly improved 5-year progression-free survival for PAs, as well as grade II diffuse and pilomyxoid astrocytomas. Younger patients with infratentorial posterior fossa PAs tend to display a high frequency of the KIAA1549-BRAF fusion (10, 21). Supratentorial tumors are less frequently fusion positive but have an increased frequency of the oncogenic BRAFV600E mutation (7, 13, 22). Currently, there are clinical trials for BRAF and MAPK inhibitors for pediatric gliomas (23).
The differential neuropathologic diagnosis for PAs includes pediatric high-grade gliomas, most of which have recently been associated with histone mutations (H3.3 K27M) (24). Thus, in addition to BRAF screening, analysis of H3.3 K27M status is a useful additional biomarker that should be negative in a histologically confirmed PA case (24).
Here, we tested for the BRAF fusion, BRAFV600E, and histone H3K27me3 biomarkers and attempted to correlate them with the clinical outcome in a cohort of pediatric PA patients.
MATERIALS AND METHODS
Clinical Cohort
The cases included 32 patients. The male:female ratio was 10:22, and the age range was 6 months to 17 years 4 months. There were 13 PAs in the cerebellum, 10 in the hypothalamus, 1 in the brainstem, 1 in the third ventricle, 1 in the fourth ventricle, 3 in the optic pathway, and 3 tumor locations were unknown (Table 1). Adjuvant therapies included radiotherapy (50–54 Gy in 28–30 fractions), chemotherapy (carboplatin, etoposide, vinblastine, vincristine), and proton therapy. In terms of completeness of resection, 9 (38%) of 24 had a documented partial resection, and 15 (62%) had a documented complete resection.
TABLE 1.
Patient Clinical Data

BRAF Fusion Reverse Transcription Polymerase Chain Reaction
RNA was extracted from macrodissected formalin-fixed paraffin-embedded (FFPE) sections using the RNeasy FFPE kit following the manufacturer’s instructions (Qiagen, Manchester, UK). RNA was reverse transcribed to cDNA using the High-Capacity cDNA RT kit following the manufacturer’s instructions (Applied Biosystems, Warrington, UK). Real- time PCR was performed using 2.5 μL of cDNA, primers, and TaqMan probes specific for the KIAA1549 and BRAF fusion as per Tian et al (4). Glyceraldehyde phosphate dehydrogenase was used as a control (Assays-on-Demand Gene Expression Product Hs02758991) using the TaqMan Gene Expression Mastermix and the ABI 7500 Real-time PCR instrument (all from Applied Biosystems). Primers were used at 0.9 mmol/L final concentration and probes at 0.25 mmol/L final concentration. Polymerase chain reaction conditions were 2 minutes at 50°C, 10 minutes at 95°C, and then 50 cycles of 15 seconds at 95°C and 1 minute at 60°C.
Fluorescence In Situ Hybridization for KIAA1549-BRAF Fusion
The BRAF fusion reverse transcription polymerase chain reaction (RT-PCR) results were confirmed by fluorescence in situ hybridization (FISH) analysis in a subset of cases. Formalin-fixed paraffin-embedded sections were deparaffinized and pretreated using the SPOT-Light Tissue Pretreatment kit (Invitrogen, Warrington, UK), and FISH was undertaken using Kreatech Poseidon BRAF-KIAA1549 (7q34) Triple-Colour Fusion probe with DAPI counterstain. At least 200 interphase nuclei were examined by 2 analysts for each sample.
Immunohistochemistry
BRAF V600E (clone VE1) and anti-histone H3.3 K27M rabbit polyclonal antibody (dilution 1:500, CAT No. ABE419; Merck Millipore, Billerica, MA) immunohistochemistry were performed as previously described (24, 25).
Statistical Analysis
The χ2 test was used to determine the association of the 15-9 fusion with tumor location. Kaplan-Meier survival analyses were used to assess age, sex, tumor location, 16-9 fusion, and 15-9 fusion associations with overall survival; p < 0.05 was considered significant.
