Abstract
Aims
In this paper, inflammatory mechanisms that link periodontal diseases to cardiovascular diseases (CVD) are reviewed.
Materials and Methods and Results
This paper is a literature review. Studies in the literature implicate a number of possible mechanisms that could be responsible for increased inflammatory responses in atheromatous lesions due to periodontal infections. These include increased systemic levels of inflammatory mediators stimulated by bacteria and their products at sites distant from the oral cavity, elevated thrombotic and hemostatic markers that promote a prothrombotic state and inflammation, cross-reactive systemic antibodies that promote inflammation and interact with the atheroma, promotion of dyslipidemia with consequent increases in proinflammatory lipid classes and subclasses, and common genetic susceptibility factors present in both disease leading to increased inflammatory responses.
Conclusions
Such mechanisms may be thought to act in concert to increase systemic inflammation in periodontal disease and to promote or exacerbate atherogenesis. However, proof that the increase in systemic inflammation attributable to periodontitis impacts inflammatory responses during atheroma development, thrombotic events, or myocardial infarction or stroke is lacking.
Keywords: Periodontitis, Cardiovascular Diseases, Atherosclerosis, Inflammation
1. Introduction: Atherosclerosis and periodontitis as inflammatory diseases
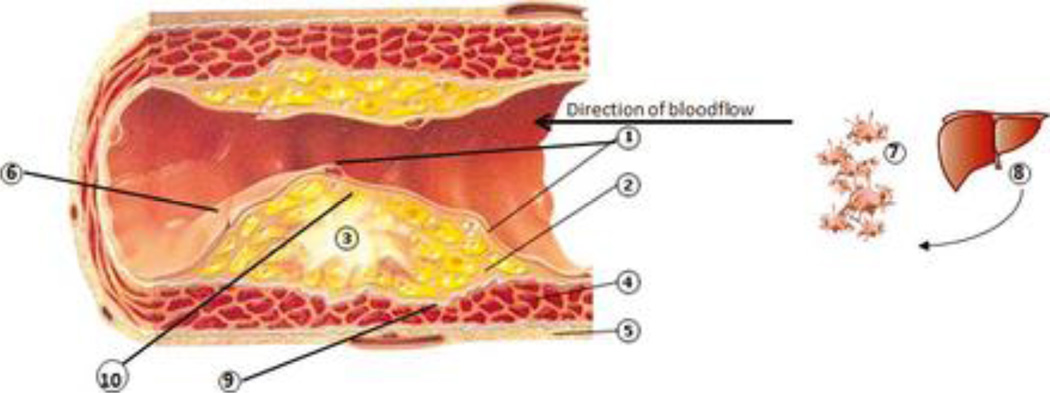
Fundamentals of inflammation in atherogenesis and atherosclerosis (Figure 1)
Figure 1. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases.
1. Dysfunctional endothelial lining: partial loss of integrity. ↑ adhesion molecules (ICAM-1, VCAM-1, E-selectin, P-selectin) and chemoattractants (e.g. IL-8, thrombin), increased platelet and leukocyte adhesion; diapedesis of monocytes, dendritic cells (both possibly with ingested bacteria) and T cells into the underlying inflammatory lesion. Activated platelets may form mini-thrombi. 2. Inflammatory lesion which may be in part initiated and/or propagated by bacteria originating from the periodontitis lesion, and also propagated by pro-inflammatory mediators (IL-1, IL-6, CRP, TNFα) and chemotactic factors (e.g. monocyte chemotactic protein-1) spilled over from the periodontal lesion, produced both in the liver and systemically. 3. Atheroma maturation. Lipid streaks and calcifications, comprised of modified low-density lipoproteins phagocytosed within macrophages/foam cells, resulting in ↑ pro-inflammatory cytokines (IL-6, IL-1, TNFa), ↑ chemoattractants (IL-8), ↑ matrix metalloproteinases (MMPs); the upregulated inflammatory lesion induces dysfunctional endothelial lining. CD4+ Th cells (↑IL-12,IL-18, IFN-γ) are also within the atheroma. 4. Smooth muscle cells (SMCs) and fibroblast with progressive fibrosis and loss of demarcation between inflammatory lesion and SMCs; development of a compensatory blood supply and increased outer muscle layer. 2 + 3 + 4 form the initimal layer of the artery. 5. Outer muscle layer. 6. Disintegrated endothelial lining (i.e. plaque rupture, exposition of underlying atherosclerotic plaque) generates thrombin from prothrombin, which in turn enzymatically generates fibrin from fibrinogen, →clotting cascade resulting in thrombosis, consequently to stroke or myocardial infarction. This process may be mediated by repeated bacteremias and pro-inflammatory state and activated inflammatory cells, such as from chronic periodontitis. 7. Activated platelets by bacteria in circulation from periodontal lesions, forming aggregates, the initiation of micro thrombus formation (out of scale with the whole picture). 8. ↑production of clotting factors in the liver due to inflammatory signals (IL-6), →pro-thrombotic state. Also increased production of acute phase reactants, including CRP, which will increase the pro-inflammatory state (out of scale with the whole picture). 9. Decreased collagen production and activation of MMPs resulting in reduction of SMC content and increased degradation of collagen that borders the fibrous cap, weakening the strength of the vessel, leading to fissuring of the atheroma. 10. In a progressed stage, the atheroma comprises of a large necrotic core which is exposed to the vasculature within the lesion, leading to contact with platelets, initiation of coagulation and ultimately, plaque rupture in so-called vulnerable lesions.
It is now widely accepted that a major component of pathology in cardiovascular disease (CVD) and particularly in atherosclerosis, involves multiple components of the innate and adaptive immune systems leading to an inflammatory response within the atheromatous lesion (Libby et al., 2009). Links between periodontitis and atherosclerosis would be predicted based upon inflammatory mechanisms initiated by bacteria associated with periodontal lesions, locally or systemically, that then influence the initiation or propagation of the atherosclerotic lesion. Such lesions may be initiated by inflammatory stimuli including systemic and locally produced inflammatory cytokines and chemotactic agents that cause changes in the endothelium such as upregulation of adhesion molecules. These changes promote interactions with leukocytes, such as monocytes, that promote leukocyte migration into the intimal layer of the artery. Lipid streaks, comprised of modified low-density lipoproteins (LDL) within macrophages and dendritic cells in the intimal layer, can initiate and propagate this inflammatory response. Upregulation of the endothelium additionally leads to release of chemotactic cytokines such as monocyte chemotactic protein-1 (MCP-1) that further attract monocytes or other cells that can transport bacteria into the lesion. Thus resident dendritic cells in susceptible locations in the vasculature (Cybulsky and Jongstra-Bilen, 2010), and monocytes attracted by chemotactic cytokines, become foam cells following ingestion of modified LDL. These cells release inflammatory cytokines, chemoattractants and matrix metalloproteinases (MMPs) which further enhance the inflammatory response in the lesion. CD4+T cells are components of this lesion, comprised of predominantly Th1 cells which amplify the inflammatory process by producing, amongst other mediators, INF-γ (Libby et al., 2009). Thus, initiation and propagation of early atherosclerotic lesions would theoretically be enhanced in periodontitis patients if periodontal microorganisms, or their effects on the host response such as initiating or propagating T cell responses, contributed to endothelial dysfunction, modification of LDL, attraction and maturation of monocytes, enhanced uptake of lipids, or attraction and promotion of Th1 T cell subsets (Andersson et al., 2010).
Maturation of the atherosclerotic lesion entails migration of smooth muscle cells (SMCs) into the intima, with progressive fibrosis. MMPs and other proteases promote SMC migration by degradation of the extracellular matrix. This permits proliferation of SMC and deposition of collagen and other proteins in the intimal layer. Thus the mature atheroma is characterized by fibrosis and calcification (Raines and Ferri, 2005). Additionally, fibrosis in the atheroma may be enhanced by endothelial-mesenchymal transition stimulated by TGF-β, the production of which is promoted by inflammation (Kumarswamy et al., 2012). Such maturing lesions express increased levels of Th1 cytokines, including interleukin (IL)-12 and IL-18, which together further promote INF-γ production and additional inflammation as well as further upregulation and dysfunction of endothelial cells, chemokine production (e.g. IL-8), cytokine production (e.g. IL-6) and MMP release.
Subsequent maturation of the atheroma, with development of a compensatory blood supply within the lesion, leads to additional activation and proliferation of inflammatory cells with inflammatory mediator release and generation of thrombin. Injury to the vasculature generates thrombin from prothrombin, which in turn enzymatically generates fibrin from fibrinogen, setting in motion the clotting cascade. Thrombin also interacts with receptors on a wide variety of cells to create a proinflammatory environment, with enhancement of smooth muscle cell and fibroblast proliferation, platelet activation, interactions with cells of the innate and adaptive immune systems including monocytes, dendritic cells, and lymphocytes, generation of mediators from endothelial cells, upregulation of endothelial cell adhesion molecules such as ICAM-1, VCAM-1, E-selectin, and P-selectin, and is chemotactic for monocytes and vascular smooth muscle cells. Thrombin generation is also associated with plaque rupture (Libby et al., 2009).
Plaque rupture, potentially leading to stroke or myocardial infarction, appears to be mediated by inflammatory cells (Imanishi and Akasaka, 2012). Decreased collagen production and reduction of smooth muscle cell content of the atheromatous lesion and increased degradation of collagen that comprises the fibrous cap by MMPs weaken the lesion, leading to fissuring of the atheroma. At this stage, the atheroma comprises a large necrotic core which is exposed to the vasculature within the lesion, leading to contact with platelets, initiation of coagulation and ultimately, plaque rupture in so-called vulnerable lesions. Thus, the more advanced stages of atherogenesis could be impacted by periodontal inflammation and infection via effects on SMC migration, promotion of Th1 responses, thrombin generation, and effects on collagen production and degradation. These processes could then promote rupture of the lesions and thrombus formation.
Rationale for mechanistic links
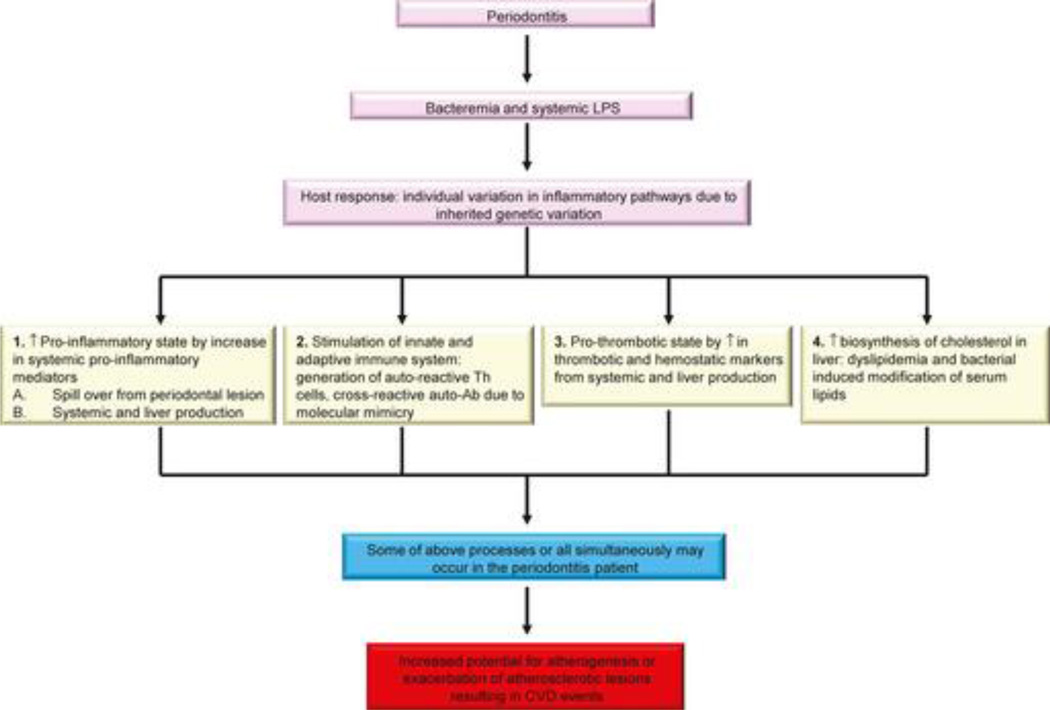
Epidemiologic links between periodontitis and CVD indicate that there is an association between these two conditions (Lockhart et al., 2012). The proposed mechanistic links are summarized in Figure 2. In this Figure we propose that the host response to bacteremias may differ between patients with periodontitis due to individual variation in inflammatory pathways. It is conceivable that inherited genetic variation could enhance these potential mechanisms explaining the links between periodontitis and CVD.
Figure 2. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases.
Schematic overview of potential inflammatory mechanisms linking periodontitis to cardiovascular diseases.
Since both periodontitis and CVD are known to be inflammatory conditions, it has been proposed that inflammation due to periodontal microorganisms, the known etiological agents of periodontitis, accounts for the contribution of periodontitis to increased CVD risk and severity.
