Abstract
Purpose/Objectives
To assess feasibility and toxicity of Helical TomoTherapy® for treating anal cancer patients.
Methods
From 2007 to 2011, 64 patients were consecutively treated with TomoTherapy® in three centres for locally advanced squamous-cell anal carcinoma (T2 > 4 cm or N positive). Prescribed doses were 45 Gy to the pelvis including inguinal nodes and 59.4 Gy to the primary site and involved nodes with fractions of 1.8 Gy, five days a week. A positional Megavoltage Computed Tomography was performed before each treatment session. All acute and late toxicities were graded according to Common Terminology Criteria for Adverse Events version 3.0. Survival analysis was performed using the Kaplan-Meier method.
Results
Median follow-up was 22.9 months. Fifty-four women and 10 men were treated (median age: 62 years). Nineteen patients (29.7 %) had T2, 16 patients (25.0 %) T3, and 27 patients (42.2 %) T4 tumours. Thirty-nine patients (60.9 %) had nodal involvement. Median tumour size was 45 mm (range, 10–110 mm). Seven patients had a colostomy before treatment initiation. Fifty-seven patients received concomitant chemotherapy (5-FU/cisplatin or 5-FU/mitomycin-based therapy). Forty-seven patients (73.4 %) experienced a complete response, 13 a partial response or local recurrence, and 11 had salvage surgery; among these, six became complete responders, three experienced metastatic failure, and two local failure. At least four patients experienced metastatic recurrence (concomitant to a local failure for one patient). The two-year overall survival was 85.6 % (95 %CI [71.1 %–93.0 %]), and the one-year disease-free survival, and colostomy-free survival were 68.7 % (95 %CI [54.4 %–79.4]), and 75.5 % (95 %CI [60.7 %–85.3 %]) respectively. Overall survival, disease-free survival and colostomy free-survival were significantly better for women than men (p = 0.002, p = 0.004, and p = 0.002 respectively). Acute grade ≥3 toxicity included dermatologic (46.9 % of patients), gastrointestinal (20.3 %), and hematologic (17.2 %) toxicity. Acute grade 4 hematologic toxicity occurred in one patient. No grade 5 event was observed.
Conclusions
TomoTherapy® for locally advanced anal cancer is feasible. In our three centres of expertise, this technique appeared to produce few acute gastrointestinal toxicities. However, high rates of dermatologic toxicity were observed. The therapeutic efficacy was within the range of expectations and similar to previous studies in accordance with the high rates of locally advanced tumours and nodal involvement.
Background
Squamous-cell carcinoma of the anus is a rather rare malignancy, accounting for 2 to 4 % of gastrointestinal (GI) malignancies, often occurring in elderly women [1, 2]. However, anal cancer incidence has been increasing over the past decades. Lymphatic spread is frequent (inguinal and iliac nodes) but metastatic evolution is rare [3–5]. Since the publication of Nigro et al., the current standard of care consists of chemoradiation, which is highly effective, achieving locoregional control and preservation of anal function without colostomy [6]. Recurrence (20–30 % of patients) is most often locoregional, with more than 80 % occurring within two years of treatment [7–11]. However, delivery of radiotherapy results in significant acute toxicity (dermatologic, GI, hematologic, and genitourinary) because of the large and complex volume to treat and the proximity to critical structures. This important toxicity sometimes leads to treatment interruptions [12]. Therefore, many institutions now use intensity-modulated radiotherapy (IMRT) in order to achieve a better planning target volume (PTV) coverage, and a better protection of organs at risk (OARs), allowing reduction of acute and late toxicities [13–15].
In July 2005, the French National Cancer Institute (INCa) launched a pilot project to study the medical impact of implementing emerging technologies in radiation oncology. This led to the installation in 2007 of three TomoTherapy® (Accuray Incorporated, Sunnyvale, CA) units in France. Before the units were operational, and since no published data on TomoTherapy® was available, treatment indications were validated based on available IMRT literature. Treatment protocols were devised for several diseases including anal canal carcinomas, for which we expected that IMRT using TomoTherapy® could reduce toxicity by effectively dealing with the complex volume to treat [16].
This work is a prospective observational study, reporting the largest published series of patients treated by TomoTherapy® for anal cancer. The primary objective was to evaluate the tolerance to chemoradiotherapy with TomoTherapy®. Secondary objectives were to evaluate overall survival (OS), disease-free survival (DFS), colostomy-free survival (CFS), and prognostic factors of recurrence.
Methods
We included all consecutive patients treated with TomoTherapy® for squamous-cell carcinoma of the anus between June 2007 and December 2011 in Bordeaux (Bergonié Institute and Bordeaux University Hospital) and in Nantes (René Gauducheau Centre), France. Institutional review board approval was obtained for this pilot study.
