Abstract
Epithelial-mesenchymal transition (EMT), a process by which differentiated epithelial cells undergo a phenotypic conversion that gives rise to the matrix-producing fibroblasts and myofibroblasts, is increasingly recognized as an integral part of tissue fibrogenesis after injury. However, the degree to which this process contributes to kidney fibrosis remains a matter of intense debate and is likely to be context-dependent. EMT is often preceded by and closely associated with chronic interstitial inflammation and could be an adaptive response of epithelial cells to a hostile or changing microenvironment. In addition to tubular epithelial cells, recent studies indicate that endothelial cells and glomerular podocytes may also undergo transition after injury. Phenotypic alteration of podocytes sets them in motion to functional impairment, resulting in proteinuria and glomerulosclerosis. Several intracellular signal transduction pathways such as TGFβ/Smad, integrin-linked kinase (ILK) and Wnt/β-catenin signaling are essential in controlling the process of EMT and presently are potential targets of antifibrotic therapy. This review highlights the current understanding of EMT and its underlying mechanisms to stimulate further discussion on its role, not only in the pathogenesis of renal interstitial fibrosis but also in the onset of podocyte dysfunction, proteinuria, and glomerulosclerosis.
Kidney fibrosis is an inevitable outcome of all kinds of progressive chronic kidney disease (CKD).1 Despite a great deal of intense study, comprehensive understanding of the pathogenesis of renal scar formation after injury remains a daunting task that poses a major obstacle toward designing effective therapeutic strategies. In the past several years, epithelial-mesenchymal transition (EMT), a process by which fully differentiated epithelial cells undergo transition to a fibroblast phenotype, has emerged as an important pathway leading to generation of matrix-producing fibroblasts and myofibroblasts in diseased kidney.
The concept of EMT, originally formulated in embryonic development and tumor metastasis,2,3 highlights the tremendous plasticity of differentiated epithelial cells and prompts an appreciation for the role of epithelia in the evolution of fibrotic lesions in adult kidney. We and others have written comprehensive reviews on EMT in kidney fibrosis approximately 6 years ago.4,5 Since then, EMT has become arguably one of the most interesting topics in the field of renal fibrosis and has attracted a great deal of attention.6–12
EMT IN KIDNEY FIBROSIS
Many studies from different laboratories illustrate that tubular epithelial cells in vitro undergo phenotypic conversion after incubation with fibrogenic TGF-β1 (TGFβ); the transition is characterized by loss of epithelial proteins such as E-cadherin, zonula occludens-1 (ZO-1) and cytokeratin, and acquisition of new mesenchymal markers including vimentin, α-smooth muscle actin (α-SMA), fibroblast-specific protein-1 (FSP1), interstitial matrix components type I collagen, and fibronectin.4,8 These alterations in protein expression are usually accompanied by morphologic changes to a fibroblastoid appearance and an enhanced migratory capacity. Several years ago, we proposed that EMT is an orchestrated, highly regulated process that consists of four key steps: loss of epithelial cell adhesion, de novo α-SMA expression and actin reorganization, disruption of tubular basement membrane, and enhanced cell migration and invasion.4,13
Conclusive demonstration of EMT in vivo in the setting of kidney diseases appears very challenging. Nevertheless, Iwano and colleagues14 provide the most convincing evidence for EMT in vivo as a source of interstitial, matrix-producing fibroblasts. Using genetically tagged proximal tubular epithelial cells, they show that up to 36% of all FSP1-positive fibroblasts within the interstitial space originate from renal proximal tubules after unilateral ureteral obstruction. This landmark study clearly illustrates the significant contribution of EMT to the pathogenesis of chronic kidney fibrosis in that model. Interestingly, recent studies using a similar cell lineage-tracing technique show that a substantial number of interstitial fibroblasts also come from capillary endothelia by endothelial-to-mesenchymal transition (EndoMT).15,16 Because endothelial cells are a specialized type of epithelia, this EndoMT represents another form of EMT that occurs in the injured kidney. This finding illustrates that the originality and multiplicity of interstitial fibroblasts in diseased kidney are much more complex than one previously thought. Evidence for EMT in vivo is emerging in various other animal models of CKD, including obstructive nephropathy,13,15 diabetic nephropathy,17,18 remnant kidney after 5/6 nephrectomy,19,20 experimental GN,21,22 nephrotoxic serum nephritis,23 and chronic allograft nephropathy.24–27