We and others propose that the link between inflammation due to periodontal microbial pathogens and inflammatory responses that impact CVD may be manifested in several ways.
- A number of inflammatory mediators and markers are present in higher concentrations in the systemic circulation of patients with periodontitis than in periodontal healthy individuals. There are hypothetically two pathway by which this could occur:
- There are ample data indicating that inflammatory cytokines and other mediators are produced in the periodontal lesion (Preshaw and Taylor, 2011). It has been hypothesized that these mediators could “spill over” into the circulation. If this does occur, and the mediators achieve sufficient concentrations with preservation of bioactivity, they would then impact tissues and organs distant from the oral cavity. In particular, these mediators from the periodontium could affect other organs, such as the liver, to initiate an acute phase response that would impact other organs. This would lead to inflammatory changes in the endothelium such as upregulation of adhesion molecules and promotion of cytokine production, and thus initiation or acceleration of atheroma development. It should be noted that there is not strong evidence supporting this mechanism for inflammatory cytokines and other mediators accessing the circulation (Teles and Wang, 2011).
- It is well known that periodontitis patients have frequent bacteremic episodes and that detectable concentrations of LPS are frequently found in the circulation. In addition, animal models of infection utilizing periodontal disease pathogens such as Porphyromonas gingivalis indicate that oral or systemic infection can promote inflammatory responses in sites distant from the oral cavity, such as in the atheroma (Gibson and Genco, 2007, Gibson et al., 2006, Hayashi et al., 2010). Thus bacteria, or their proinflammatory components, may stimulate systemic inflammatory responses as well as local inflammatory responses in atheromatous lesions (Teles and Wang, 2011). This would follow their association with or modification of serum lipids, engagement of receptors on inflammatory cells and endothelium, invasion of endothelial cells, or seeding of atheromatous lesions with bacteria or bacterial components. Bacteria or their products could then promote inflammatory changes that would contribute to the development of atheromatous lesions.
Several antibodies that may impact pathogenic inflammatory responses in atherosclerosis have been identified. Several of these antibodies are examples of “molecular mimicry” wherein cross-reactive antibodies induced by periodontal pathogens recognize host antigens and modulate their function. In some cases, these antibodies increase the risk for or accelerate atherosclerosis by enhancing endothelial inflammation, promoting uptake of lipids into macrophages, or blocking anti-atherogenic effects of protective molecules.
Several studies indicate that serum concentrations of potentially inflammatory lipids, including LDLs, triglycerides (TGs), and very low density lipoproteins (vLDLs) are elevated in periodontitis patients. These lipid subforms may more easily enter the blood vessel wall, may be more susceptible to modification and therefore more likely to be incorporated in to the atherosclerotic lesion. This would accelerate development of the local lesions and promote the maturation of the lesions.
Some or all of these mechanisms together may be operant in individual patients with their summative effects impacting on cardiovascular inflammation.
A summary of these hypothesized mechanisms is presented in Figure 2, emphasizing that some or all of them may be ongoing within periodontitis patients at any given time. What follows below in Section 2–6 are summaries of studies that lend credence to these potential mechanisms, with emphasis on clinical studies that support, refute, or illustrate the potential for these mechanisms to occur. We discuss:
systemic biomarkers and inflammatory mediators noted to have particular relevance to the pathology of atherosclerosis
relevant thrombotic and hemostatic markers with known links to inflammatory processes
antibodies of relevance to atherogenesis that can be induced by oral microorganisms and promote inflammation in the vasculature and the atheroma
serum lipids whose levels and potential modification by oral infection may influence atherogenesis, and
genetic markers that may explain individual variation in the inflammatory response in both periodontal infection and atherosclerosis
2. Increased systemic mediators of inflammation
A large number of studies demonstrate that there are increased circulating levels of inflammatory mediators in patients with periodontal diseases compared to healthy controls. Elevated levels of many of these mediators are statistically associated with increased cardiovascular risk and are therefore thought to be potential mechanistic links between periodontal infection and CVD, either as disease markers or as participants in inflammatory responses in endothelial tissue and atheromatous lesions. A summary of the studies discussed below can be found in Table 1.
Table 1.
Clinical studies suggesting the role of biomarkers and increased systemic mediators of inflammation in periodontitis as a link to inflammation in CVD
| Inflammatory Mediator or marker |
Association(s) with CVD | References |
|---|---|---|
| C-reactive protein | Serum levels increased in chronic periodontitis | Ebersole et al., 1997; Loos et al., 2000; Slade et al., 2000; Noack et al., 2001; Offenbacher, 2002; Ebersole et al., 2002; Buhlin et al., 2003; Beck and Slade et al., 2003; Amar et al., 2003; Yoshii et al., 2009; Gomes-Filho et al., 2011; Pejcic et al., 2011 |
| Serum levels increased in aggressive periodontitis | Salzberg et al., 2006; Sun et al., 2009 | |
| Serum levels increased in patients with CVD and chronic periodontitis compared to either condition alone | Glurich et al., 2002; Persson et al., 2005; Amabile et al., 2008; Malali et al., 2010; Liu et al., 2010 | |
| Serum levels decrease along with improvement in surrogate measures of cardiovascular health (brachial artery flow-mediated dilation (FMD), hypertension, Framingham Risk Score) following therapy | Seinost et al., 2005; D'Aiuto et al., 2006; Higashi et al., 2008; Higashi et al., 2009 | |
| C-reactive protein, fibrinogen, interleukin-6, and other markers | Serum levels decreased following therapy | Iwamoto et al., 2003; D'Aiuto et al., 2004; Montebugnoli et al., 2005; Taylor et al., 2006; Hussain Bokhari et al., 2009; Vidal et al., 2009; Nakajima et al., 2010 |
| Transient increase in serum levels shortly after therapy (24h) and impairment of FMD | Tonetti et al., 2007 | |
| Serum levels unchanged following therapy | Ide et al., 2003; Yamazaki et al., 2005 | |
| Interleukin-6 | Serum levels increased in chronic periodontitis | Buhlin et al., 2009 |
| Haptoglobin | ||
| Fibrinogen | ||
| Interleukin-18 | Serum levels decreased in chronic periodontitis | |
| Interleukin-4 | ||
| Serum amyloid A | Serum levels increased in patients with CVD and chronic periodontitis compared to periodontitis alone | Glurich et al., 2002 |
| Alpha 1 anti-chymotrypsin | ||
| Matrix metalloproteinase (MMP)-9 | Serum levels decreased following therapy; high levels associated with changes in carotid IMT in chronic periodontitis | Behle et al., 2009; Soder et al., 2009 |
| Platelet activating factor (PAF) and PAF-acetylhydrolase (AH) | PAF levels elevated in serum and gingiva, PAF-AH levels decrease following therapy | Noguchi et al., 1989; Losche et al., 2005; Zheng et al., 2006; Chen et al., 2010 |
C-reactive protein
Of particular interest, and worth extra comment, is C-reactive protein (CRP). CRP is an acute phase reactant that is mainly produced in the liver in response to a variety of inflammatory cytokines such as IL-6. It therefore serves as a marker for systemic inflammation in a variety of conditions (Abd et al., 2011). Pertinent to periodontal diseases and their putative impact on CVD, serum CRP concentration has been proposed to be a risk marker for CVD and its serum levels are elevated in patients with periodontitis. However, the validity of serum CRP measurements as a risk predictor for atherosclerosis, and even its pathologic role in the development or progression of disease, is controversial (Anand and Yusuf, 2010).
CRP, originally discovered due to its ability to bind to phosphorylcholine on pneumococcal C-polysaccharide, can bind to modified LDL, vLDL, and the lipid mediator platelet-activating factor (PAF). CRP activates the complement system and is present in atheromas. Thus, it possesses properties implicating it as playing a direct role in the inflammatory responses attendant to atheroma formation. However, proof of a definitive role for CRP in the pathogenesis of atherosclerosis appears to be lacking (Ridker, 2009).
The interpretation of data suggesting that elevated CRP levels in periodontitis is a link between periodontal inflammation and atherosclerosis depends upon acceptance of the concept that CRP itself is pathologic or that it influences downstream pathology impacting the atherosclerotic lesion. Alternatively, it may merely be a marker for systemic inflammation whose magnitude is modestly impacted by periodontal inflammation. Analysis of this controversial area is beyond the scope of this paper, but arguments and evidence on either side of this issue are summarized in recent companion review articles (Anand and Yusuf, 2010, Bisoendial et al., 2010)
a. CRP, IL-6 and other acute-phase reactants and inflammatory mediators in periodontitis
Levels of inflammatory mediators in periodontitis
There is ample evidence that serum CRP and other acute phase reactant and inflammatory cytokine concentrations are higher in otherwise healthy individuals with chronic and aggressive periodontitis than in periodontally healthy controls. Acute-phase reactants such as CRP and haptoglobin are elevated in chronic and aggressive periodontitis patients compared to healthy controls based on early studies (Ebersole et al., 2002, Ebersole et al., 1997), and later studies confirmed these findings (Amar et al., 2003, Yoshii et al., 2009). Further studies have provided evidence for elevated systemic levels of additional proinflammatory mediators and markers in chronic periodontitis including fibrinogen, haptoglobin, and IL-18, as well as decreased levels of anti-inflammatory mediators including IL-4 (Buhlin et al., 2009). Furthermore, CRP levels may be elevated in sera from patients with aggressive periodontitis based on 2 studies (Salzberg et al., 2006, Sun et al., 2009).
Since CVD and periodontitis share some common risk factors such as smoking, investigators have attempted to account for these covariates in further analyses of the these associations. Most studies observed that associations of serum levels of key mediators such as CRP and IL-6 with periodontitis remained significant following statistical correction for these factors. (Loos et al., 2000, Gomes-Filho et al., 2011, Pejcic et al., 2011, Noack et al., 2001, Buhlin et al., 2003). Analysis of NHANES-3 data indicated that CRP is elevated in the US population in individuals with periodontal disease and in edentulous subjects after accounting for other explanatory variables for elevated CRP (Slade et al., 2000). Further, in a series of analyses of the Atherosclerosis Risk in Communities (ARIC) study, the association of CRP with periodontal measures such as pocket depth was observed following correction for a variety of CVD risk factors (Beck and Offenbacher, 2002, Slade et al., 2003).
Levels of mediators in CVD patients with periodontitis
Since the observation that acute-phase reactants are elevated in periodontitis suggested a possible role for oral inflammation in the pathology of CVD, studies have been carried out that compared populations of patients with or without both disease entities. Most found that the levels of mediators such as CRP found in individuals with both CVD and periodontitis were additive relative to levels founds in patients with either condition (Glurich et al., 2002, Malali et al., 2010, Persson et al., 2005). For example, Glurich (Glurich et al., 2002), reporting data from the Erie County Periodontal Epidemiology Study, noted a hierarchy in levels of CRP, serum amyloid A, and alpha 1-antichymotrypsin wherein the highest levels were found in patients with both periodontitis and CVD compared to either condition alone. Other markers such as ceruloplasmin and sVCAM-1 were elevated only in CVD while some including sICAM-1 were not elevated. Similar observations have since been made in other populations, including a Chinese population with relatively low CRP concentrations (Liu et al., 2010).
Impact of periodontal therapy on systemic inflammatory mediators
Periodontal therapy has been shown in several studies to decrease levels of some inflammatory and acute-phase markers, further implicating the periodontium as a source of systemic inflammatory mediators. Most such studies employed conservative therapies such as scaling, root planing and antibiotic treatment to show decreases in mediators such as CRP, TNF-α, and IL-6 (Iwamoto et al., 2003, D'Aiuto et al., 2004, Montebugnoli et al., 2005). In a study in which patients with advanced periodontitis were treated by full-mouth extraction, it was noted that there was a significant decrease in CRP as well as plasminogen-activator inhibitor-1, fibrinogen, and WBC counts within 12 weeks following treatment (Taylor et al., 2006). Subsequent treatment studies examining the impact of periodontal therapy on periodontitis patients with concurrent CVD noted similar reductions in key mediators of systemic inflammation (Hussain Bokhari et al., 2009, Vidal et al., 2009, Nakajima et al., 2010).
It is noteworthy that some studies failed to show changes in acute phase reactants following periodontal therapy. Ide (Ide et al., 2003) observed that conservative periodontal therapy, including scaling and root planing, failed to alter serum levels of CRP, fibrinogen, or inflammatory cytokines 6 weeks following treatment. In a study of Japanese subjects, it was observed that CRP and IL-6 levels at baseline were lower than had been previously reported in other populations, and that treatment did not significantly alter serum levels of these markers (Yamazaki et al., 2005). These studies may indicate that specific populations behave differently with respect to susceptibility to inflammatory stimulants or response to therapy, and that the relationships between periodontitis and CVD may not be uniform or universal.