Inclusion criteria
Treatment indications included locally advanced squamous-cell carcinomas, T2 larger than 4 cm, T3, T4 or positive lymph nodes. For these locally advanced cancers, it is recommended to treat inguinal and iliac (internal and external) lymphatic areas. All patients underwent a physical examination including anuscopy with biopsy and rectal examination, a computed tomography of the chest, abdomen, and pelvis; and a magnetic resonance imaging of the pelvis. A positron emission tomography scan was not routinely carried out for patient staging. Only patients presenting a negative serology for Human Immunodeficiency Virus infection were included.
Chemoradiotherapy
Doses prescribed were 45 Gy to the lymphatic areas at risk (inguinal, mesorectal, and internal and external iliac), and 59.4 Gy to the tumour and the involved nodes with fractions of 1.8 Gy, five days a week. A break in fractions at 45 Gy was introduced in case of toxicity, however this was not mandatory. Chemotherapy involved a combination of cisplatin and 5-FU on weeks one and five. This regimen was the standard regimen for anal cancer treatment in France at that time, and was also used for the control arm of another French trial (ACCORD 03 trial) [17].
Target delineation and TomoTherapy® planning
Patients were in supine position with knee and foot support. CT scans were performed with contiguous 2.5-mm-thick slices with and without contrast infusion. Delineation was performed by a radiation oncologist and included the following target volumes: the gross tumour volume (GTV: primary tumour and involved nodes) and the clinical target volume (CTV: GTV and lymphatic areas at risk: inguinal, mesorectal, and the internal and external iliac areas). Rules for the delineation of the CTV were as follows: delineation of obturator, external and internal iliac lymphatic areas including vessels plus a 7 mm margin modified by exclusion of bones and muscular structures, inclusion of the totality of the mesorectum and presacral area, and inclusion of ischio-rectal fossa and anal margin. Inguinal areas included inguinal vessels and all visible groins. In case of anal margin involvement, perianal skin was included with a wide margin of 2 cm. In case of posterior vaginal involvement, the entire vagina was included in the CTV. A 10 mm isotropic margin was added to CTV and GTV to create PTV1 and PTV2, respectively. The following OARs were delineated: bladder, genitals, femoral heads, and intestines. The dose was prescribed to the median PTV, with 95 % of the volume receiving at least 95 % of the prescribed dose, 98 % of the volume receiving at least 90 % of the prescribed dose, and 3 % of the volume receiving less than 107 % of the prescribed dose. Dose constraints were defined for femoral heads (D2% <40 Gy), bladder (D2 % <45 Gy, V30 < 20 %), genitals (D2% <50 Gy, V40 < 60 %), and intestines (D2 % <50 Gy, V30 < 20 %). The usual planning parameters were a 2.5 cm field width, a 0.3 pitch and a planned modulation factor of 2–2.5. Some additional dummy structures were delineated such as upper and lower volumes to constrain the dose gradient outside the PTV. Dummy volumes were also created between each separate target volumes. During plan optimization, the first priority was PTV coverage, and then the doses to the OARs and normal tissues were lowered as much as possible without compromising PTV coverage. Quality assurance consisted of in-phantom verification and in-vivo dosimetry using plain film and ion chamber measurements before the first fraction of each treatment and before any treatment plan modification to correlate actual dose and planned dose.
Clinical course and follow-up
All patients were monitored weekly for acute hematologic, dermatologic, GI (diarrhoea and nausea), and urinary toxicity. After completion of the treatment, all patients were evaluated by a radiation oncologist within six to eight weeks, every four months for the following two years, and every six months during the three following years. All acute and late toxicities were graded according to Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE V3).
Statistical analysis
Survival was estimated using the Kaplan-Meier method from the date of initiation of radiotherapy. OS was defined as time to death, DFS and CFS were defined as time to progressive disease or recurrence or death and time to colostomy or death, respectively. All patients who did not experience the event of interest were censored at the last follow-up date. Log-rank test was used to determine factors associated with OS, DFS and CFS. Associations between violation of the OAR dose constraints and acute toxicities were estimated using Fisher exact test. All analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA). A 2-sided P value of less than 0.05 was considered statistically significant.
Results
Patient and tumour characteristics
Sixty-nine patients were consecutively treated between July 2007 and November 2011 (first day of radiotherapy) for anal cancer: among them five patients presented with metastatic disease and were excluded. In total 64 patients with locally advanced anal cancer were included and analysed. There were 10 men and 54 women; median age was 62 years (range, 32–89 years). Median tumour size was 45 mm (range, 10–110 mm) and 67.2 % of these tumours were T3 or T4 tumours. Sixty-one percent of the patients had lymph node involvement. A colostomy was needed prior to treatment for seven patients (10.9 %) (Table 1).
Table 1.