RESULTS
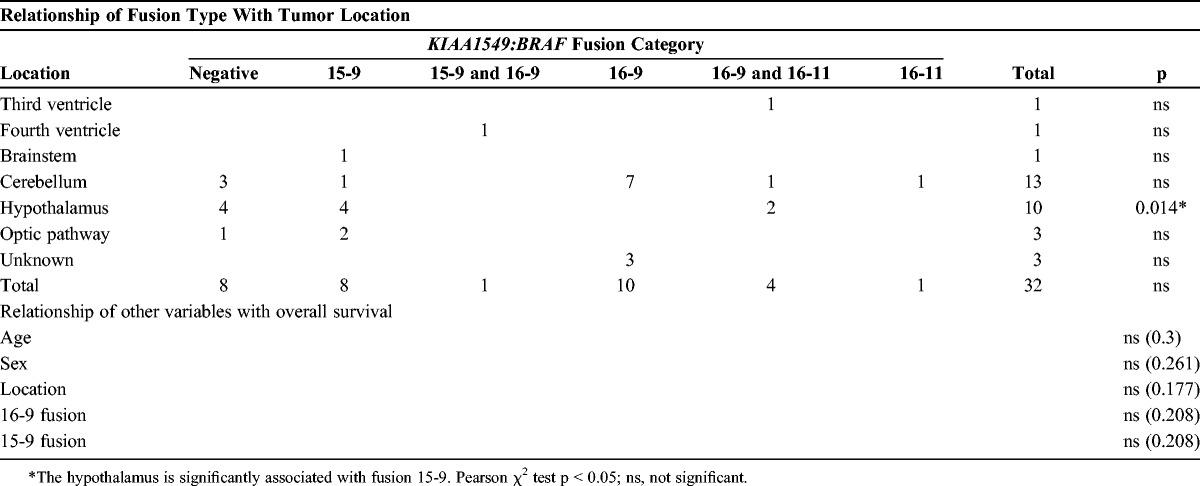
A cohort composed of 32 PA patients was successfully tested using the BRAF fusion RT-PCR assay, BRAF V600E, and histone H3.3 K27M. In the cohort of 32 patients, the BRAF fusion was identified in 24 cases (75%). All cases were negative for the H3.3 K27M, as expected. All tumors were also negative for BRAF V600E (Table 1). Of the BRAF fusion-positive cases, 10 (42%) had only the 16-9 fusion and 8 (33%) had only the 15-9 fusion. Five (21%) of the 24 patients had multiple fusions, 4 (17%) showed both the 16-9 fusion and a low-level 16-11 fusion, and 1 (4%) showed both the 16-9 fusion and a 15-9 fusion (Table 2). One patient (4%) exclusively had the 16-11 fusion. Multiple biopsy samples across different time points were available for 8 patients. In 7 (87%) of these 8 patients, the results from the multiple biopsies were identical. In 1 (13%) of the 8 patients, both biopsies sampled had the 16-9 fusion but the 16-11 fusion was detected at a very low level in only one of the biopsies.
TABLE 2.
Relationships of Fusion Type With Tumor Location
Clinical follow-up showed that 7 (22%) of the 32 patients were deceased, and 25 (78%) were alive between 2 and 14 years after initial biopsy. The deceased group had a male: female ratio of 3:4, with an age at diagnosis range of 2 years to 13 years 6 months.
In 2 (29%) of the 7 deceased patients, the original tumor site was not noted. In the remaining 5 (71%) of this group, the tumors originated in the hypothalamus/third ventricle or optic pathway. The site of origin in the patients who were alive was recorded in 24 cases (96%). Of these, 13 (55%) were in the cerebellum, 8 (33%) were in the hypothalamus, and 1 each (4%) was in the optic pathway, brainstem, or fourth ventricle.
Of the deceased group, 1 (14%) had a complete resection and, of the remaining 6 patients (86%), the extent of surgical resection was unknown. Of the alive group, the extent of surgical resection was recorded for 23 (92%) out of 25 patients. Of these, 8 (35%) had partial resection and 15 (65%) had complete resection. As expected, tumor location and complete or partial resection were significantly related in the cohort (p < 0.001).
Of the deceased group, 2 patients (28%) had recorded adjuvant therapy: 1 received chemotherapy alone and 1 received chemotherapy and radiotherapy. Of the alive group, 22 patients (88%) had recorded adjuvant therapy: 2 received chemotherapy and radiotherapy, 2 received radiotherapy, 1 received chemotherapy alone, 1 received chemotherapy and proton therapy, and 16 (72%) of these patients received no therapy.
The RT-PCR fusion test was positive for 5 (71%) out of 7 of the deceased group. Within this group, the type of fusion present included 2 out of 5 with 16-9 fusion, 2 out of 5 with 16-9 and 16-11 fusions, and 1 with 15-9 fusion. Of the alive group, 19 (76%) cases had a positive result for the RT-PCR and, of these, 8 (42%) had the 16-9 fusion, 7 (37%) had the 15-9 fusion, 1 (5%) had only the 16-11 fusion, and 1 (5%) was positive for both the 16-9 and the 15-9 fusions. In addition, 3 (16%) showed 16-9 fusions also with low levels of 16-11 fusions also (Table 1).