Meta-analyses of CRP levels in periodontitis
A meta-analysis published in 2006 by Ioannidou (Ioannidou et al., 2006) failed to find a significant decrease in serum CRP levels due to scaling and root planing. A later systematic review which examined CRP levels in periodontitis and the impact of periodontal therapy on CRP levels was published in 2008 (Paraskevas et al., 2008). This analysis included studies utilizing the hsCRP assay in which the full range of serum CRP values can be evaluated, and which has been proposed to identify patients at risk for CVD (Ridker and Silvertown, 2008). This review of cross-sectional case-control studies and of periodontal treatment studies indicated that there is ample evidence in the literature to conclude that CRP serum concentrations are elevated in individuals with periodontitis compared to control subjects, while there is a modest but statistically significant impact of therapy on CRP levels. Further, in a comparison of standard treatment of scaling and root planing compared with intensive treatment in which antibiotic usage and an accelerated time-frame for therapy was included, there was no difference in result. Significantly, mean hsCRP levels in untreated patients with periodontitis exceeded the 3 mg/L threshold proposed as a cutoff for determination of elevated risk for CVD in otherwise healthy individuals.
Studies incorporating measures of cardiovascular outcome
Several studies examined outcomes related to cardiovascular health in tandem with measures of CRP and other inflammatory markers. In a study of the impact of periodontal therapy on endothelial dysfunction, as assessed by brachial artery flow-mediated dilation (FMD) (Seinost et al., 2005), improvement in FMD and a concomitant significant decrease in serum CRP levels were noted. In a trial comparing the impact of routine scaling and root planing with similar therapy that included local delivery antibiotics (D'Aiuto et al., 2006) it was observed that therapy that included antimicrobials resulted in enhanced reduction of CRP and IL-6 as well as enhanced clinical outcome in terms of decrease in blood pressure and Framingham Risk Score. Similarly, Highashi (Higashi et al., 2009, Higashi et al., 2008) demonstrated improved endothelial function following periodontal therapy, with significantly decreased levels of CRP and IL-6, in patients with periodontitis. Interestingly, in a study that assessed serum inflammatory markers and FMD in patients following periodontal therapy, a significant increase in CRP, IL-6, sE-selectin and von Willebrand factor concentrations and impairment of FMD within 24 hours after treatment was observed, noting that these effects were transient upregulatory alterations of systemic inflammation (Tonetti et al., 2007).
Animal studies of inflammatory mediators
A limited number of animal studies have assessed inflammatory serum markers in models of CVD. In non-human primates, it has been demonstrated that induction and progression of ligature-induced periodontitis results in elevated acute-phase proteins CRP and fibrinogen, which was reversed following treatment (Ebersole et al., 2002). Administration of Aggregatibacter actinomycetemcomitans or its LPS to ApoE−/− mice resulted in increased CRP as well increased small dense low density lipoprotein (LDL) and matrix metalloproteinase-9 (MMP-9) expression in the aorta (Tuomainen et al., 2008). Similarly, Zhang (Zhang et al., 2010) reported both elevation of the serum markers IL-6, IL-8, TNF-a, and MCP-1, as well as increased size of atherosclerotic plaques, in ApoE−/− mice infused with A. actinomycetemcomitans. A recent study sought to examine the impact of bacteremia with Porphyromonas gingivalis on CVD by analyzing mechanisms of inflammation within the myocardium (Akamatsu et al., 2011). It was observed that infusion of P. gingivalis into mice induced myocardial infarction or myocarditis. They further found that no inflammation was observed in mice genetically deficient in IL-17A suggesting a role for Th17 associated inflammatory pathways in P. gingivalis-induced cardiovascular inflammation.
b. Other markers: MMPs and PAF
MMPs are thought to play a key role in both periodontal destruction (Page, 1998) and in CVD due to their association with rupture of the atherosclerotic plaque, and can be induced by oral bacterial products (Hajishengallis et al., 2002). It has been proposed that P. gingivalis proteases (gingipains) can both stimulate MMP production and process latent MMPs to become activated (Imamura et al., 2003). Thus, there is a hypothetical link between periodontitis and CVD through this pathway.
There are far fewer clinical studies implicating MMPs in the inflammatory link between periodontitis and CVD than for other mediators. Decreased serum MMP-9 levels in patients shortly following initiation of periodontal treatment has been noted (Behle et al., 2009), and associations between high MMP-9 and tissue inhibitor of metalloptroteinase-1 (TIMP-1) concentrations in periodontitis patients and changes in carotid IMT measures have been observed (Soder et al., 2009). Animal model studies of MMPs in this regard, which have examined both local and systemic associations between infection, MMPs, and atherogenesis, have given mixed results. For example, in a model of atherosclerosis induction by intravenous injection of A. actinomycetemcomitans in ApoE−/− mice (Tuomainen et al., 2008), it was observed that there was increased atherogenesis accompanied by increased expression of aortic MMP-9, increased serum gelatinase activity, and decreased serum levels of pro-MMP-9 compared to uninfected control animals. However, it was observed in a model of P. gingivalis-induced bone loss following oral infection that particle size of HDL and VLDL are increased in MMP-8 deficient mice, implying that MMP-8 might play a protective, anti-inflammatory role with respect to systemic lipid profiles in P. gingivalis infection (Kuula et al., 2009). These limited data to date, utilizing differing modes of infection, fail to implicate MMPs as an important inflammatory factor linking periodontal infection and CVD.
Similarly, only a limited number of studies provide data supporting a hypothesis that PAF and PAF-acetyl hydrolase (PAF-AH), both associated with cardiovascular risk, are possible inflammatory factors linking periodontitis and CVD. PAF is known to be present at high levels in gingival tissue and GCF (Noguchi et al., 1989), and the PAF receptor is known to be a portal of entry for invasion of endothelial cells by certain bacteria including A. actinomycetemcomitans (Schenkein et al., 2000). It was observed (Losche et al., 2005) that serum concentrations of PAF-AH correlated with bleeding on probing, pocket depth, and attachment level in periodontitis patients and that treatment reduced levels significantly. The authors suggested that these data, and those indicating that these mediators have been shown to be independent risk markers of CVD, indicate their importance in mediating periodontal effects on systemic disease. It has been further reported (Zheng et al., 2006) that sera from periodontitis patients contained significantly greater concentrations of PAF than samples from gingivitis or healthy control subjects. Furthermore, Chen (Chen et al., 2010) reported that serum PAF levels are elevated to the same extent in patients with periodontitis and with CVD.
Thus, although these inflammatory factors are known to be important in atherogenesis and outcomes such as stroke and MI, there are insufficient data at this point to implicate them in the link between periodontitis and CVD.
3. Thrombotic and hemostatic markers influencing inflammation
The coagulation and fibrinolytic systems are intimately associated with vascular inflammation and play an important role in atherogenesis and thrombosis (Davalos and Akassoglou, 2012, Popovic et al., 2012). A number of hemostatic factors are associated with development of atherosclerosis including fibrinogen, von Willebrand factor, tissue plasminogen activator (tPA), plasminogen activator inhibitor-1 (PAI-1), and factors VII and VIII.
Elevated fibrinogen is an indicator of systemic inflammation and is a risk marker for atherosclerosis, and results in increased blood viscosity and thus shear stress which can promote endothelial cell activation and platelet aggregation. Fibrinogen can interact with cellular integrin receptors CD11b/CD18 and CD11c/CD18 to stimulate production of proinflammatory cytokines or through TLR4 to induce MCP-1, MIP-1α and β, IL-6, IL-8, TNF-α, MMP-1, and MMP-9. Fibrinogen and its degradation products can be localized to atheromas as a structural component of the lesion where it, and its degradation products, can induce inflammatory cytokine production as well as promote platelet aggregation (Davalos and Akassoglou, 2012).
The association of periodontitis with hemostatic factors has been reported by a number of investigators. An early report indicated that patients with periodontitis have higher plasma fibrinogen levels and white blood cell counts than age-matched controls, and suggested a link to myocardial infarction (Kweider et al., 1993). Subsequent studies likewise noted increased fibrinogen levels in periodontitis (Sahingur et al., 2003) including a report that there is an association between the number of periodontal pockets and fibrinogen levels, even after correction for a number of covariates associated with CVD risk and systemic inflammation (Schwahn et al., 2004). Subjects with >15 pockets had significantly elevated fibrinogen while, interestingly, edentulous patients did not demonstrate elevated levels. Full-mouth tooth extraction in patients with advanced periodontitis was reported to result in significant decreases in hemostatic factors including PAI-1 and fibrinogen (Taylor et al., 2006). In a cohort of patients with coronary artery disease (CAD), those with periodontitis had higher levels of fibrinogen as well as CRP and serum amyloid A than patients without CAD (Amabile et al., 2008). Elevated levels of fibrinogen were observed in patients with severe periodontitis (Buhlin et al., 2009) while decreased fibrinogen as well as IL-6 and CRP concentrations were found following periodontal therapy in patients with refractory hypertension (Vidal et al., 2009). Similarly, a decrease in plasma fibrinogen levels following non-surgical periodontal therapy in subjects with or without CVD has been observed (Hussain Bokhari et al., 2009). Alexander (Alexander et al., 2011) reported that gamma fibrinogen, an isoform of fibrinogen that may be associated with CVD, correlates with both CRP and the extent of gingival inflammation. Thus, clinical studies consistently show that fibrinogen levels are elevated in periodontitis patients, even those with CVD, and are decreased following periodontal therapy.
Other thrombotic and hemostatic factors have also been implicated in the link between periodontitis and CVD. PAI-1 is a protease inhibitor that decreases fibrinolysis by inhibiting tPA (tissue plasminogen activator) and uPA (urokinase). These properties of PAI-1 are associated with increased risk for atherosclerosis. In a study that examined a variety of risk factors in periodontitis patients with CVD, significant but weak associations between periodontal indices and von Willebrand factor and PAI-1 levels were reported, but a follow-up study of the impact of scaling and root planing failed to note significant changes in levels of hemostatic factors (Montebugnoli 2005). It was speculated that this result may be due to the preexisting CVD status of the subjects in this study and difficulty in modifying these factors in such patients. Bizzarro (Bizzarro et al., 2007) measured a series of thrombotic markers in periodontitis patients including PAI-1, vWF, prothrombin cleavage fragments, and D-dimer. They found that PAI-1 was elevated in patients with advanced periodontitis. Interestingly, a study by Bretz (Bretz et al., 2005) in an elderly population failed to demonstrate an association between PAI-1 levels and periodontitis despite significant increases in CRP, IL-6 and TNF-a. The importance of these factors as links between periodontitis and CVD therefore remains an open question.
Platelets contribute to atheroma formation and thrombosis due to their aggregation, proinflammatory mediator release upon activation, and their binding to thrombi at advanced stages of atheroma development and breakdown. Papapanagiotou (Papapanagiotou et al., 2009) examined platelet activation in periodontitis patients, by first measuring plasma concentrations of sP-selectin (sCD62P) and sCD40 ligand and then examining platelet-bound P-selectin and expression of activated glycoprotein IIb/IIIa. After adjustment for confounders, sP-selectin was significantly increased in periodontitis patients. Furthermore, the percentage of platelets expressing activated glycoprotein IIb/IIIa and the density of receptor expression, both indicating platelet activation, was elevated in periodontitis patients compared to controls and correlated with the proportion of teeth with >50% bone loss Increased surface P-selectin on platelets from aggressive periodontitis patients as well as elevated CD18 on phagocytes resulting in increased aggregates of platelets with monocytes and PMN’s has also been reported (Fredman et al., 2011). These phenomena were reversed in the presence of Resolvin E1 implicating an impairment of inflammation resolution in these patients and increased susceptibility to systemic inflammation (Fredman and Serhan, 2011). Few corroborating animal studies relating thrombotic and hemostatic markers to periodontitis have been carried out. Ebersole reported an increase in plasma fibrinogen levels in experimental periodontitis in subhuman primates (Ebersole et al., 2002). Thus, there is some evidence for in vivo systemic platelet activation in periodontitis patients, but direct links to CVD risk due to periodontitis have not been studied.
A summary of clinical studies of thrombotic and hemostatic markers in periodontitis can be found in Table 2.
Table 2.
Clinical studies suggesting a role of thrombotic and hemostatic mediators and markers in periodontitis as a link to inflammation in CVD
| Thrombotic or hemostatic marker or mediator |
Association(s) with CVD | References |
|---|---|---|
| Plasminogen-activator inhibitor (PAI)-1 | Serum levels in patients with advanced periodontitis decrease following full-mouth extraction | Taylor et al., 2006 |
| Serum levels increased in chronic periodontitis | Bizzarro et al., 2007 | |
| No association of serum levels with periodontitis | Bretz et al., 2005 | |
| Fibrinogen | Serum levels increased in chronic periodontitis | Kweider et al., 1993; Sahingur et al., 2003; Schwahn et al., 2004; Buhlin et al., 2009 |
| Serum levels in patients with advanced periodontitis decrease following full-mouth extraction | Taylor et al., 2006 | |
| Serum levels decreased following periodontal therapy | Vidal et al., 2009 | |
| Serum levels increased in patients with CVD and chronic periodontitis compared to either condition alone | Amabile et al., 2008 | |
| Serum levels decreased following therapy in periodontitis patients with or without CVD | Hussain Bokhari et al., 2009 | |
| von Willebrand factor and PAI-1 | Significant association with periodontal measures in periodontitis patients with CVD | Montebugnoli 2005 |
| sP-selectin, P-selectin, CD18, activated glycoprotein IIb/IIIa (platelet activation markers) | Plasma and cell-surface levels elevated in periodontitis, reversed by resolvin | Papapanagiotou et al., 2009; Fredman et al., 2011; Fredman and Serhan, 2011 |
4. Antibodies
Patients with periodontitis are known to have elevated systemic antibody responses to a variety of periodontal microorganisms, and several such organisms are known to be able to induce cross-reactive and specific antibodies of relevance to atherosclerosis risk. These antibodies in turn may promote or influence inflammatory responses systemically and within atheromatous lesions. Measures of such antibodies have both been associated with increased cardiovascular risk in periodontitis.