Patient and tumour characteristics (N = 64)
| Characteristic | Number | Percent |
|---|---|---|
| Age, years, median (range) | 62 | (32–89) |
| Female gender | 54 | (84.4) |
| Perianal involvement | 25 | (39.1) |
| Inguinal Node Status | ||
| Negative | 45 | (60.9) |
| Positive | 19 | (39.1) |
| Vaginal involvement | 22 | (34.4) |
| T Category | ||
| T1 | 2 | (3.1) |
| T2 | 19 | (29.7) |
| T3 | 16 | (25.0) |
| T4 | 27 | (42.2) |
| N Category | ||
| N0 | 25 | (39.1) |
| N1 | 15 | (23.4) |
| N2 | 16 | (25.0) |
| N3 | 8 | (12.5) |
| M Category | ||
| M0 | 64 | (100.0) |
| Colostomy prior to treatment | 7 | (10.9) |
Data are shown as number (percentage), except when specified otherwise
Treatment characteristics and dosimetric parameters
Median total dose was 59.4 Gy (range, 45.0–67.1 Gy) and median dose per fraction was 1.8 Gy (Fig. 1). Dosimetric parameters concerning PTV coverage and conformity index are summarized in Table 2 and results of dose volume histogram (DVH) analysis for OARs are reported in Table 3. Median overall treatment time (OTT) was 57 days (range, 35–113 days). Thirty patients experienced a treatment break of a mean duration of 5.9 days: in two centres, the treatment break was indicated only based on toxicity for 12 patients whereas in the third centre the break was prospectively planned for 18 patients. Chemotherapy was combined with radiotherapy for 57 patients (89.2 %) and consisted of cisplatin and 5-FU for 49 patients, mitomycine and 5-FU for three patients. For five patients, a combination of eloxatine and 5-FU (folfox regimen) or oral 5-FU in the form of capecitabine were prescribed because of cardiac or renal comorbidities (contra-indication for cisplatin). Seven patients did not receive any concomitant chemotherapy because of comorbidities and age.
Fig. 1.
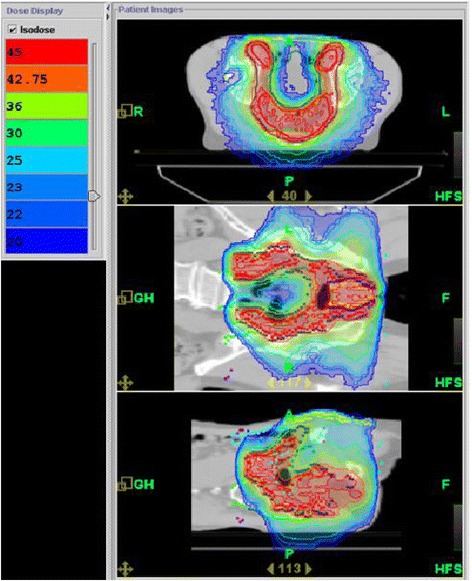
Dose distribution on planning CT with Tomotherapy for the first plan of treatment (45 Gy) for a patient with anal cancer
Table 2.
Dosimetric parameters (N = 64)
| Mean | (SD) | |
|---|---|---|
| Planning Target Volume 1 | ||
| Dose, Gy | 44.04 | (5.60) |
| Conformity Index (opt =1) | 1.00 | (0.39) |
| CO (opt =1) | 0.93 | (0.03) |
| HI (opt = 0) | 0.12 | (0.13) |
| Planning Target Volume 2 | ||
| Dose, Gy | 60.36 | (2.92) |
| Conformity Index (opt =1) | 0.57 | (0.39) |
| CO | 0.96 | (0.03) |
| HI | 0.07 | (0.14) |
SD, standard deviation
Table 3.