Clinical studies utilizing human kidney biopsies also suggest that EMT likely plays a role in the pathogenesis of human CKD. Tubular expression of mesenchymal markers such as vimentin and FSP1 is found in various progressive kidney diseases including diabetic nephropathy,28,29 lupus nephritis,30 pauci-immune crescentic GN,31 IgA nephropathy,32 and chronic allograft nephropathy.26,33,34 Furthermore, the expression of these transitional proteins in tubular epithelial cells often is well correlated with declining renal function.28,34
The extent to which EMT contributes to renal fibrosis in vivo remains a matter of intense debate and is likely to be context-dependent.35–37 There are numerous reasons why EMT is often underestimated in injured kidney, as discussed previously.4 Although loss of epithelial markers such as E-cadherin, ZO-1, and cytokeratin is a universal feature of EMT, fibroblastic conversion has been more difficult to define because of a lack of specificity of many available phenotypic markers.38 Commonly used mesenchymal markers include vimentin, α-SMA, FSP1, desmin, collagen I, fibronectin, N-cadherin, the transcription factor Snail, and matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9, respectively). However, most of these markers are not absolutely specific for fibroblasts because they are also present in other cells such as inflammatory cells and endothelial cells. Furthermore, tubular epithelial cells and endothelial cells after injury in vivo may not undergo a complete EMT in many circumstances; rather, they undergo a partial EMT, also known as pre-EMT or in situ EMT, in which these cells only change one or two phenotypic markers but not actually leave their local microenvironment.34 The discussion surrounding the relative role, even the very existence, of EMT in renal fibrogenesis is likely to continue, particularly in human CKD, because it is impossible to obtain conclusive evidence by utilizing the cell lineage-tracing technique in humans for the obvious reasons.
EMT AND PERITUBULAR MICROENVIRONMENT
Renal fibrosis is generally considered the result of a failed tissue injury/repair response and the entire process can be arbitrarily divided into several phases.1,39,40 Tubular epithelial cells initially produce various chemokines and cytokines in response to various environmental stresses, including high ambient glucose, protein overload, hypoxia, and increased reactive oxygen species, as well as other injurious stimuli such as persistent infection, auto-immune reactions, and chemical insults. The chemokine gradients built up around tubular and capillary compartments attract and direct the influx of inflammatory cells, including monocytes/macrophages and lymphocytes (particularly T cells), to the tubulointerstitial space. Infiltrating cells in turn activate and produce a mixture of soluble factors, including proinflammatory, profibrotic cytokines and MMPs.1,40 This cytokine milieu creates a hostile microenvironment for tubular epithelial and endothelial cells, rendering them adaptable to changing cell phenotype for the sake of escaping apoptosis. Not surprisingly, many EMT regulatory genes such as Snail also play an important role in modulating cell survival.41,42 In that regard, EMT could be viewed as an adaptive response of epithelial cells after chronic stress/injury.
Many factors and extracellular cues in the tubular and capillary microenvironment clearly play a critical role in regulating EMT in different phases of renal fibrogenesis.4 The list of these EMT regulatory factors is constantly growing (Table 1, A and B) and includes various cytokines, growth factors, and proteases as well as other environmental cues. In the early inflammatory phase of renal fibrosis, cytokines produced by infiltrating cells play a decisive role in initiating EMT. This notion is substantiated by a study in which oncostatin M from conditioned media of activated peripheral blood mononuclear cells promotes tubular EMT in vitro.43,44 Likewise, proinflammatory cytokine IL-1 is an important regulator of EMT.45 Of interest, some proinflammatory cytokines such as a mixture of IL-1, TNFα, and IFN-γ profoundly potentiate tubular EMT triggered by TGFβ by inducing TGFβ receptor expression, although they have little effect by themselves.46 TNFα-dependent NF-κB activation also stabilizes the transcription factor Snail by blocking its ubiquitination, providing another molecular linkage between inflammation and EMT.47 The significance of renal inflammation in initiating and promoting EMT is also manifested by many observations that renal fibrosis is almost always preceded by and closely associated with chronic interstitial inflammation.48–51
Table 1.