In the 5 deceased patients, 2 (40%) had multiple fusions, whereas 4 (21%) out of 19 alive patients had multiple fusions. The small numbers of patients in this cohort precluded determining relationships of the presence of multiple fusions with clinical features and survival.
Interestingly, the 15-9 fusion was significantly associated with tumor location in the midline outside of the cerebellum (p = 0.014) (Tables 2, 3). Tumors located in the cerebellum were negatively associated with fusion 15-9 (p = 0.008). Tumors in the cerebellum were not significantly associated with fusion 16-9 (p = 0.080). Age (p = 0.300), sex (p = 0.261), tumor location (p = 0.177), 16-9 fusion (p = 0.208), and 15-9 fusion (p = 0.208) were not significantly associated with overall survival.
TABLE 3.
Cross-Tabulation of Tumor Location Versus 15-9 Fusion

DISCUSSION
In this study of 32 PA patients, the KIAA1549-BRAF fusion detection rate was (24 of 32) 75% overall. This is comparable to that in the literature, with previous studies showing that 60% to 80% of PAs harbor BRAF fusions (10, 20). The BRAF V600E mutation and H3K27me3 histone methylation were not detected in the cohort.
The neuropathologic distinction of PA from malignant glioma can be challenging because biopsies of PAs tend to be small and show features such as necrosis and microvascular proliferation that are also seen in high-grade gliomas (1). Distinguishing these entities is key for appropriate treatment and accurate prognostic information for pediatric glioma patients. The KIAA1549-BRAF fusion is increasingly used as a diagnostic marker to aid neuropathologic diagnosis in low-grade gliomas because of its high frequency in these tumors, particularly PAs, and its absence in high-grade tumors such as anaplastic astrocytoma (WHO grade III) and glioblastoma (WHO grade IV) (15).
In our cohort, the KIAA1459-BRAF 16-9 fusion was the most common, accounting for 42%; the 15-9 fusion accounted for 33% and the 16-11 fusion only accounted for 4% (3–6). Twenty-one percent of our cohort had multiple fusions, with 17% showing both the 16-9 fusion and a low-level 16-11 fusion and 4% showing both the 16-9 fusion and a 15-9 fusion. Similarly, Tian et al (4) also described patients expressing more than 1 fusion variant, that is, 9% of their cohort expressed both 16-9 and 15-9 fusions, and 3% expressed both 16-9 and 16-11 fusions. The primers used in our study were identical to those used by Tian et al (4), so this could be a genuine finding or a technical artifact of the assay. Taha et al (26) reported 3 PA patients with fusions of both 16-9 and 15-9 using a different RT-PCR assay. The presence of multiple fusion transcripts may be caused by the production of several different RNA transcripts from alternative splicing or could reflect different subpopulations of the tumor, each expressing a different fusion isoform. In our cohort, it is unclear whether multiple fusions may predict a worse clinical outcome because of the small numbers with multiple fusions.
In our cohort, the KIAA1549-BRAF 15-9 fusion was significantly associated with tumor location in the midline (p = 0.014) and PAs located within the cerebellum were negatively associated with fusion 15-9 (p = 0.008). There is some evidence of tumors in the cerebellum being associated with fusion 16-9, but this does not achieve significance (p = 0.080). Tumors located in the hypothalamus are traditionally difficult to resect; indeed, complete resection has been reported in 94% of cerebellar PAs compared with only 3.2% of hypothalamic and chiasmatic tumors, with an overall tumor recurrence rate of 19% (27, 28). Our findings of an association of fusion with tumor location were not seen in the large cohort of PAs described by Jones et al; however, that study had only 2 out of 96 PAs that arose in the hypothalamus. In addition, in contrast to other studies, the cohort by Jones et al (3) did not show multiple fusions within a single tumor, raising the possibility that this finding could be an artifact of PCR.
Other studies have shown an association between location and BRAF alterations, but the present study is the first to demonstrate an association between BRAF fusion subtype and location. The KIAA1549-BRAF fusions are more common in posterior fossa PAs (10, 21), whereas supratentorial PAs are less frequently fusion positive but have an increased frequency of the oncogenic BRAFV600E mutation (7, 13, 22). More specifically, Hawkins et al (20) showed that midline supratentorial low-grade gliomas (which are usually unresectable) had a higher frequency of KIAA1549-BRAF fusions than lobar tumors.