Doses to critical organs (N = 64)
| Dose Volume Histogram for organs at risk | Mean | SD | Minimum | Median | Maximum |
|---|---|---|---|---|---|
| Genitalia | |||||
| D98 % | 15.69 | 8.39 | 2.80 | 13.52 | 40.23 |
| D2 % | 40.53 | 12.67 | 18.63 | 37.94 | 66.82 |
| Mean D | 24.76 | 9.82 | 10.48 | 22.05 | 58.10 |
| V40 (%) | 14.92 | 23.06 | 0.05 | 5.58 | 98.27 |
| V40 (cm3) | 4.78 | 5.46 | 0.04 | 3.55 | 23.22 |
| Volume (cm3) | 78.40 | 65.62 | 10.98 | 58.54 | 271.2 |
| Right Femoral Head | |||||
| D98 % | 19.29 | 7.43 | 2.42 | 18.71 | 47.25 |
| D2 % | 39.63 | 4.86 | 27.37 | 40.57 | 50.07 |
| Mean D | 26.90 | 5.07 | 15.68 | 26.47 | 43.60 |
| Volume (cm3) | 112.50 | 50.98 | 32.30 | 122.60 | 209.20 |
| Left Femoral Head | |||||
| D98 % | 20.31 | 6.30 | 4.12 | 20.50 | 40.28 |
| D2 % | 39.96 | 5.02 | 26.93 | 40.70 | 49.36 |
| Mean D | 27.38 | 5.28 | 15.14 | 27.44 | 43.41 |
| Volume (cm3) | 108.00 | 53.02 | 16.80 | 114.40 | 207.40 |
| Bladder | |||||
| D98 % | 19.78 | 5.32 | 10.45 | 19.40 | 33.31 |
| D2 % | 51.56 | 5.90 | 39.20 | 50.67 | 64.83 |
| Mean D | 32.18 | 7.39 | 22.19 | 30.73 | 51.63 |
| V30 (%) | 50.11 | 25.12 | 9.40 | 46.38 | 99.80 |
| V30 (cm3) | 65.68 | 61.91 | 15.33 | 45.02 | 357.70 |
| Volume (cm3) | 141.9 | 132.80 | 45.50 | 94.51 | 635.20 |
| Intestine | |||||
| D98 % | 5.45 | 5.13 | 0.50 | 3.50 | 19.20 |
| D2 % | 45.27 | 9.17 | 3.74 | 45.65 | 57.47 |
| Mean D | 20.64 | 6.63 | 1.54 | 21.69 | 31.03 |
| V30 (%) | 23.50 | 10.45 | 4.06 | 22.17 | 42.86 |
| V30 (cm3) | 219.30 | 123.00 | 41.90 | 187.50 | 580.60 |
| volume (cm3) | 863.20 | 512.00 | 231.00 | 713.50 | 2295.00 |
SD, standard deviation
Clinical outcomes
Sixty-four patients were evaluated at the first follow-up visit: 34 (54.0 %) had complete response and 30 patients had partial response at the first follow-up visit (median interval, 1.6 months; range, 0.4–5.7 months). Eighteen patients who were considered partial responders at first follow-up evaluation were considered complete responders at the following evaluations (up to 14.2 months after the end of treatment). The median interval between end of radiotherapy and end of follow-up was 22.9 months (range, 3.5–52.0 months). Forty-seven patients (73.4 %) experienced a complete response and 17 patients experienced recurrence after a median 5.4 months following the end of radiotherapy (range, 3.2–9.3 months): three patients had distant recurrence, one patient experienced both local and distant recurrence and 13 patients experienced local failure as first recurrence. Among these 13 patients, 11 were considered partial responders and two were considered complete responders at first evaluation, before diagnosis of disease progression. Eleven patients had to undergo salvage surgery consisting of abdomino-perineal resection: among them, seven were in complete remission, two experienced local failure, one experienced distant failure, and one experienced both local and distant failure. Overall, six patients experienced metastatic failure, and for three patients it was the only recurrence. Among the 17 patients who experienced local or distant recurrence, two patients did not receive concomitant chemotherapy and four patients had a modified chemotherapy regimen (eloxatine based regimen for three patients and capecitabine alone for one patient). Nine patients (14.1 %) died during follow-up, at a median 11.5 months (range, 5.4–28.7 months). One-year OS was 88.4 % (95 %CI [75.9 %–94.7 %]) and two-year OS was 85.6 % (95 %CI [71.1 %–93.0 %]) (Fig. 2). OS was significantly better for women than men (p = 0.0017) (Fig. 3). OS was not associated with age, tumour size, nodal status, break in treatment, concomitant chemotherapy or overall treatment time (longer or shorter than 56 days).
Fig. 2.
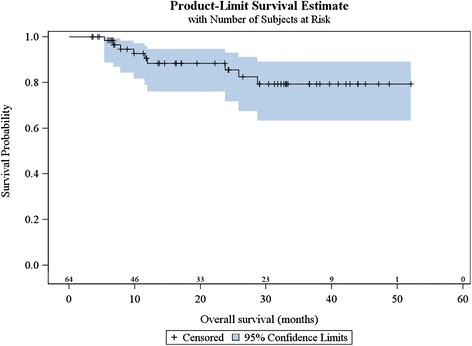
Kaplan Meier estimates of overall survival (n = 64)
Fig. 3.
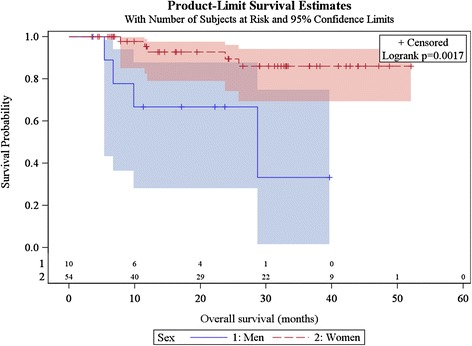
Kaplan Meier estimates of overall survival according to gender (n = 64)
Among the 57 patients without colostomy at inclusion, ten patients (17.5 %) underwent colostomy, at a median of 6.5 months (range, 4.2–13.1 months). One-year CFS was 75.5 % (95 %CI [60.7 %–85.3 %]), and CFS was significantly better for women than for men (p = 0.0036) (Fig. 4). There was a trend for increased median age among men (66 years vs. 62 for women), there was no difference in terms of size of tumours between genders, there was a trend towards more frequent treatment break among men (60 % of men experienced a break in treatment vs. 44 % of women), and 4 men (40 %) did not receive concomitant chemotherapy (vs. 5.6 % of women; p = 0.01).