Heat-shock proteins
Microbial heat-shock proteins (HSPs) and the immune response to these proteins represent a hypothesized pathway linking bacterial infections and atherosclerosis. Human HSPs are molecular chaperones that transport protective proteins to the cell surface. Stressed human tissues, such as those at a site of inflammation, will express HSPs which in turn are subject to regulation by both innate and adaptive arms of the immune system. For example, HSP-reactive T-cells can be found in the circulation and in atherosclerotic lesions, and anti-HSP reactive antibodies can be detected in serum of patients with atherosclerosis. In addition, HSPs can interact directly with TLRs and thereby induce inflammatory responses in macrophages and endothelial cells.
Most bacteria also express stress-induced antigens that sufficiently resemble human HSPs so as to be able induce the production of antibodies and T cells that react with human HSP’s. This form of molecular mimicry, via induction of cross-reactive cells and antibodies, may be a link between infection and atherosclerosis. A scenario whereby periodontal microorganisms can induce inflammatory responses via induction of immunity may be the following. Following upregulation of endothelial cell HSP60 due to the stress of well-known risk factors such as high blood cholesterol levels, modified LDL, hypertension, diabetes, and smoking, patients with preexisting bacterial infections may have elevated levels of cross-reactive anti-HSP antibodies and circulating lymphocytes. Along with true autoantibodies and autoreactive T-cells, these can infiltrate early atherosclerotic lesions and enhance the inflammatory response. Oral pathogens express such antigens and are capable of inducing such responses to enhance inflammation in the atheroma (Van Eden et al., 2007).
Periodontal pathogens including P. gingivalis express HSPs such as HSP60 (GroEL) (Lu and McBride, 1994, Maeda et al., 1994, Vayssier et al., 1994). In periodontitis patients, GroEL stimulates inflammatory cytokines from macrophages via TLR (Ueki et al., 2002) and anti-P. gingivalis GroEL is elevated compared to healthy individuals. Patients with mild periodontitis have elevated serum levels of HSP60 and elevated small dense LDL compared to healthy controls. Serum HSP60 concentrations correlate directly with serum triglyceride levels and inversely with HDL levels (Rizzo et al., 2012). Elevated serum levels of anti-F. nucleatum GroEL has also been described in periodontitis patients (Lee et al., 2012). F. nucleatum GroEL itself has properties consistent with atheroma formation and pathology including enhanced foam cell formation, activation of endothelial cells with increased monocyte adhesion and migration, and promotion of coagulation (Lee et al., 2012).
Cross-reactivity of bacteria-stimulated anti-HSPs with human HSPs would suggest that they could react with human HSP60 expressed on endothelial cells. Anti-Tannerella forsythia, anti-A. actinomycetemcomitans, and anti-P. gingivalis HSPs are cross reactive with each other and with human HSP (Hinode et al., 1998). P. gingivalis HSP60 contains both B- and T-cell epitopes cross-reactive with hHSP60 (Choi et al., 2004). Furthermore, T cell lines derived from atherosclerotic plaques are cross-reactive between human HSP and GroEL. (Ford et al., 2005). In addition, antibody levels to GroEL and human HSP60 were found to be higher in atherosclerosis patients in comparison to periodontitis patients and healthy subjects, and GroEL-specific T cells were detected in both the circulation and in some atherosclerotic lesions in atherosclerosis patients (Yamazaki et al., 2004). Additionally, T cell lines established from atherosclerotic plaques that are specific for GroEL and for human HSP60 have similar cytokine profiles and phenotypic characteristics to P. gingivalis-specific lines from periodontitis patients (Ford et al., 2005). These studies support the notion that bacterial HSP can induce immune responses that would be expected to promote inflammation in the atheroma itself.
Finally, animal studies support a potential interaction of bacterial HSPs and promotion of atherosclerosis. Mori (Mori et al., 2000) found that immunization of mice on a high-cholesterol diet with human HSP increased both fatty streak formation and periodontal inflammation, and Lee and coworkers (Lee et al., 2012) found that immunization of ApoE−/− mice with F. nucleatum GroEL promoted increased lipid deposition in atherosclerotic plaques in the aorta. These studies thus support the ability of anti-HSP induced either by human or bacterial immunogens to interact with both periodontal and atheromatous tissues.
Anti-cardiolipin
Pathogenic anti-cardiolipin (anti-CL) antibodies, most commonly found in patients with systemic lupus erythematosis (SLE) or the antiphospholipid syndrome (APS), are known to be associated with systemic sequelae that include vascular thrombosis and early atherosclerosis. The target antigen for these autoantibodies is on the serum protein β2-glycoprotein 1 (β2GP1), which binds to anionic lipids such as cardiolipin to form a complex that is recognized by these antibodies. It is thought that a physiologic function of β2GP1 may be to protect damaged endothelial cell surfaces from promoting inappropriate coagulation, though the function of this protein is still not clearly established. It is believed that anti-CL disrupts this protective function. In vitro treatment of endothelial cells with anti-CL leads to upregulation of adhesion molecules and production of inflammatory cytokines, thus potentially linking the presence of these antibodies to enhanced vascular inflammation. In addition, the binding specificity of β2GP1 is not limited to cardiolipin; β2GP1 also binds to modified LDL and can be detected in atheromatous lesions. Hence, anti-CL binding to β2GP1 may increase risk for early CVD in SLE patients. This phenomenon termed “autoimmune atherosclerosis” entails uptake of modified lipids into macrophages and promotion of inflammatory mechanisms resulting from the formation of these immune complexes within the atheroma (Kobayashi et al., 2005, Matsuura and Lopez, 2008).
It has been shown that a variety of microbial pathogens are capable of inducing pathogenic anti-CL because of their similarities to peptide sequences in β2GP1. One such sequence in β2GP1 is TLRVYK, and many microorganisms contain homologous peptide sequences to TLRVYK. It has been hypothesized that some anti-CL found in patients without autoimmune disease may result from molecular mimicry of microbial origin (Blank et al., 2002). In fact, Wang (Wang et al., 2008) demonstrated that patients with A. actinomycetemcomitans infection displayed elevated antibody concentrations to the peptide SIRVYK, a sequence found in A. actinomycetemcomitans that is homologous to TLRVYK. Furthermore, anti-SIRVYK correlated with anti-TLRVYK in patients colonized with A. actinomycetemcomitans. Thus, it was reasoned that infection with A. actinomycetemcomitans contributes to the content of antibody reactive with β2GP1.
Patients with chronic or aggressive periodontitis demonstrate a higher prevalence of elevated levels of anti-CL than healthy subjects without periodontitis (Schenkein et al., 2003). Between 15% and 20% of periodontitis patients contain elevated levels IgG or IgM antibodies, raising questions as to the source of these antibodies. In addition, Gunupati (Gunupati et al., 2011) has shown that periodontal therapy in patients who have experienced an acute myocardial infarction leads to significant decreases in serum concentrations of IgG and IgM anti-CL. These studies, and those demonstrating cross-reactivity between oral bacterial antigens and β2GP1, implicate the oral microflora as a source of anti-CL.
Additional clinical studies have shown an association between levels of serum anti-CL and serum markers of vascular inflammation, including sICAM-1, sVCAM-1, and sE-selectin, in periodontitis patients (Schenkein et al., 2007). Furthermore, Turkoglu (Turkoglu et al., 2008) demonstrated that periodontitis patients with hypertension have elevated IgM anti-CL serum antibodies and speculated that these antibodies may impart increased risk for atherosclerosis in these patients. Finally, Pussinen (Pussinen et al., 2004b) noted that in patients with severe periodontitis and high serum LPS concentrations, periodontal therapy resulted in a decrease in serum anti- β2GP1 concentration, implicating gram negative bacterial infection in the production of anti- β2GP1 in periodontitis.
Other antibodies
Other antibodies that link periodontitis with CVD include anti-phosphorylcholine (anti-PC) and anti-oxidized LDL (anti-oxLDL). These have the common attributes that they have been shown to be inducible by periodontal pathogens and both react with neoepitopes revealed on LDL following modification. Antibodies of both specificities have been implicated in both protection against and risk for CVD.
IgM anti-PC is a component of the innate immune system and thus present in all sera (Briles et al., 1987, Scott et al., 1987). In addition, IgG anti-PC is present at higher levels in sera from patients with periodontal attachment loss than in healthy subjects and is locally produced in the periodontal lesion (Schenkein et al., 1999). IgG anti-PC is cross –reactive between many oral bacteria and oxLDL (Schenkein et al., 2004, Schenkein et al., 2001). In mice, high levels of IgM anti-PC is associated with protection against atherosclerosis (Binder et al., 2003, Shaw et al., 2003), but this has not been demonstrated in humans. Thus, the overall impact of elevated levels of anti-PC due to stimulation with periodontal bacterial antigens is not known.
Anti-oxLDL is present in sera of patients with CVD and has been proposed to be a marker for cardiovascular risk. Such antibodies are also locally produced in the gingiva and present in GCF (Schenkein et al., 2004). It has been recently demonstrated that natural IgM antibodies and monoclonal antibodies raised against modified LDL recognized epitopes on the arginine-specific gingipain (Rgp) of P. gingivalis. These are not phosphorylcholine-containing epitopes but rather unique antigens that cross-react with modified LDL (Turunen et al., 2012).
It has been observed that the serum levels of anti-oxLDL were significantly higher in CVD patients with greater severity of periodontal disease (Montebugnoli et al., 2004, Montebugnoli et al., 2005), and Monteiro (Monteiro et al., 2009) demonstrated that periodontitis patients’ sera contain higher concentrations of antibodies against oxidized low-density lipoprotein than healthy controls. It has been proposed that oxLDL that is opsonized by antibodies such as anti-PC, anti-oxLDL, and anti-CL, could promote systemic inflammation through interactions with dendritic cells (DC’s), which produce IL-12 and IL-18 leading to IFN-γ production. The production of IL-12 in this manner, along with stimulation by oxLDL-containing immune complexes, could lead to monocyte maturation and promotion of inflammatory responses in the endothelium, as well as increased foam-cell formation. Since oral bacteria can induce these cross-reactive antibodies, and they in turn bind to these bacteria, this represents a potential mechanistic link between periodontal infection and inflammation in the atheroma (Tew et al., 2012).
In summary, cross-reactive and autoreactive antibodies can enhance or even precipitate chronic inflammatory reactions in early or advanced atherosclerotic lesions. A major source for the generation of these antibodies seems to be the microbiota of periodontal infections and mimicry of the host antigens in periodontitis. These studies are summarized in Table 3.
Table 3.