| Table 1A. Factors in the peritubular microenvironment that induce or promote EMT
| |
|---|---|
| Factors | References |
| Cytokines | |
| IL-1 | 45 |
| oncostatin M | 43, 44 |
| Growth factors | |
| TGFβ1 | 6, 13, 38 |
| FGF-2 | 52 |
| connective tissue growth factor | 17 |
| Component of renin-angiotensin system | |
| angiotensin II | 54 |
| Proteases | |
| MMP-2 | 57 |
| tissue-type plasminogen activator | 61 |
| plasmin | 60 |
| Environmental stresses | |
| hypoxia/reactive oxygen species | 27, 66, 68 |
| advanced glycation end products | 17, 72 |
| Table 1B. Factors in the peritubular microenvironment that suppress EMT
| |
|---|---|
| Factors | References |
| Growth factors | |
| hepatocyte growth factor | 74 |
| bone morphogenic protein-7 | 23 |
| Nuclear receptor activator | |
| Vitamin D | 75 |
| Renin-angiotensin system inhibitors | |
| angiotensin II receptor blocker | 76 |
| Other | |
| statin | 13, 77 |
| rapamycin | 78, 79 |
The major driving force behind EMT during the fibrogenic phase of renal fibrosis appears to be various profibrotic growth factors, including TGFβ,6,13 basic fibroblast growth factor,52 and connective tissue growth factor,17,53 as well as angiotensin II,54 the principal component of the renin-angiotensin system. Produced by stressed/injured tubular epithelial cells, infiltrating inflammatory cells, and/or residential activated fibroblasts, these factors establish a fibrogenic niche in the interstitial space that drives tubular epithelial and endothelial cells to transition. Among them, TGFβ is the most widely studied and likely the most potent EMT inducer.6,38 As a sole factor, TGFβ can initiate and complete the entire course of EMT processes.13 The essential role of TGFβ in EMT is also consistent with the observation that its expression is universally upregulated in every kind of CKD in experimental models and in clinical settings.55
The fibrogenic phase is often followed by tissue repair and remodeling.40,56 In this stage, many matrix-degrading proteases are activated, secreted into the tubulointerstitial space, and participate in extracellular matrix remodeling. Surprisingly, these proteases also play a crucial role in promoting EMT. It has been reported that MMP-2 is necessary and sufficient for inducing tubular EMT in vitro, and overexpression of MMP-2 in transgenic mice promotes renal fibrosis.57,58 MMPs also induce the proteolytic shedding of E-cadherin, which causes the nuclear translocation of β-catenin and the induction of Snail2 (Slug), leading to EMT in tubular epithelial cells.59 Intriguingly, plasmin is demonstrated to bind to the tubular cell membrane receptor, protease-activated receptor-1, and initiates a cascade of signal transduction events leading to induction of EMT.60 We have also reported that tissue-type plasminogen activator facilitates tubular EMT by inducing MMP-9 expression and promoting the destruction of tubular basement membrane integrity.61,62
Sustained injury eventually causes peritubular endothelial dysfunction and/or EndoMT, leading to hypoxia. In the advanced stage of CKD, renal parenchymal hypoxia, generation of reactive oxygen species, advanced glycation end products, and advanced oxidation protein products are predominant pathologic features.27,63–67 In an elegant study by Higgins and colleagues,68 the condition of hypoxia, through hypoxia-inducible factor-1 (HIF-1), is a powerful cue for inducing tubular EMT in vivo and in vitro. Stable expression of HIF-1α in tubular epithelial cells through ablation of von Hippel-Lindau tumor suppressor, a ubiquitin ligase responsible for HIF-1α degradation, consistently promotes interstitial fibrosis.69 Recent studies further demonstrate that HIF-1α directly induces Twist, a key EMT regulatory transcription factor in kidney tubular cells subjected to hypoxia.70 Likewise, advanced glycation end products are shown to induce tubular EMT in a TGFβ-dependent and -independent manner.71,72 Although not tested, it would not be surprising if advanced oxidation protein products also induce EMT in diseased kidney.