Fig. 4.
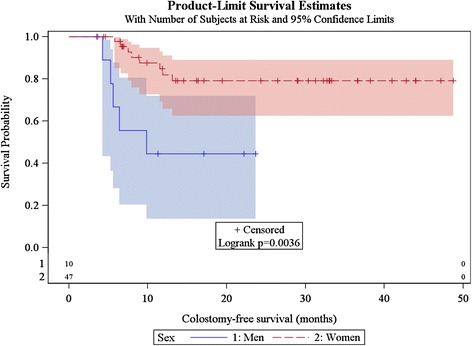
Kaplan Meier estimates of colostomy-free survival according to gender (n = 64)
Seventeen patients (26.6 %) presented progressive disease or recurrence, at a median of 6.8 months (range, 3.2–16.1 months). One-year DFS was 68.7 % (95 %CI [54.4 %–79.4 %]) among the entire population, and women presented a significantly better DFS than men (p = 0.0019) (Fig. 5).
Fig. 5.
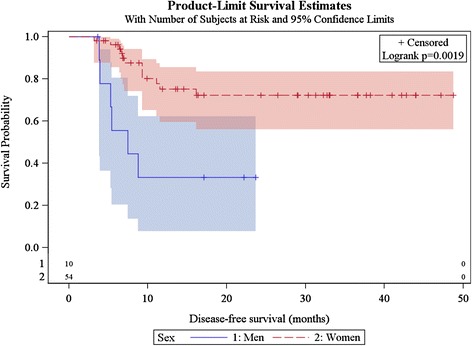
Kaplan Meier estimates of disease-free survival according to gender (n = 64)
Toxicity
Acute toxicities are summarized in Table 4. A Grade ≥3 acute GI toxicity (such as diarrhoea), occurred in 20.3 %, grade ≥3 of acute skin toxicity (dermatitis) in 46.9 % and grade 3 hematologic toxicity in 17.2 % of patients. One patient experienced an acute grade 4 hematologic toxicity. A break in treatment at 45 Gy became necessary for 30 patients and mean duration of the break was 5.9 days (range, 0–23 days). Analysis of the relationship between violation of the OAR dose constraints and grade 3 or higher acute toxicity indicated a positive association between bladder V30 > 20 % and occurrence of an acute grade 3 bladder toxicity (83.3 % vs. 0.0 %; p = 0.008).
Table 4.
Distribution of Acute Toxicity (n = 64)
| Toxicity Category | Toxicity Grade, n (%) | ||||
|---|---|---|---|---|---|
| 0–1 | 2 | 3 | 4 | 5 | |
| Weight loss | 55 (85.9) | 8 (12.5) | 1 (1.6) | 0 (0.0) | 0 (0.0) |
| Nausea / vomiting | 54 (84.4) | 5 (7.8) | 5 (7.8) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 36 (56.3) | 15 (23.4) | 13 (20.3) | 0 (0.0) | 0 (0.0) |
| Anal mucositis | 11 (17.2) | 26 (40.6) | 27 (42.2) | 0 (0.0) | 0 (0.0) |
| Cystitis | 56 (87.5) | 7 (10.9) | 1 (1.6) | 0 (0.0) | 0 (0.0) |
| Dermatitis | 8 (12.5) | 26 (40.6) | 29 (45.3) | 1 (1.6) | 0 (0.0) |
| Haematological toxicity | 47 (73.4) | 6 (9.4) | 10 (15.6) | 1 (1.6) | 0 (0.0) |
All acute and late toxicities were graded according to Common Terminology Criteria for Adverse Events, version 3.0
Discussion
Several studies have demonstrated the dosimetric advantages of IMRT over 3D conformal radiotherapy and some studies suggested a decreased toxicity while achieving at least equivalent outcomes [14, 18–20]. The present analysis was performed in the context of the INCa pilot project on the medical impact of implementing new technologies and resulted in a series of 64 patients treated for anal cancer with TomoTherapy® at three institutions. The two-year OS of 85.6 % in our cohort is similar to recent studies, such as Koerber et al. (87.4 % in the IMRT group) or Vieillot et al. (89 %) [15, 21]. However one-year DFS of 68.7 % could seem low compared to recent results in the literature: it is important to note that our study included locally advanced anal cancers and that two thirds of tumours were T3–T4 tumours, and 61 % were node positive. One could consider that these results are in the expected range given the high rate of locally advanced tumours and nodal involvement. However the question remains about role and type of concomitant chemotherapy since in our study 6 patients among the 11 partial responders had no chemotherapy or an adapted eloxatine based regimen because of comorbidities. Furthermore, the Cisplatin based chemotherapy (standard regimen in France at the time of our study) could also be responsible for this poor DFS since superiority of mitomycine and 5-FU has been demonstrated by the RTOG 98–11 trial [8]. The publication in 2012 of the updated RTOG 98–11 trial results has led to change the recommended chemotherapy regimen in France to mitomycine and 5-FU. An interesting point is the significant association of gender to OS, DFS, and CFS. Such a difference has already been highlighted in other trials: male gender has been observed as a poor prognostic factor in RTOG 98–11 trial and more recently by Koerber et al. [8, 15]. In our study, 10 patients were men; one was on immunosuppressant treatment, suggesting this patient might have a more aggressive form of anal cancer. Median age was higher for men (66 years vs. 62 for women), there was no difference in terms of size of tumours. There were some differences regarding characteristics of treatment that could explain such a survival difference: 4 men (40 %) did not receive concomitant chemotherapy (vs. 5.6 % of women; p = 0.01) and there was a trend towards more frequent treatment break among men (60 % of men experienced a break in treatment vs. 44 % of women).