Studiesimplicating antibodies in periodontitis as a link to inflammation in CVD
| Antibody | Association(s) with CVD | References |
|---|---|---|
| Anti-Heat Shock Proteins (HSPs) | Periodontal pathogens express HSPs such as HSP-60 (GroEL) | Lu and McBride, 1994, Maeda et al., 1994, Vayssier et al., 1994 |
| Anti-P. gingivalis GroEL and anti-F. nucleatum GroEL, elevated in periodontitis patients, stimulates inflammatory cytokine production as well as monocyte and endothelial cell activation | Ueki et al., 2002, Lee et al., 2012 | |
| Serum HSP60 correlates with serum triglycerides, inversely with HDL levels | Rizzo et al., 2012 | |
| Anti-Tannerella forsythia, anti-A. actinomycetemcomitans, and anti-P. gingivalis HSPs are cross reactive with each other and with human HSP | Hinode et al., 1998 | |
| T cell lines derived from human atheromas specific for GroEL and HSP60 functionally similar to P. gingivalis-specific T cell lines from periodontitis patients | Ford et al., 2005 | |
| Anti-cardiolipin | Elevated levels in periodontitis patients | Schenkein et al., 2003 |
| Elevated levels in periodontitis patients with hypertension | Turkoglu et al., 2008 | |
| Serum levels decreased following periodontal therapy | Gunupati et al., 2011, Pussinen et al., 2004b | |
| Elevated serum levels in periodontitis patients associated with elevated serum markers of endothelial cell activation | Schenkein et al., 2007 | |
| Periodontal pathogens can produce antibodies cross-reactive with anti-cardiolipin | Wang et al., 2008 | |
| Anti-phosphorylcholine (PC) | Elevated levels in periodontitis patients, cross-reacts with oxidized low density lipoproteins (oxLDL) and oral bacteria | Schenkein et al., 1999; Schenkein et al., 2001; Schenkein et al., 2004; |
| Anti-oxLDL | Locally produced in gingiva, found in GCF | Schenkein et al., 2004 |
| Cross react with P. gingivalis Rgp protease | Turunen et al., 2012 | |
| Higher in serum of periodontitis patients, and in periodontitis patients with CVD | Montebugnoli et al., 2004; Montebugnoli et al., 2005; Monteiro et al., 2009 |
5. Dyslipidemia, lipid peroxidation and inflammation
Increased serum concentrations of total cholesterol or especially subsets of serum lipids including LDL, vLDL, and TGs are considered to be proatherogenic. LDLs, which diffuse freely into the intimal layer of blood vessels, can be modified in a number of ways, including by oxidative or proteolytic mechanisms, so as to be recognizable by cellular receptors on phagocytes. Thus macrophages in the subendothelial environment can become engorged with modified lipids to become foam cells during the early stages of atheroma formation. Activation of macrophages, with release of inflammatory mediators, can stimulate endothelial cells to release chemotactic cytokines such as MCP-1 and to upregulate cell-surface receptors involved in further monocyte recruitment into the atheromatous lesions. Clinical studies have demonstrate dyslipidemia in periodontitis patients that may be reduced following periodontal therapy, implicating periodontal inflammation as a link to atherogenesis. Furthermore, a limited number of in vitro studies in animal models indicate that periodontitis is associated with changes in serum lipid concentrations and with lipid modification that would favor atherogenesis.
Relationships between infection, serum lipid levels and structure, and inflammatory mechanisms affecting atherogenesis suggest mechanisms linking periodontitis and CVD. Though cholesterol biosynthesis and transport are fundamental physiological processes, the appearance and properties of serum lipids are influenced by infectious processes. Pertinent to periodontitis, the presence of LPS in plasma and acute phase responses to systemic dissemination of bacteria could promote elevated biosynthesis of cholesterol in the liver, which in turn is transported as serum lipids capable of binding to bacterial LPS. In this manner, a pathway can be envisioned in which periodontal infection both promotes dyslipidemia and interacts with serum lipids so as to enhance their atherogenicity.
Clinical studies of systemically healthy and diabetic patients demonstrated associations between periodontitis and dyslipidemia. Losche and coworkers (Losche et al., 2000) reported that chronic periodontitis (CP) patients exhibited higher levels of total cholesterol and serum LDL than control subjects. This observation has been repeated in other studies (Katz et al., 2001). Nibali and coworkers (Nibali et al., 2007) observed elevation in serum LDL levels as well as decreases in HDL levels in periodontitis patients who were otherwise healthy, along with significantly elevated white cell counts. These relationships were seen in non-smokers as well as in smokers. Comparison of serum lipid levels between patients with CP and healthy controls revealed increased triglycerides and decreased HDL concentrations in CP, as well as increased serum levels of oxLDL and modified LDL (Monteiro et al., 2009). Studies evaluating the density of LDL in periodontitis patients have also found higher levels of atherogenic small dense LDL in chronic periodontitis patients (Rizzo et al., 2012) and in aggressive periodontitis patients (Rufail et al., 2007). In diabetic subjects, Nishimura (Nishimura et al., 2006) observed that total cholesterol and serum LDL was associated with antibody titer to P. gingivalis. Other investigators, however, have failed to observe these relationships (Machado et al., 2005, Valentaviciene et al., 2006).
Several studies have examined modification of serum lipid profiles via periodontal therapy. Losche and coworkers (Losche et al., 2005) did not observe alterations in total cholesterol, LDL, HDL, or TGs following therapy, while Oz (Oz et al., 2007) and Duan (Duan et al., 2009) found that total cholesterol and LDL levels decreased. Montebugnoli observed decreased circulating oxLDL levels following intensive periodontal therapy (Montebugnoli et al., 2005).
Proposed mechanisms that link alteration of lipid profiles due to periodontal inflammation to enhanced inflammatory mechanisms in CVD suggest interactions with serum lipids that promote enhanced uptake of modified lipids by macrophages. A series of studies by Pussinen and coworkers have suggested pathways connecting periodontal and cardiovascular inflammation. They isolated serum lipids from periodontitis patients before and after periodontal therapy and determined their uptake in vitro by macrophages. They found that both the concentration of oxLDL as well as the production of TNF-α by macrophages were both correlated with serum LPS concentrations. In addition, lower concentration of HDL, smaller particle size of LDL, and induction of inflammatory cytokines by LDL, were found in patients with more severe periodontitis (Pussinen et al., 2004b). Treatment effects were dependent upon baseline LPS levels, with increases in HDL and LDL particle size following therapy. Using similar methodologies, they found that HDL promoted the enhanced efflux of cholesterol from macrophages following therapy, especially in patients in which CRP levels were also reduced by periodontal treatment (Pussinen et al., 2004a). Kallio (Kallio et al., 2008) observed that in periodontitis patients, elevated serum LPS levels persisted following therapy and that LPS was associated with highly atherogenic lipids, including vLDL and intermediate density lipoproteins (IDL). Thus, LPS associates with proatherogenic lipids in periodontitis, and this pattern is not changed following treatment, indicating that the disease, or colonization by Gram negative bacteria, persisted even after therapy.
Further mechanisms whereby periodontitis could impact the atherogenicity and proinflammatory potential of serum lipids were suggested by in vitro studies. A series of studies by Kuramitsu (Kuramitsu et al., 2003, Miyakawa et al., 2004) demonstrated the ability of P. gingivalis to induce in vitro foam cell formation in the presence of exogenous LDL. This was replicated by other investigators and a role for P. gingivalis fimbriae (Giacona et al., 2004) and proteases (Miyakawa et al., 2004) was confirmed for these in vitro phenomena. Miyakawa demonstrated aggregation of LDL by P. gingivalis and outer membrane vesicles, degradation of ApoB-100, and enhanced foam cell formation (Miyakawa et al., 2004).
The ability of periodontal pathogens, such as P. gingivalis, to induce atheroma formation in animal models, has been established (Jain et al., 2003, Li et al., 2002). These models have been used to study the impact of periodontal infection on lipid levels and lipid modification leading to increased atherogenesis. In a study of the impact of intravenous administration of P. gingivalis into ApoE−/− mice, enhanced atheroma production and increases in serum cholesterol and LDL and decreased LDL were noted (Hashimoto et al., 2006). It was further noted that incorporation of protease inhibitors, or an Rgp-defective mutant of P. gingivalis, led to decreased atheroma formation implicating P. gingivalis protease in the response. This study demonstrated the ability of Rgp of P. gingivalis to modify apoB-100 (the primary apolipoprotein component of LDL), which leads to increased uptake of LDL into macrophages. Likewise, Maekawa (Maekawa et al., 2011) observed increased atheroma formation in apoE−/− mice utilizing an oral infection model, noting elevation in LDL, vLDL, and total cholesterol as well as decreased HDL. They noted that these changes do not occur in wild-type mice implying the importance of a susceptible host in this model. Uchiumi (Uchiumi et al., 2004) noted that chronic infusion of LPS in rats increased serum TG levels.
In summary (Table 4), there is evidence from clinical studies that patients with periodontitis can demonstrate elevated levels of serum cholesterol as well as of LDL, small dense LDL, vLDL, IDL, and TGs, in concert with decreased levels of HDL, thus presenting with a more atherogenic lipid risk profile. Studies show that patients with periodontitis have low levels of circulating LPS, as well as episodes of bacteremia, and that LPS can be circulating in a bound form to atherogenic serum lipids. In addition, oxLDL can be found at higher levels in periodontitis. Both oxLDL and LPS-LDL are modified forms of lipid that would tend to enhance lipid uptake into macrophages to enhance the inflammatory response in the atheroma. These concepts are supported by in vitro demonstration of modification of lipids due to association with LPS and proteolytic modification of lipids by bacterial proteases with enhanced formation of foam cells. Finally, animal models indicate that similar alterations in the lipid profile can occur due to infections with periodontal pathogens and that promotion of atheroma development can occur in animals prone to hyperlipidemia due to genetic factors or dietary factors.
Table 4.
Studies implicating the role of serum lipids in periodontitis patients as a link to inflammation in CVD
| Lipid | Association(s) with CVD | References |
|---|---|---|
| Total serum cholesterol, serum lipid profile | Chronic periodontitis patients have higher total serum cholesterol than controls | Losche et al., 2000; Katz et al., 2001; Nibali et al., 2007 |
| Periodontal therapy can improve serum lipid profiles | Pussinen et al., 2004b; Montebugnoli et al., 2005; Oz et al., 2007; Duan et al., 2009 | |
| Low-density lipoprotein (LDL) | Chronic periodontitis patients have higher serum LDL than controls | Losche et al., 2000; Katz et al., 2001; Nibali et al., 2007; |
| Chronic periodontitis patients have higher oxLDL than controls | Monteiro et al., 2009 | |
| Chronic and aggressive periodontitis patients have higher small dense LDL than controls | Pussinen et al., 2004b; Rufail et al., 2007; Rizzo et al., 2012 | |
| P. gingivalis induces in vitro foam cell formation in the presence of exogenous LDL | Kuramitsu et al., 2003; Miyakawa et al., 2004; Giacona et al., 2004; Miyakawa et al., 2004 | |
| High-density lipoprotein (HDL) | Chronic periodontitis patients have lower HDL than controls | Nibali et al., 2007; Monteiro et al., 2009; Pussinen et al., 2004b |
| Triglycerides | Chronic periodontitis patients have higher serum triglycerides than controls | Monteiro et al., 2009 |
| Very (v)LDL, Intermediate density lipoprotein (IDL) | In chronic periodontitis, serum LPS is associated with atherogenic lipids (vLDL, IDL), and remains so after periodontal therapy | Kallio et al., 2008 |
6. Common genetic risk factors impacting inflammation
Of interest are recent findings that suggest a common genetic background for CVD and periodontitis, which could dictate the way the host responds in general to certain types of inflammatory processes. The underlying notion is that periodontitis and CVD are both associated with common inflammatory mechanisms or that they interact by influencing inflammatory processes that impact the disease process. Several susceptibility loci for CVD have been identified by various genome-wide association studies (GWAS) (2007, Helgadottir et al., 2007, McPherson et al., 2007, Samani et al., 2007) and the ANRIL locus is the best replicated coronary heart disease (CHD) associated risk locus to date (McPherson, 2010, Palomaki et al., 2010, Schunkert et al., 2008, Schunkert et al., 2011). In addition, variants within this region were independently found to be associated with type 2 diabetes, (Saxena et al., 2007, Scott et al., 2007, Zeggini et al., 2007) abdominal aortic and intracranial aneurysms (Helgadottir et al., 2008), ischemic stroke (Matarin et al., 2008), Alzheimer’s disease and vascular dementia (Emanuele et al., 2011), and high-grade glioma susceptibility (Wrensch et al., 2009). Significantly, Schaefer et al (Schaefer et al., 2011, Schaefer et al., 2009), and replicated by Ernst et al (Ernst et al., 2010), observed a highly increased risk for aggressive periodontitis (AgP) with variants of ANRIL.
ANRIL is a long intergenic noncoding RNA within the Chr9p21 locus. ANRIL is expressed in tissues and cell types that are affected by atherosclerosis Long intergenic noncoding RNAs, like ANRIL, do not have an obvious open reading frame and are arbitrarily considered to be longer than 200 nucleotides (Mercer et al., 2009). These RNAs have diverse cellular functions and regulate gene expression by RNA-RNA, RNA-DNA, or RNA-protein interactions (Hung and Chang, 2010). Based on functional studies it is suggested that ANRIL might constitute a regulator of epigenetic modification and gene expression and thereby modulate cardiovascular risk (Holdt and Teupser, 2012); its role in inflammatory processes is still elusive. Nevertheless, similar processes might play a role in periodontitis, thus yielding the possibility that these two diseases have common inflammatory pathways and in this way, a certain proportion of the population might be susceptible to both diseases. The causative variant(s) and functional role of ANRIL are as yet unknown despite extensive fine-mapping and functional studies (Jarinova et al., 2009, Schunkert et al., 2008), and therefore a causal role for ANRIL variants in both periodontitis patients and CVD patients is not yet determined; a role in inflammatory pathways seems logical. Another important difficulty we face to date, is the limited evidence of increased frequencies of ANRIL variants in patients with chronic periodontitis (CP), who often tend to be at ages when cardiovascular events take place. The frequency of some ANRIL variants were significantly higher in Dutch CP individuals, while not significantly increased in German CP patients (Schaefer et al. 2011). Also it remains to be determined what ANRIL gene variant frequencies are in both AgP and CP populations (and respective controls) of other ethnic descents than Northern Europe. Further research will shed light on the functional significance of these as of yet limited, but promising observations and of the contribution of genetic risk to the relationship between periodontitis and CVD..