In this study, age, sex, tumor location, 16-9 fusion, and 15-9 fusion were not significantly associated with overall survival; however, the small sample size, absence of some data, and small number of deaths may have precluded detection of a significant association. Two previous studies have shown that the KIAA1549-BRAF fusion is associated with an improved outcome in pediatric low-grade gliomas (13, 20), whereas 4 studies have reported no effect on outcome (3, 13, 16, 27).
Currently, there are several clinical trials in glioma involving agents that inhibit the MAPK pathway such as BRAF, RAF1, and MEK inhibitors (15). Although a recent phase II trial of sorafenib (multikinase inhibitor) in pediatric low-grade astrocytoma was discontinued because of rapid progression of the disease (29), combined therapy of sorafenib and an mTOR inhibitor was successful in a single patient with a spindle cell neoplasm harboring the KIAA1549-BRAF fusion (30). Combination therapy may therefore be a useful approach in molecularly targeting BRAF alterations in pediatric gliomas and should be the focus of clinical trials in this area, particularly in patients with tumors that are difficult to resect.
The BRAF fusions are a useful diagnostic biomarker and a potential prognostic biomarker in pediatric low-grade glioma. In the future, the identification of BRAF fusions may also be important for patient treatment as more targeted molecular therapies become available. It is therefore important that a diagnostic test for these fusions is readily available in a routine diagnostic setting. The KIAA1549-BRAF RT-PCR assay used here is very straightforward to interpret, quick to perform, amenable to FFPE tissue (some samples tested were cases more than 20 years old), and also gives additional information as to the type of fusion variant identified. However, because the RT-PCR assay detects only the 3 most common fusion variants, there could be other fusion variants present that are not detected by the assay such as KIAA1549-BRAF fusions involving different exons and fusions between RAF genes and a different gene; rare FAM131B-BRAF, SRGAP3-RAF1, FXR1-BRAF, BRAF-MACF and QK1-RAF1 fusions have also been described (6, 15). Together, these rare variants not detected by the RT-PCR assay account for approximately 4% of all reported fusion variants.
In terms of reproducibility of the RT-PCR assay, in 7 (87%) of 8 of the patients with multiple biopsies, the results were identical, whereas in 1 (13%) of the 8 cases, the 16–11 fusion was detected at a very low level. The reason for this discrepancy is unclear, but it could possibly be caused by the very low expression of the 16-11 fusion in the relatively small tumor samples (4) or heterogeneity between the tumor samples. In terms of technical failure rate, in our initial validation of the RT-PCR assay, 5 patients were excluded because of low RNA concentration extracted from very small biopsy samples.
The RT-PCR is increasingly being implemented over or alongside FISH in a diagnostic setting. This is because FISH results are often very difficult to interpret because the KIAA1549 and BRAF genes lie in close proximity on chromosome 7q34, and it is difficult to distinguish a fusion signal from a normal signal (4). Moreover, analysis may also be complicated by amplification of the 7q34 region in some tumors without gene fusion (10). In addition, identification of the type of fusion present may give further prognostic information in PAs, so an assay that can identify the fusion variant such as RT-PCR may be useful to implement in a diagnostic setting. Fluorescence in situ hybridization may be a useful adjunct for cases where a fusion variant has not been identified by RT-PCR or where RNA extraction is unsuccessful.
In conclusion, we have identified KIAA1549-BRAF fusions in 75% of patients with PA using an RT-PCR assay from FFPE tissue. In our small cohort, the KIAA1549-BRAF 15-9 fusion was significantly associated with PAs located outside of the cerebellum in the midline. This is the first study to demonstrate an association between BRAF fusion subtype and tumor location. Tumors of the midline are traditionally difficult to resect and are prone to recurrence, so further research is required to examine this observation in more detail.
ACKNOWLEDGMENTS
The authors thank the Pathological Society & Jean Shanks Foundation Pathological Research Training Fellowship, the Showering Grant, North Bristol Trust, Small Grants Scheme North Bristol Trust, British Neuropathological Society.
Footnotes
This study was funded by the Pathological Society & Jean Shanks Foundation Pathological Research Training Fellowship; the Showering Grant, North Bristol Trust; Small Grants Scheme North Bristol Trust; British Neuropathological Society.
David Capper declares shared inventorship of BRAF V600E mutant-specific antibody VE1 and has applied for a patent on the diagnostic use of VE1 immunohistochemistry. All terms are being managed by the German Cancer Research Centre in accordance with its conflict of interest policies.