Concerning toxicity, our results are consistent with results reported by studies involving IMRT [13, 15, 18]. Two grade 4 toxicities were observed: hematologic toxicity for a patient treated with mitomycine-based chemotherapy and dermatitis for another patient. The level of hematologic toxicity we observed was comparable to ACCORD 3 trial and lower than that in RTOG but this toxicity was also in part due to chemotherapy [7, 9, 13, 17]. Severe acute GI toxicity was lower (18 % of patients) compared to the RTOG study (36 % of the patients). We did not encounter a relationship between compliance with the dose constraints and GI toxicity. This could be explained by the heterogeneity of intestinal volumes among patients and the fact that intestinal dose constraints were specified in terms of proportion and not in terms of absolute volume. For instance, in our study, intestinal volumes ranged from 231–2500 cc (mean, 917 cc) depending on the upper level of the CT scan and on whether the colon was also contoured along with the small intestine. The only relationship between following dose constraints and toxicity was found for acute grade 3 bladder toxicity and V30 > 20 %, which is not a usual dose constraint for bladder, but was adopted in order to minimize the dose to the central pelvic zone.
Rates of dermatologic toxicities were high: 46 % of the patients suffered from grade 3 skin toxicity, which seems similar to that reported in other studies except the RTOG 0529 trial [13]. We must take into account the fact that we did not define any dose constraint for the skin, which could be added in the future in order to reduce this toxicity. We could also study the possibility to reduce our set-up margin. However we have to be cautious, because the skin cannot be avoided when nodes are just below it or in the case of anal margin extension, limitation of which could result in failure.
Another crucial point when using TomoTherapy® is the ability to decrease the overall treatment time (OTT) by avoiding treatment breaks. In the study by Franco et al., OTT was 44 days (range 37–55) similar to that in the RTOG 0529 trial (43 days; range 32–59), which could have been reduced by the use of IMRT compared to the RTOG 98–11 trial (49 days; range 4–100) [8, 22, 23]. In our study, OTT was 57 days (range 35–113), which is related to the higher doses and delivery in two plans. Thirty patients experienced a treatment break of a mean duration of 5.9 days. In the two centres where the break was decided only in case of toxicity, this break was necessary for 12 patients (26 % of 46 patients) suggesting that TomoTherapy® could allow treatment without break for 74 % of patients. In the study reported by Franco et al., 9 patients underwent a treatment break (17 %) with a shorter mean duration (3.9 days) whereas the treatment break was necessary for 40 % of patients in RTOG 0529 [22, 23]. Several publications have shown a relationship between OTT and survival for squamous cell carcinomas of the uterine cervix, oropharyngeal cancers, and anal cancer [12, 18, 24, 25]. However, we did not observe any relationship between break vs. no break or OTT being longer or shorter than 56 days and OS, DFS or CF, similarly to the results reported by Dewas et al. [26].
Conclusions
TomoTherapy® in locally advanced anal cancer is feasible. At our three centres of expertise in anal carcinoma, this technique seems to reduce acute GI toxicity; nevertheless, high rates of dermatologic toxicities remain prevalent. The therapeutic efficacy is in the range of expectation and similar to previous techniques taking into account the high rates of locally advanced tumours and nodal involvement in the cohort.