7. Concluding remarks: Periodontal inflammation, systemic inflammatory mediators, immune mediators and CVD; alternative hypotheses
The preponderance of the data appears to support the concept that periodontitis can contribute to systemic levels of inflammatory mediators and markers associated with increased risk for CVD. Studies in this regard support the concept that a variety of mechanisms that depend upon exposure of the oral microflora or components thereof to organs distant from the oral cavity are likely to account for these findings. Such organs likely include the liver, elements of the innate and adaptive immune systems, components of the coagulation and fibrinolytic systems, and the atheromatous lesion itself, leading to enhanced systemic levels of inflammatory mediators.
In otherwise healthy periodontitis patients, CRP levels are generally above the level shown in epidemiologic and intervention studies to be associated with elevated risk for CVD (Ridker and Silvertown, 2008). Treatment studies appear to show (Paraskevas et al., 2008) a modest decrease in CRP, indicating that either periodontitis makes a modest contribution to CRP levels in patients with other predisposing factors to systemic inflammation, or that end-points of periodontal therapy are difficult to reach even with aggressive treatment. The contribution of CRP mechanistically to the disease process of CVD is itself debatable, despite many biological functions of CRP that conceptually can be thought to be operant in disease. Thus, this mechanistic linkage remains open.
Numerous inflammatory reactions, with release of a myriad of mediators, occur in the periodontal tissues. It has been proposed that local periodontal lesions might produce sufficient quantities of relevant mediators that enter the circulation so as to enhance their levels, but incisive studies demonstrating that this occurs are not available. Alternatively, it is certainly the case that periodontitis patients have frequent bacteremias and that sera from such individuals contain elevated LPS. Thus, promotion of a systemic inflammatory response with production of CRP or other mediators most likely occurs.
What appears to be missing in the mechanistic argument that periodontal disease causes systemic inflammation leading to increased atherogenesis, or increased risk for a cardiovascular event is proof that the increase in systemic inflammation attributable to periodontitis impacts the atheromatous lesion or occurrence of thrombosis. Studies of periodontal therapy, some of which demonstrate an impact on surrogate measures of CVD including systemic levels of inflammatory mediators, have not addressed CVD end-points that would be convincing in this regard. A recent systematic review (Lockhart et al., 2012) correctly noted that there are numerous papers demonstrating hypothetical links between CVD and periodontitis, mainly through studies showing correlations between these markers and both diseases. However, the absence of prospective clinical trials and incisive studies that show causal relationships and implicate specific pathways have not been carried out. Additionally, the concept that elevation of key markers such as CRP have a mechanistic role in CVD inflammation, despite their diagnostic or prognostic value, is not itself proven or universally accepted.
From the above review, it seems clear that several mechanistic pathways may exist that explain how periodontitis might be causally linked to CVD. Some or all of these proposed biological explanations most likely occur simultaneously and could be the direct or indirect consequence of the pathogenic microbiota in periodontal lesions. In addition, genetic variations in inflammatory pathways common to both diseases could indirectly explain some of the findings outlined in the current review.
In the current paper we have summarized potential host response mechanisms to incident bacteremias. In addition to genetic variations within individuals (as discussed for ANRIL), we emphasize that individual host response mechanisms are also affected by population-specific differences related, for example, to ethnicity, dietary habits and nutritional availabilities, and life style factors. These considerations make it difficult to generalize at this point potential host response mechanisms to might form the causal link between periodontitis and CVD.
Clinical Relevance.
Scientific Rationale for Study
There is a need to determine whether inflammatory mechanisms explain the links between periodontitis and cardiovascular diseases (CVD).
Principal Findings
Many studies show that periodontitis is associated with increased systemic inflammation and that a variety of mechanisms may account for these observations. Treatment of periodontitis generally decreases levels of inflammatory mediators. Thus it is possible that inflammation due to untreated periodontitis contributes to the development of inflammatory lesions in atheromatous plaques leading to CVD.
Practical Implications
The relative degree of increased levels due to periodontitis of well documented inflammatory risk markers such as C-reactive protein is sufficient to theoretically increase risk for CVD, but levels are only modestly, and possibly transiently, reduced by periodontal therapy. Additionally, there are little data relating inflammatory factors to clinical end-points such as thrombotic events or myocardial infarction. Thus, it remains to be shown that this enhancement of systemic inflammation significantly affects risk for or development of CVD.
Acknowledgments
Source of Funding Statement
This paper was supported in part by grant RO1DE018125 from the National Institute of Dental and Craniofacial Research to HAS, and by a grant from the University of Amsterdam focusing on “Oral infection and inflammation” to BL.
Footnotes
Conflict of Interest
The authors declare that they have are no conflicts of interest related to this paper.
Contributor Information
Harvey A. Schenkein, Virginia Commonwealth University, Department of Periodontics, PO Box 980566, Richmond, VA 23298-0566, U.S.A., Telephone: 804-828-9185, FAX: 804-828-5787, haschenk@vcu.edu
Bruno G. Loos, Department of Periodontology, Academic Centre for Dentistry Amsterdam (ACTA), University of Amsterdam and VU University Amsterdam, Gustav Mahlerlaan 3004, 1081 LA Amsterdam, The Netherlands, b.g.loos@acta.nl
References
- Abd TT, Eapen DJ, Bajpai A, Goyal A, Dollar A, Sperling L. The role of C-reactive protein as a risk predictor of coronary atherosclerosis: implications from the JUPITER trial. Current atherosclerosis reports. 2011;13:154–161. doi: 10.1007/s11883-011-0164-5. [DOI] [PubMed] [Google Scholar]
- Akamatsu Y, Yamamoto T, Yamamoto K, Oseko F, Kanamura N, Imanishi J, Kita M. Porphyromonas gingivalis induces myocarditis and/or myocardial infarction in mice and IL-17A is involved in pathogenesis of these diseases. Archives of oral biology. 2011;56:1290–1298. doi: 10.1016/j.archoralbio.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Alexander KS, Madden TE, Farrell DH. Association between gamma' fibrinogen levels and inflammation. Thrombosis and haemostasis. 2011;105:605–609. doi: 10.1160/TH10-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabile N, Susini G, Pettenati-Soubayroux I, Bonello L, Gil JM, Arques S, Bonfil JJ, Paganelli F. Severity of periodontal disease correlates to inflammatory systemic status and independently predicts the presence and angiographic extent of stable coronary artery disease. Journal of internal medicine. 2008;263:644–652. doi: 10.1111/j.1365-2796.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- Anand SS, Yusuf S. C-reactive protein is a bystander of cardiovascular disease. European heart journal. 2010;31:2092–2096. doi: 10.1093/eurheartj/ehq242. [DOI] [PubMed] [Google Scholar]
- Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clinical immunology. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. Relationships among clinical measures of periodontal disease and their associations with systemic markers. Annals of periodontology / the American Academy of Periodontology. 2002;7:79–89. doi: 10.1902/annals.2002.7.1.79. [DOI] [PubMed] [Google Scholar]
- Behle JH, Sedaghatfar MH, Demmer RT, Wolf DL, Celenti R, Kebschull M, Belusko PB, Herrera-Abreu M, Lalla E, Papapanou PN. Heterogeneity of systemic inflammatory responses to periodontal therapy. Journal of clinical periodontology. 2009;36:287–294. doi: 10.1111/j.1600-051X.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nature medicine. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ES, Kastelein JJ. C-reactive protein is a mediator of cardiovascular disease. European heart journal. 2010;31:2087–2091. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- Bizzarro S, van der Velden U, ten Heggeler JM, Leivadaros E, Hoek FJ, Gerdes VE, Bakker SJ, Gans RO, Ten Cate H, Loos BG. Periodontitis is characterized by elevated PAI-1 activity. Journal of clinical periodontology. 2007;34:574–580. doi: 10.1111/j.1600-051X.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, Tobar A, Shoenfeld Y. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. The Journal of clinical investigation. 2002;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz WA, Weyant RJ, Corby PM, Ren D, Weissfeld L, Kritchevsky SB, Harris T, Kurella M, Satterfield S, Visser M, Newman AB. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. Journal of the American Geriatrics Society. 2005;53:1532–1537. doi: 10.1111/j.1532-5415.2005.53468.x. [DOI] [PubMed] [Google Scholar]
- Briles DE, Scott G, Gray B, Crain MJ, Blaese M, Nahm M, Scott V, Haber P. Naturally occurring antibodies to phosphocholine as a potential index of antibody responsiveness to polysaccharides. The Journal of infectious diseases. 1987;155:1307–1314. doi: 10.1093/infdis/155.6.1307. [DOI] [PubMed] [Google Scholar]
- Buhlin K, Gustafsson A, Pockley AG, Frostegard J, Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. European heart journal. 2003;24:2099–2107. doi: 10.1016/j.ehj.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Buhlin K, Hultin M, Norderyd O, Persson L, Pockley AG, Rabe P, Klinge B, Gustafsson A. Risk factors for atherosclerosis in cases with severe periodontitis. Journal of clinical periodontology. 2009;36:541–549. doi: 10.1111/j.1600-051X.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Zheng P, Zhu H, Zhu J, Zhao L, El Mokhtari NE, Eberhard J, Lins M, Jepsen S. Platelet-activating factor levels of serum and gingival crevicular fluid in nonsmoking patients with periodontitis and/or coronary heart disease. Clinical oral investigations. 2010;14:629–636. doi: 10.1007/s00784-009-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, Kim SJ. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. Journal of dental research. 2004;83:936–940. doi: 10.1177/154405910408301209. [DOI] [PubMed] [Google Scholar]
- Consortium WTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky MI, Jongstra-Bilen J. Resident intimal dendritic cells and the initiation of atherosclerosis. Current opinion in lipidology. 2010;21:397–403. doi: 10.1097/MOL.0b013e32833ded96. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. American heart journal. 2006;151:977–984. doi: 10.1016/j.ahj.2005.06.018. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. Journal of periodontal research. 2004;39:236–241. doi: 10.1111/j.1600-0765.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Seminars in immunopathology. 2012;34:43–62. doi: 10.1007/s00281-011-0290-8. [DOI] [PubMed] [Google Scholar]
- Duan JY, Ou-Yang XY, Zhou YX. Effect of periodontal initial therapy on the serum level of lipid in the patients with both periodontitis and hyperlipidemia. Beijing da xue xue bao. Yi xue ban = Journal of Peking University. Health sciences. 2009;41:36–39. [PubMed] [Google Scholar]
- Ebersole JL, Cappelli D, Mathys EC, Steffen MJ, Singer RE, Montgomery M, Mott GE, Novak MJ. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Annals of periodontology / the American Academy of Periodontology. 2002;7:102–111. doi: 10.1902/annals.2002.7.1.102. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clinical and experimental immunology. 1997;107:347–352. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele E, Lista S, Ghidoni R, Binetti G, Cereda C, Benussi L, Maletta R, Bruni AC, Politi P. Chromosome 9p21.3 genotype is associated with vascular dementia and Alzheimer's disease. Neurobiology of aging. 2011;32:1231–1235. doi: 10.1016/j.neurobiolaging.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Ernst FD, Uhr K, Teumer A, Fanghanel J, Schulz S, Noack B, Gonzales J, Reichert S, Eickholz P, Holtfreter B, Meisel P, Linden GJ, Homuth G, Kocher T. Replication of the association of chromosomal region 9p21.3 with generalized aggressive periodontitis (gAgP) using an independent case-control cohort. BMC medical genetics. 2010;11:119. doi: 10.1186/1471-2350-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford P, Gemmell E, Walker P, West M, Cullinan M, Seymour G. Characterization of heat shock protein-specific T cells in atherosclerosis. Clinical and diagnostic laboratory immunology. 2005;12:259–267. doi: 10.1128/CDLI.12.2.259-267.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PloS one. 2011;6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. The Biochemical journal. 2011;437:185–197. doi: 10.1042/BJ20110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacona MB, Papapanou PN, Lamster IB, Rong LL, D'Agati VD, Schmidt AM, Lalla E. Porphyromonas gingivalis induces its uptake by human macrophages and promotes foam cell formation in vitro. FEMS microbiology letters. 2004;241:95–101. doi: 10.1016/j.femsle.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Gibson FC, 3rd, Genco CA. Porphyromonas gingivalis mediated periodontal disease and atherosclerosis: disparate diseases with commonalities in pathogenesis through TLRs. Current pharmaceutical design. 2007;13:3665–3675. doi: 10.2174/138161207783018554. [DOI] [PubMed] [Google Scholar]