REFERENCES
- 1. Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst 2001; 17: 503– 11 [DOI] [PubMed] [Google Scholar]
- 2. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005; 109: 93– 108 [DOI] [PubMed] [Google Scholar]
- 3. Jones DTW, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res 2008; 68: 8673– 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian Y, Rich BE, Vena N, et al. Detection of KIAA1549-BRAF fusion transcripts in formalin-fixed paraffin-embedded pediatric low-grade gliomas. J Mol Diagn 2011; 13: 669– 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawson AR, Tatevossian RG, Phipps KP, et al. RAF gene fusions are specific to pilocytic astrocytoma in a broad paediatric brain tumour cohort. Acta Neuropathol 2010; 120: 271– 73 [DOI] [PubMed] [Google Scholar]
- 6. Cin H, Meyer C, Herr R, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol 2011; 121: 763– 74 [DOI] [PubMed] [Google Scholar]
- 7. Jones DT, Gronych J, Lichter P, et al. MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci 2012; 69: 1799– 811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: A signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol 2009; 218: 172– 81 [DOI] [PubMed] [Google Scholar]
- 9. Dimitriadis E, Alexiou GA, Tsotsou P, et al. BRAF alterations in pediatric low-grade gliomas and mixed neuronal-glial tumors. J Neurooncol 2013; 113: 353– 58 [DOI] [PubMed] [Google Scholar]
- 10. Jacob K, Albrecht S, Sollier C, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer 2009; 101: 722– 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dougherty MJ, Santi M, Brose MS, et al. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol 2010; 12: 621– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koelsche C, Sahm F, Paulus W, et al. BRAF V600E expression and distribution in desmoplastic infantile astrocytoma/ganglioglioma. Neuropathol Appl Neurobiol 2014; 40: 337– 44 [DOI] [PubMed] [Google Scholar]
- 13. Horbinski C, Nikiforova MN, Hagenkord JM, et al. Interplay among BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro Oncol 2012; 14: 777– 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez FJ, Schniederjan MJ, Nicolaides T, Tihan T, Burger PC, Perry A. High rate of concurrent BRAF-KIAA1549 gene fusion and 1p deletion in disseminated oligodendroglioma-like leptomeningeal neoplasms (DOLN). Acta Neuropathol 2015; 129: 609– 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penman CL, Faulkner C, Lowis SP, Kurian KM. Current understanding of BRAF alterations in diagnosis, prognosis, and therapeutic targeting in pediatric low-grade gliomas. Front Oncol 2015; 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol 2012; 71: 66– 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013; 340: 626– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tatevossian RG, Lawson AR, Forshew T, et al. MAPK pathway activation and the origins of pediatric low-grade astrocytomas. J Cell Physiol 2010; 222: 509– 14 [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 2013; 45: 602– 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawkins C, Walker E, Mohamed N, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res 2011; 17: 4790– 98 [DOI] [PubMed] [Google Scholar]
- 21. Hasselblatt M, Riesmeier B, Lechtape B, et al. BRAF-KIAA1549 fusion transcripts are less frequent in pilocytic astrocytomas diagnosed in adults. Neuropathol Appl Neurobiol 2011; 37: 803– 6 [DOI] [PubMed] [Google Scholar]
- 22. Nicolaides TP, Li H, Solomon DA, et al. Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res 2011; 17: 7595– 604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A 2013; 110: 5957– 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bechet D, Gielen GG, Korshunov A, et al. Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta Neuropathol 2014; 128: 733– 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koelsche C, Sahm F, Wöhrer A, et al. BRAF-mutated pleomorphic xanthoastrocytoma is associated with temporal location, reticulin fiber deposition and CD34 expression. Brain Pathol 2014; 24: 221– 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taha H, Yehia M, Mahmoud M, El-Beltagy M, Ghabriel M, El-Naggar S. Incidence of kiaa1549-braf fusion gene in Egyptian pediatric low-grade glioma. Clin Transl Med 2015; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colin C, Padovani L, Chappé C, et al. Outcome analysis of childhood pilocytic astrocytomas: A retrospective study of 148 cases at a single institution. Neuropathol Appl Neurobiol 2013; 39: 693– 705 [DOI] [PubMed] [Google Scholar]
- 28. Stokland T, Liu JF, Ironside JW, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: A population-based cohort study (CCLG CNS9702). Neuro Oncol 2010; 12: 1257– 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karajannis MA, Legault G, Fisher MJ, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol 2014; 16: 1408– 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Subbiah V, Westin SN, Wang K, et al. Targeted therapy by combined inhibition of the RAF and mTOR kinases in malignant spindle cell neoplasm harboring the KIAA1549-BRAF fusion protein. J Hematol Oncol 2014; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