Abbreviations
- MVCT
Megavoltage Computed Tomography
- CTCAE V3
Common Terminology Criteria for Adverse Events version 3.0
- CR
Complete response
- PR
Partial response
- LR
Local recurrence
- OS
Overall survival
- DFS
Disease-free survival
- CFS
Colostomy-free survival
- GI
Gastro-intestinal
- IMRT
Intensity-modulated radiotherapy
- GTV
Gross tumor volume
- CTV
Clinical target volume
- PTV
Planning target volume
- OARs
Organs at risk
- INCa
Institut National du Cancer (National Institute of Cancer)
- DVH
Dose Volume Histograms
- RTOG
Radiation Therapy Oncology Group
- OTT
Overall Treatment Time
Footnotes
J. P. Maire Deceased.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VV conceived and coordinated the study, acquired and analysed the data and drafted the manuscript. BH participated in acquisition of the data, and helped to draft the manuscript. ER participated in acquisition of the data and analysis. JB, SB, AL, and NM participated in the design of the study and acquisition of data. EF and AD performed the statistical analysis. GK, MAM and JPM participated in the design, coordination and revision of the study. All authors read and approved the final manuscript.
Contributor Information
V. Vendrely, Email: veronique.vendrely@chu-bordeaux.fr
B. Henriques de Figueiredo, Email: b.henriques@bordeaux.unicancer.fr
E. Rio, Email: Emmanuel.Rio@ico.unicancer.fr
J. Benech, Email: julie.benech@chu-bordeaux.fr
S. Belhomme, Email: S.Belhomme@bordeaux.unicancer.fr
A. Lisbona, Email: Albert.Lisbona@ico.unicancer.fr
E. Frison, Email: eric.frison@isped.u-bordeaux2.fr
A. Doussau, Email: Adelaide.Doussau@isped.u-bordeaux2.fr
N. Nomikossoff, Email: Natacha.NOMIKOSSOFF@ap-hm.fr
M. A. Mahé, Email: Marc-Andre.Mahe@ico.unicancer.fr
G. Kantor, Email: G.Kantor@bordeaux.unicancer.fr
J. P. Maire, Email: renaud.trouette@chu-bordeaux.fr
References
- 1.Grulich AE, Poynten IM, Machalek DA, Jin F, Templeton DJ, Hillman RJ. The epidemiology of anal cancer. Sex Health. 2012;9:504–508. doi: 10.1071/SH12070. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: The Surveillance, Epidemiology, and End Results experience, 1973–2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Human papillomavirus-associated cancers - United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–261. [PubMed] [Google Scholar]
- 4.Abramowitz L, Jacquard A-C, Jaroud F, Haesebaert J, Siproudhis L, Pradat P, Aynaud O, Leocmach Y, Soubeyrand B, Dachez R, Riethmuller D, Mougin C, Pretet J-L, Denis F. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int J Cancer. 2011;129:433–439. doi: 10.1002/ijc.25671. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, Gillison M, Bruni L, Ronco G, Wentzensen N, Brotherton J, Qiao Y-L, Denny L, Bornstein J, Abramowitz L, Giuliano A, Tommasino M, Monsonego J. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131(9):1969–82. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigro ND, Vaitkevicius VK, Considine BJ. Combined therapy for cancer of the anal canal: a preliminary report. 1974. Diseases of the Colon & Rectum. 1993;36:709–711. doi: 10.1007/BF02238600. [DOI] [PubMed] [Google Scholar]
- 7.James R, Meadows H, Wan S. ACT II: the second UK phase III anal cancer trial. Clin Oncol (R Coll Radiol) 2005;17:364–366. doi: 10.1016/j.clon.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB, Thomas CR, Mayer RJ, Haddock MG, Rich TA, Willett CG. Long-term update of US GI intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–4351. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, Thomas CR, Mayer RJ, Haddock MG, Rich TA, Willett C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 10.Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M, Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 11.Northover J, Glynne-Jones R, Sebag-Montefiore D, James R, Meadows H, Wan S, Jitlal M, Ledermann J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer. 2010;102:1123–1128. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glynne-Jones R, Sebag-Montefiore D, Adams R, McDonald A, Gollins S, James R, Northover JMA, Meadows HM, Jitlal M, UKCCCR Anal Cancer Trial Working Party “Mind the gap--”the impact of variations in the duration of the treatment gap and overall treatment time in the first UK Anal Cancer Trial (ACT I) Int J Radiat Oncol Biol Phys. 2011;81:1488–1494. doi: 10.1016/j.ijrobp.2010.07.1995. [DOI] [PubMed] [Google Scholar]