- Gibson FC, 3rd, Yumoto H, Takahashi Y, Chou HH, Genco CA. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. Journal of dental research. 2006;85:106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- Glurich I, Grossi S, Albini B, Ho A, Shah R, Zeid M, Baumann H, Genco RJ, De Nardin E. Systemic inflammation in cardiovascular and periodontal disease: comparative study. Clinical and diagnostic laboratory immunology. 2002;9:425–432. doi: 10.1128/CDLI.9.2.425-432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Filho IS, Freitas Coelho JM, da Cruz SS, Passos JS, Teixeira de Freitas CO, Aragao Farias NS, Amorim da Silva R, Silva Pereira MN, Lima TL, Barreto ML. Chronic periodontitis and C-reactive protein levels. Journal of periodontology. 2011;82:969–978. doi: 10.1902/jop.2010.100511. [DOI] [PubMed] [Google Scholar]
- Gunupati S, Chava VK, Krishna BP. Effect of phase I periodontal therapy on anti-cardiolipin antibodies in patients with acute myocardial infarction associated with chronic periodontitis. Journal of periodontology. 2011;82:1657–1664. doi: 10.1902/jop.2011.110002. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Annals of periodontology / the American Academy of Periodontology. 2002;7:72–78. doi: 10.1902/annals.2002.7.1.72. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kadowaki T, Tsukuba T, Yamamoto K. Selective proteolysis of apolipoprotein B-100 by Arg-gingipain mediates atherosclerosis progression accelerated by bacterial exposure. Journal of biochemistry. 2006;140:713–723. doi: 10.1093/jb/mvj202. [DOI] [PubMed] [Google Scholar]
- Hayashi C, Gudino CV, Gibson FC, 3rd, Genco CA. Review: Pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Molecular oral microbiology. 2010;25:305–316. doi: 10.1111/j.2041-1014.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jaaskelainen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsater A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemela M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nature genetics. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Goto C, Hidaka T, Soga J, Nakamura S, Fujii Y, Hata T, Idei N, Fujimura N, Chayama K, Kihara Y, Taguchi A. Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis. 2009;206:604–610. doi: 10.1016/j.atherosclerosis.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, Takemoto H, Nakamura S, Soga J, Chayama K, Yoshizumi M, Taguchi A. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51:446–453. doi: 10.1161/HYPERTENSIONAHA.107.101535. [DOI] [PubMed] [Google Scholar]
- Hinode D, Nakamura R, Grenier D, Mayrand D. Cross-reactivity of specific antibodies directed to heat shock proteins from periodontopathogenic bacteria and of human origin [corrected] Oral microbiology and immunology. 1998;13:55–58. doi: 10.1111/j.1399-302x.1998.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Holdt LM, Teupser D. Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:196–206. doi: 10.1161/ATVBAHA.111.232678. [DOI] [PubMed] [Google Scholar]
- Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA biology. 2010;7:582–585. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Bokhari SA, Khan AA, Tatakis DN, Azhar M, Hanif M, Izhar M. Non-surgical periodontal therapy lowers serum inflammatory markers: a pilot study. Journal of periodontology. 2009;80:1574–1580. doi: 10.1902/jop.2009.090001. [DOI] [PubMed] [Google Scholar]
- Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. Journal of clinical periodontology. 2003;30:334–340. doi: 10.1034/j.1600-051x.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- Imamura T, Travis J, Potempa J. The biphasic virulence activities of gingipains: activation and inactivation of host proteins. Current protein & peptide science. 2003;4:443–450. doi: 10.2174/1389203033487027. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Akasaka T. Biomarkers associated with vulnerable atheromatous plaque. Current medicinal chemistry. 2012;19:2588–2596. doi: 10.2174/092986712800492922. [DOI] [PubMed] [Google Scholar]
- Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A. Effect of periodontal treatment on serum C-reactive protein levels: a systematic review and meta-analysis. Journal of periodontology. 2006;77:1635–1642. doi: 10.1902/jop.2006.050443. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Nishimura F, Soga Y, Takeuchi K, Kurihara M, Takashiba S, Murayama Y. Antimicrobial periodontal treatment decreases serum C-reactive protein, tumor necrosis factor-alpha, but not adiponectin levels in patients with chronic periodontitis. Journal of periodontology. 2003;74:1231–1236. doi: 10.1902/jop.2003.74.8.1231. [DOI] [PubMed] [Google Scholar]
- Jain A, Batista EL, Jr, Serhan C, Stahl GL, Van Dyke TE. Role for periodontitis in the progression of lipid deposition in an animal model. Infection and immunity. 2003;71:6012–6018. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarinova O, Stewart AF, Roberts R, Wells G, Lau P, Naing T, Buerki C, McLean BW, Cook RC, Parker JS, McPherson R. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1671–1677. doi: 10.1161/ATVBAHA.109.189522. [DOI] [PubMed] [Google Scholar]
- Kallio KA, Buhlin K, Jauhiainen M, Keva R, Tuomainen AM, Klinge B, Gustafsson A, Pussinen PJ. Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate immunity. 2008;14:247–253. doi: 10.1177/1753425908095130. [DOI] [PubMed] [Google Scholar]
- Katz J, Chaushu G, Sharabi Y. On the association between hypercholesterolemia, cardiovascular disease and severe periodontal disease. Journal of clinical periodontology. 2001;28:865–868. doi: 10.1034/j.1600-051x.2001.028009865.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Lopez LR, Shoenfeld Y, Matsuura E. The role of innate and adaptive immunity to oxidized low-density lipoprotein in the development of atherosclerosis. Annals of the New York Academy of Sciences. 2005;1051:442–454. doi: 10.1196/annals.1361.086. [DOI] [PubMed] [Google Scholar]
- Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:361–369. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- Kuramitsu HK, Kang IC, Qi M. Interactions of Porphyromonas gingivalis with host cells: implications for cardiovascular diseases. Journal of periodontology. 2003;74:85–89. doi: 10.1902/jop.2003.74.1.85. [DOI] [PubMed] [Google Scholar]
- Kuula H, Salo T, Pirila E, Tuomainen AM, Jauhiainen M, Uitto VJ, Tjaderhane L, Pussinen PJ, Sorsa T. Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis. Infection and immunity. 2009;77:850–859. doi: 10.1128/IAI.00873-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweider M, Lowe GD, Murray GD, Kinane DF, McGowan DA. Dental disease, fibrinogen and white cell count; links with myocardial infarction? Scottish medical journal. 1993;38:73–74. doi: 10.1177/003693309303800304. [DOI] [PubMed] [Google Scholar]
- Lee HR, Jun HK, Kim HD, Lee SH, Choi BK. Fusobacterium nucleatum GroEL induces risk factors of atherosclerosis in human microvascular endothelial cells and ApoE(−/−) mice. Molecular oral microbiology. 2012;27:109–123. doi: 10.1111/j.2041-1014.2011.00636.x. [DOI] [PubMed] [Google Scholar]
- Li L, Messas E, Batista EL, Jr, Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105:861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American College of Cardiology. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu Y, Ding Y, Meng S, Ge S, Deng H. Evaluation of serum levels of C-reactive protein and lipid profiles in patients with chronic periodontitis and/or coronary heart disease in an ethnic Han population. Quintessence international. 2010;41:239–247. [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, Wilson WR, Smith SC, Jr, Baddour LM. Periodontal Disease and Atherosclerotic Vascular Disease: Does the Evidence Support an Independent Association?: A Scientific Statement From the American Heart Association. Circulation. 2012 doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. Journal of periodontology. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- Losche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. Journal of clinical periodontology. 2000;27:537–541. doi: 10.1034/j.1600-051x.2000.027008537.x. [DOI] [PubMed] [Google Scholar]
- Losche W, Marshal GJ, Apatzidou DA, Krause S, Kocher T, Kinane DF. Lipoprotein-associated phospholipase A2 and plasma lipids in patients with destructive periodontal disease. Journal of clinical periodontology. 2005;32:640–644. doi: 10.1111/j.1600-051X.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- Lu B, McBride BC. Stress response of Porphyromonas gingivalis. Oral microbiology and immunology. 1994;9:166–173. doi: 10.1111/j.1399-302x.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Machado AC, Quirino MR, Nascimento LF. Relation between chronic periodontal disease and plasmatic levels of triglycerides, total cholesterol and fractions. Brazilian oral research. 2005;19:284–289. doi: 10.1590/s1806-83242005000400009. doi:/S1806-83242005000400009. [DOI] [PubMed] [Google Scholar]
- Maeda H, Miyamoto M, Hongyo H, Nagai A, Kurihara H, Murayama Y. Heat shock protein 60 (GroEL) from Porphyromonas gingivalis: molecular cloning and sequence analysis of its gene and purification of the recombinant protein. FEMS microbiology letters. 1994;119:129–135. doi: 10.1111/j.1574-6968.1994.tb06879.x. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Takahashi N, Tabeta K, Aoki Y, Miyashita H, Miyauchi S, Miyazawa H, Nakajima T, Yamazaki K. Chronic oral infection with Porphyromonas gingivalis accelerates atheroma formation by shifting the lipid profile. PloS one. 2011;6:e20240. doi: 10.1371/journal.pone.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malali E, Basar I, Emekli-Alturfan E, Elemek E, Oktay S, Ayan F, Emekli N, Noyan U. Levels of C-reactive protein and protein C in periodontitis patients with and without cardiovascular disease. Pathophysiology of haemostasis and thrombosis. 2010;37:49–54. doi: 10.1159/000318189. [DOI] [PubMed] [Google Scholar]
- Matarin M, Brown WM, Singleton A, Hardy JA, Meschia JF. Whole genome analyses suggest ischemic stroke and heart disease share an association with polymorphisms on chromosome 9p21. Stroke; a journal of cerebral circulation. 2008;39:1586–1589. doi: 10.1161/STROKEAHA.107.502963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura E, Lopez LR. Autoimmune-mediated atherothrombosis. Lupus. 2008;17:878–887. doi: 10.1177/0961203308093553. [DOI] [PubMed] [Google Scholar]
- McPherson R. Chromosome 9p21 and coronary artery disease. The New England journal of medicine. 2010;362:1736–1737. doi: 10.1056/NEJMcibr1002359. [DOI] [PubMed] [Google Scholar]
- McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boerwinkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nature reviews. Genetics. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Honma K, Qi M, Kuramitsu HK. Interaction of Porphyromonas gingivalis with low-density lipoproteins: implications for a role for periodontitis in atherosclerosis. Journal of periodontal research. 2004;39:1–9. doi: 10.1111/j.1600-0765.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C. Poor oral health is associated with coronary heart disease and elevated systemic inflammatory and haemostatic factors. Journal of clinical periodontology. 2004;31:25–29. doi: 10.1111/j.0303-6979.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C, Melandri G. Periodontal health improves systemic inflammatory and haemostatic status in subjects with coronary heart disease. Journal of clinical periodontology. 2005;32:188–192. doi: 10.1111/j.1600-051X.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- Monteiro AM, Jardini MA, Alves S, Giampaoli V, Aubin EC, Figueiredo Neto AM, Gidlund M. Cardiovascular disease parameters in periodontitis. Journal of periodontology. 2009;80:378–388. doi: 10.1902/jop.2009.080431. [DOI] [PubMed] [Google Scholar]
- Mori Y, Kitamura H, Song QH, Kobayashi T, Umemura S, Cyong JC. A new murine model for atherosclerosis with inflammation in the periodontal tissue induced by immunization with heat shock protein 60. Hypertension research : official journal of the Japanese Society of Hypertension. 2000;23:475–481. doi: 10.1291/hypres.23.475. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Honda T, Domon H, Okui T, Kajita K, Ito H, Takahashi N, Maekawa T, Tabeta K, Yamazaki K. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary heart disease. Journal of periodontal research. 2010;45:116–122. doi: 10.1111/j.1600-0765.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- Nibali L, D'Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. Journal of clinical periodontology. 2007;34:931–937. doi: 10.1111/j.1600-051X.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- Nishimura F, Taniguchi A, Yamaguchi-Morimoto M, Soga Y, Iwamoto Y, Kokeguchi S, Kuroe A, Fukushima M, Nakai Y, Seino Y. Periodontal infection and dyslipidemia in type 2 diabetics: association with increased HMG-CoA reductase expression. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2006;38:530–535. doi: 10.1055/s-2006-949525. [DOI] [PubMed] [Google Scholar]
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. Journal of periodontology. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Morita I, Murota S. The detection of platelet-activating factor in inflamed human gingival tissue. Archives of oral biology. 1989;34:37–41. doi: 10.1016/0003-9969(89)90044-7. [DOI] [PubMed] [Google Scholar]
- Oz SG, Fentoglu O, Kilicarslan A, Guven GS, Tanrtover MD, Aykac Y, Sozen T. Beneficial effects of periodontal treatment on metabolic control of hypercholesterolemia. Southern medical journal. 2007;100:686–691. doi: 10.1097/SMJ.0b013e31802fa327. [DOI] [PubMed] [Google Scholar]
- Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Annals of periodontology / the American Academy of Periodontology. 1998;3:108–120. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- Palomaki GE, Melillo S, Bradley LA. Association between 9p21 genomic markers and heart disease: a meta-analysis. JAMA : the journal of the American Medical Association. 2010;303:648–656. doi: 10.1001/jama.2010.118. [DOI] [PubMed] [Google Scholar]