- 13.Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran H, Willet C. RTOG 0529: A Phase 2 Evaluation of Dose-Painted Intensity Modulated Radiation Therapy in Combination With 5-Fluorouracil and Mitomycin-C for the Reduction of Acute Morbidity in Carcinoma of the Anal Canal. Radiat Oncol Biol. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Servagi-Vernat S, Giraud P, Fenoglietto P, Azria D, Lisbona A, de La Rochefordiere A, Zefkili S, Fau P, Resbeut M, Huger S, Peiffert D, Meyer P, Noël G, Mazurier J, Latorzeff I, Biston MC, Pommier P, Ledu D, Garcia R, Chauvet B, Dudouet P, Belhomme S, Kantor G, Mahé MA. Apport de la RCMI rotationnelle et de la tomothérapie hélicoïdale dans les cancers pelviens : étude dosimétrique prospective sur 51 patients. Cancer Radiother. 2014;18(2):1–8. doi: 10.1016/j.canrad.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Koerber SA, Slynko A, Haefner MF, Krug D, Schoneweg C, Kessel K, Kopp-Schneider A, Herfarth K, Debus J, Sterzing F. Efficacy and toxicity of chemoradiation in patients with anal cancer - a retrospective analysis. Radiat Oncol. 2014;9:1–8. doi: 10.1186/1748-717X-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantor G, Mahé MA, Giraud P, Alapetite C, Durdux C, Fourquet A, Gardner M, Le Prisé E, Maire JP, Richaud P, Vendrely V, Caron J, Dejean C, Lisbona A, Munos C, Zefkili S, Mazal A. Évaluation nationale de la tomothérapie hélicoïdale: description des indications, des contraintes de dose et des seuils de repositionnement. Cancer / Radiother. 2007;11:331–337. doi: 10.1016/j.canrad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Peiffert D, Tournier-Rangeard L, Gérard J-P, Lemanski C, François E, Giovannini M, Cvitkovic F, Mirabel X, Bouché O, Luporsi E, Conroy T, Montoto-Grillot C, Mornex F, Lusinchi A, Hannoun-Lévi J-M, Seitz J-F, Adenis A, Hennequin C, Denis B, Ducreux M. Induction Chemotherapy and Dose Intensification of the Radiation Boost in Locally Advanced Anal Canal Carcinoma: Final Analysis of the Randomized UNICANCER ACCORD 03 Trial. J Clin Oncol. 2012;30:1941–1948. doi: 10.1200/JCO.2011.35.4837. [DOI] [PubMed] [Google Scholar]
- 18.Bazan JG, Hara W, Hsu A, Kunz PA, Ford J, Fisher GA, Welton ML, Shelton A, Kapp DS, Koong AC, Goodman KA, Chang DT. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer. 2011;117:3342–3351. doi: 10.1002/cncr.25901. [DOI] [PubMed] [Google Scholar]
- 19.Clivio A, Fogliata A, Franzetti-Pellanda A, Nicolini G, Vanetti E, Wyttenbach R, Cozzi L. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92(1):1–7. doi: 10.1016/j.radonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Joseph KJ, Syme A, Small C, Warkentin H, Quon H, Ghosh S, Field C, Pervez N, Tankel K, Patel S, Usmani N, Severin D, Nijjar T, Fallone G, Pedersen J. A treatment planning study comparing helical tomotherapy with intensity-modulated radiotherapy for the treatment of anal cancer. Radiother Oncol. 2010;94:60–66. doi: 10.1016/j.radonc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Vieillot S, Fenoglietto P, Lemanski C, Moscardo CL, Gourgou S, Dubois J-B, Aillères N, Azria D. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol. 2012;7:45. doi: 10.1186/1748-717X-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franco P, Mistrangelo M, Arcadipane F, Munoz F, Sciacero P, Spadi R, et al. Intensity-Modulated Radiation Therapy with Simultaneous Integrated Boost Combined with Concurrent Chemotherapy for the Treatment of Anal Cancer Patients: 4-Year Results of a Consecutive Case Series. Cancer Invest. 2015. [DOI] [PubMed]
- 23.Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, Willins JD, Ryan DP, Hong TS. Dose-Painted Intensity-Modulated Radiation Therapy for Anal Cancer: A Multi-Institutional Report of Acute Toxicity and Response to Therapy. Int J Radiat Oncol Biol Phys. 2012;82:153–158. doi: 10.1016/j.ijrobp.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Josef E, Moughan J, Ajani JA, Flam M, Gunderson L, Pollock J, Myerson R, Anne R, Rosenthal SA, Willett C. Impact of Overall Treatment Time on Survival and Local Control in Patients With Anal Cancer: A Pooled Data Analysis of Radiation Therapy Oncology Group Trials 87–04 and 98–11. J Clin Oncol. 2010;28:5061–5066. doi: 10.1200/JCO.2010.29.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantinou EC, Daly W, Fung CY, Willett CG, Kaufman DS, DeLaney TF. Time-dose considerations in the treatment of anal cancer. Radiat Oncol Biol. 1997;39:651–657. doi: 10.1016/S0360-3016(97)00329-5. [DOI] [PubMed] [Google Scholar]
- 26.Dewas CV, Maingon P, Dalban CC, Petitfils AL, Peignaux K, Truc G, Martin E, Khoury CD, Dewas S, hange GC. Does gap-free intensity modulated chemoradiation therapy provide a greater clinical benefit than 3D conformal chemoradiation in patients with anal cancer? Radiat Oncol. 2012;7:1–1. doi: 10.1186/1748-717X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