- Papapanagiotou D, Nicu EA, Bizzarro S, Gerdes VE, Meijers JC, Nieuwland R, van der Velden U, Loos BG. Periodontitis is associated with platelet activation. Atherosclerosis. 2009;202:605–611. doi: 10.1016/j.atherosclerosis.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. Journal of clinical periodontology. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- Pejcic A, Kesic LJ, Milasin J. C-reactive protein as a systemic marker of inflammation in periodontitis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2011;30:407–414. doi: 10.1007/s10096-010-1101-1. [DOI] [PubMed] [Google Scholar]
- Persson GR, Pettersson T, Ohlsson O, Renvert S. High-sensitivity serum C-reactive protein levels in subjects with or without myocardial infarction or periodontitis. Journal of clinical periodontology. 2005;32:219–224. doi: 10.1111/j.1600-051X.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Popovic M, Smiljanic K, Dobutovic B, Syrovets T, Simmet T, Isenovic ER. Thrombin and vascular inflammation. Molecular and cellular biochemistry. 2012;359:301–313. doi: 10.1007/s11010-011-1024-x. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? Journal of clinical periodontology. 2011;38(Suppl 11):60–84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Jauhiainen M, Vilkuna-Rautiainen T, Sundvall J, Vesanen M, Mattila K, Palosuo T, Alfthan G, Asikainen S. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. Journal of lipid research. 2004a;45:139–147. doi: 10.1194/jlr.M300250-JLR200. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G, Palosuo T, Jauhiainen M, Sundvall J, Vesanen M, Mattila K, Asikainen S. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arteriosclerosis, thrombosis, and vascular biology. 2004b;24:2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- Raines EW, Ferri N. Thematic review series: The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. Journal of lipid research. 2005;46:1081–1092. doi: 10.1194/jlr.R500004-JLR200. [DOI] [PubMed] [Google Scholar]
- Ridker PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clinical chemistry. 2009;55:209–215. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. Journal of periodontology. 2008;79:1544–1551. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Cappello F, Marfil R, Nibali L, Marino Gammazza A, Rappa F, Bonaventura G, Galindo-Moreno P, O'Valle F, Zummo G, Conway de Macario E, Macario AJ, Mesa F. Heat-shock protein 60 kDa and atherogenic dyslipidemia in patients with untreated mild periodontitis: a pilot study. Cell stress & chaperones. 2012 doi: 10.1007/s12192-011-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufail ML, Schenkein HA, Koertge TE, Best AM, Barbour SE, Tew JG, van Antwerpen R. Atherogenic lipoprotein parameters in patients with aggressive periodontitis. Journal of periodontal research. 2007;42:495–502. doi: 10.1111/j.1600-0765.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- Sahingur SE, Sharma A, Genco RJ, De Nardin E. Association of increased levels of fibrinogen and the −455G/A fibrinogen gene polymorphism with chronic periodontitis. Journal of periodontology. 2003;74:329–337. doi: 10.1902/jop.2003.74.3.329. [DOI] [PubMed] [Google Scholar]
- Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, Schenkein HA. C-reactive protein levels in patients with aggressive periodontitis. Journal of periodontology. 2006;77:933–939. doi: 10.1902/jop.2006.050165. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Genomewide association analysis of coronary artery disease. The New England journal of medicine. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Dommisch H, Reinartz M, Nothnagel M, Noack B, Laine ML, Folwaczny M, Groessner-Schreiber B, Loos BG, Jepsen S, Schreiber S. CDKN2BAS is associated with periodontitis in different European populations and is activated by bacterial infection. Journal of medical genetics. 2011;48:38–47. doi: 10.1136/jmg.2010.078998. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, El Mokhtari NE, Loos BG, Jepsen S, Schreiber S. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS genetics. 2009;5:e1000378. doi: 10.1371/journal.pgen.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA, Barbour SE, Berry CR, Kipps B, Tew JG. Invasion of human vascular endothelial cells by Actinobacillus actinomycetemcomitans via the receptor for platelet-activating factor. Infection and immunity. 2000;68:5416–5419. doi: 10.1128/iai.68.9.5416-5419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA, Berry CR, Burmeister JA, Brooks CN, Barbour SE, Best AM, Tew JG. Anti-cardiolipin antibodies in sera from patients with periodontitis. Journal of dental research. 2003;82:919–922. doi: 10.1177/154405910308201114. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Berry CR, Burmeister JA, Brooks CN, Best AM, Tew JG. Locally produced anti-phosphorylcholine and anti-oxidized low-density lipoprotein antibodies in gingival crevicular fluid from aggressive periodontitis patients. Journal of periodontology. 2004;75:146–153. doi: 10.1902/jop.2004.75.1.146. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Berry CR, Purkall D, Burmeister JA, Brooks CN, Tew JG. Phosphorylcholine-dependent cross-reactivity between dental plaque bacteria and oxidized low-density lipoproteins. Infection and immunity. 2001;69:6612–6617. doi: 10.1128/IAI.69.11.6612-6617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA, Best AM, Brooks CN, Burmeister JA, Arrowood JA, Kontos MC, Tew JG. Anti-cardiolipin and increased serum adhesion molecule levels in patients with aggressive periodontitis. Journal of periodontology. 2007;78:459–466. doi: 10.1902/jop.2007.060305. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Gunsolley JC, Best AM, Harrison MT, Hahn CL, Wu J, Tew JG. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infection and immunity. 1999;67:4814–4818. doi: 10.1128/iai.67.9.4814-4818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, Gotz A, Braund P, McGinnis R, Tregouet DA, Mangino M, Linsel-Nitschke P, Cambien F, Hengstenberg C, Stark K, Blankenberg S, Tiret L, Ducimetiere P, Keniry A, Ghori MJ, Schreiber S, El Mokhtari NE, Hall AS, Dixon RJ, Goodall AH, Liptau H, Pollard H, Schwarz DF, Hothorn LA, Wichmann HE, Konig IR, Fischer M, Meisinger C, Ouwehand W, Deloukas P, Thompson JR, Erdmann J, Ziegler A, Samani NJ. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008;117:1675–1684. doi: 10.1161/CIRCULATIONAHA.107.730614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ, Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schafer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M, Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WH, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JC, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, Marz W, Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O'Donnell CJ, McPherson R, Erdmann J, Samani NJ. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nature genetics. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwahn C, Volzke H, Robinson DM, Luedemann J, Bernhardt O, Gesch D, John U, Kocher T. Periodontal disease, but not edentulism, is independently associated with increased plasma fibrinogen levels. Results from a population-based study. Thrombosis and haemostasis. 2004;92:244–252. doi: 10.1160/TH04-02-0092. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MG, Briles DE, Shackelford PG, Smith DS, Nahm MH. Human antibodies to phosphocholine. IgG anti-PC antibodies express restricted numbers of V and C regions. Journal of immunology. 1987;138:3325–3331. [PubMed] [Google Scholar]
- Seinost G, Wimmer G, Skerget M, Thaller E, Brodmann M, Gasser R, Bratschko RO, Pilger E. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. American heart journal. 2005;149:1050–1054. doi: 10.1016/j.ahj.2004.09.059. [DOI] [PubMed] [Google Scholar]
- Shaw PX, Goodyear CS, Chang MK, Witztum JL, Silverman GJ. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. Journal of immunology. 2003;170:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Archives of internal medicine. 2003;163:1172–1179. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the US population. Journal of dental research. 2000;79:49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- Soder PO, Meurman JH, Jogestrand T, Nowak J, Soder B. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in blood as markers for early atherosclerosis in subjects with chronic periodontitis. Journal of periodontal research. 2009;44:452–458. doi: 10.1111/j.1600-0765.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Meng HX, Shi D, Xu L, Zhang L, Chen ZB, Feng XH, Lu RF, Ren XY. Elevation of C-reactive protein and interleukin-6 in plasma of patients with aggressive periodontitis. Journal of periodontal research. 2009;44:311–316. doi: 10.1111/j.1600-0765.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, Elliott MJ, Kull AD, Ward C, Schenck K. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. Journal of dental research. 2006;85:74–78. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- Teles R, Wang CY. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral diseases. 2011;17:450–461. doi: 10.1111/j.1601-0825.2010.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew JG, El Shikh ME, El Sayed RM, Schenkein HA. Dendritic cells, antibodies reactive with oxLDL, and inflammation. Journal of dental research. 2012;91:8–16. doi: 10.1177/0022034511407338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. The New England journal of medicine. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Tuomainen AM, Jauhiainen M, Kovanen PT, Metso J, Paju S, Pussinen PJ. Aggregatibacter actinomycetemcomitans induces MMP-9 expression and proatherogenic lipoprotein profile in apoE-deficient mice. Microbial pathogenesis. 2008;44:111–117. doi: 10.1016/j.micpath.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Turkoglu O, Baris N, Kutukculer N, Senarslan O, Guneri S, Atilla G. Evaluation of serum anti-cardiolipin and oxidized low-density lipoprotein levels in chronic periodontitis patients with essential hypertension. Journal of periodontology. 2008;79:332–340. doi: 10.1902/jop.2008.070321. [DOI] [PubMed] [Google Scholar]
- Turunen SP, Kummu O, Harila K, Veneskoski M, Soliymani R, Baumann M, Pussinen PJ, Horkko S. Recognition of Porphyromonas gingivalis Gingipain Epitopes by Natural IgM Binding to Malondialdehyde Modified Low-Density Lipoprotein. PloS one. 2012;7:e34910. doi: 10.1371/journal.pone.0034910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiumi D, Kobayashi M, Tachikawa T, Hasegawa K. Subcutaneous and continuous administration of lipopolysaccharide increases serum levels of triglyceride and monocyte chemoattractant protein-1 in rats. Journal of periodontal research. 2004;39:120–128. doi: 10.1111/j.1600-0765.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumour necrosis factor-alpha in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clinical and experimental immunology. 2002;127:72–77. doi: 10.1046/j.1365-2249.2002.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentaviciene G, Paipaliene P, Nedzelskiene I, Zilinskas J, Anuseviciene OV. The relationship between blood serum lipids and periodontal condition. Stomatologija / issued by public institution "Odontologijos studija" … [et al.] 2006;8:96–100. [PubMed] [Google Scholar]
- Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Annals of the New York Academy of Sciences. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- Vayssier C, Mayrand D, Grenier D. Detection of stress proteins in Porphyromonas gingivalis and other oral bacteria by western immunoblotting analysis. FEMS microbiology letters. 1994;121:303–307. doi: 10.1111/j.1574-6968.1994.tb07117.x. [DOI] [PubMed] [Google Scholar]
- Vidal F, Figueredo CM, Cordovil I, Fischer RG. Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension. Journal of periodontology. 2009;80:786–791. doi: 10.1902/jop.2009.080471. [DOI] [PubMed] [Google Scholar]
- Wang D, Nagasawa T, Chen Y, Ushida Y, Kobayashi H, Takeuchi Y, Umeda M, Izumi Y. Molecular mimicry of Aggregatibacter actinomycetemcomitans with beta2 glycoprotein I. Oral microbiology and immunology. 2008;23:401–405. doi: 10.1111/j.1399-302X.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA, Ballman KV, Berger M, Buckner JC, Chang S, Giannini C, Halder C, Kollmeyer TM, Kosel ML, LaChance DH, McCoy L, O'Neill BP, Patoka J, Pico AR, Prados M, Quesenberry C, Rice T, Rynearson AL, Smirnov I, Tihan T, Wiemels J, Yang P, Wiencke JK. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nature genetics. 2009;41:905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Honda T, Oda T, Ueki-Maruyama K, Nakajima T, Yoshie H, Seymour GJ. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. Journal of periodontal research. 2005;40:53–58. doi: 10.1111/j.1600-0765.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Ohsawa Y, Itoh H, Ueki K, Tabeta K, Oda T, Nakajima T, Yoshie H, Saito S, Oguma F, Kodama M, Aizawa Y, Seymour GJ. T-cell clonality to Porphyromonas gingivalis and human heat shock protein 60s in patients with atherosclerosis and periodontitis. Oral microbiology and immunology. 2004;19:160–167. doi: 10.1111/j.0902-0055.2004.00134.x. [DOI] [PubMed] [Google Scholar]
- Yoshii S, Tsuboi S, Morita I, Takami Y, Adachi K, Inukai J, Inagaki K, Mizuno K, Nakagaki H. Temporal association of elevated C-reactive protein and periodontal disease in men. Journal of periodontology. 2009;80:734–739. doi: 10.1902/jop.2009.080537. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS immunology and medical microbiology. 2010;59:143–151. doi: 10.1111/j.1574-695X.2010.00674.x. [DOI] [PubMed] [Google Scholar]
- Zheng P, Chen H, Shi S, Jepsen S, Eberhard J. Periodontal parameters and platelet-activating factor levels in serum and gingival crevicular fluid in a Chinese population. Journal of clinical periodontology. 2006;33:797–802. doi: 10.1111/j.1600-051X.2006.00987.x. [DOI] [PubMed] [Google Scholar]