Extensive studies also identify a diverse array of factors that negatively regulate EMT (Table 1). Hepatocyte growth factor and bone morphogenic protein-7 directly target TGFβ/Smad signaling and prevent, and even reverse in some cases, EMT and renal fibrosis.23,73,74 The antifibrotic effects of several therapeutic agents such as vitamin D analogues, renin-angiotensin system inhibitors, statin, and rapamycin are, at least to some extent, attributable to their action in suppressing EMT.75–79
PODOCYTE EMT AND PROTEINURIA
In addition to tubular EMT and EndoMT as discussed above, recent findings indicate that glomerular podocytes also undergo phenotypic conversion, characterized by loss of podocyte-specific markers and gain of transitional features, a process reminiscent of EMT.80 This implies that EMT in kidney diseases goes beyond the tubulointerstitial compartment. It is attractive to speculate that the transition of podocytes after injury may play a critical role in causing podocyte dysfunction, which ultimately leads to a defective glomerular filtration, proteinuria, and glomerulosclerosis.
Podocytes are specialized visceral epithelial cells that reside on the glomerular basement membrane (GBM) outside of the glomerular capillaries.81,82 Similar to the cells in most parts of the nephron, podocytes are developmentally derived from the metanephric mesenchyme through mesenchymal to epithelial transdifferentiation. In this context, it seems not completely surprising that podocytes also undergo EMT, a process of reverse embryogenesis, under pathologic conditions. Recent studies demonstrate that podocytes in culture, upon incubation with TGFβ, reduce the slit diaphragm-associated proteins P-cadherin, ZO-1, and nephrin, changes consistent with loss of the epithelial feature.80 Meanwhile, these cells begin to express the intermediate filament protein desmin, secret MMP-9, produce the interstitial matrix components fibronectin and collagen I, and upregulate the transcription factor Snail.80 As a result, these alterations in cell phenotype eventually impair podocytes’ filtration barrier function, as demonstrated by a paracellular albumin flux assay.80
Podocyte EMT also occurs in proteinuric kidney diseases.80,83 In human biopsy samples of diabetic nephropathy and focal and segmental glomerulosclerosis, loss of nephrin and ZO-1 expression in glomerular podocytes is a common feature, whereas these cells express mesenchymal markers such as desmin, FSP1, MMP-9, and key EMT regulators Snail and integrin-linked kinase (ILK).80,83,84 In a rat model of puromycin aminonucleoside nephropathy, several mesenchymal intermediate filament proteins such as desmin, vimentin, and nestin are upregulated predominantly in injured podocytes.85 In an innovative cell lineage tracing study,86 genetically tagged podocytes completely lost their molecular signatures such as Wilms tumor protein 1, synaptopodin, nephrin, and podocin, and presumably migrate to and repopulate in glomerular crescents during the early phases of cellular crescent formation in anti-GBM GN. These studies provide compelling evidence for profound phenotypic changes of podocytes in vivo under pathologic conditions. Of particular interest, several key EMT regulatory intracellular signal transduction pathways, including Wnt/β-catenin signaling,87 ILK,88 Snail,80,89 and Jaggad/Notch signaling,90,91 are often activated specifically in glomerular podocytes in various proteinuric kidney diseases, suggesting an active EMT program at work in podocytes after various insults.
The hypothesis of podocyte EMT offers a novel explanation for how injury causes podocyte dysfunction and a defective glomerular filtration barrier. We envision that EMT is an integral part of the spectrum of podocyte responses after injury. As illustrated in Figure 1, in response to injurious stimuli, podocytes undergo a range of adaptive changes, including hypertrophy, dedifferentiation, detachment, and apoptosis, depending on the severity and duration of the injury.80,92 The initial response may be cell hypertrophy, an adaptive change in cell size in an attempt to compensate for any lost function.93 However, if the injury is progressive, podocytes will undergo EMT to escape from apoptosis, which results in the loss of highly specialized podocyte features and acquisition of new mesenchymal markers. This leads to an impaired glomerular filtration barrier, thereby ensuring the onset of proteinuria. More severe and/or longer injury induces podocyte detachment from GBM and/or apoptosis, resulting in podocyte loss, which certainly exacerbates proteinuria and leads to glomerulosclerosis. The involvement and relative contribution of these podocyte responses may not only depend on the severity and duration of a particular injury, but also vary in different disease models. For instance, podocyte hypertrophy may be a predominant feature in aging nephropathy, whereas cell loss could be a major finding in nephrotoxin-induced proteinuric glomerular diseases. We suspect that for many common glomerular diseases such as diabetic nephropathy, EMT could be a primary pathway leading to podocyte dysfunction and detachment, proteinuria, and glomerulosclerosis.
Figure 1.
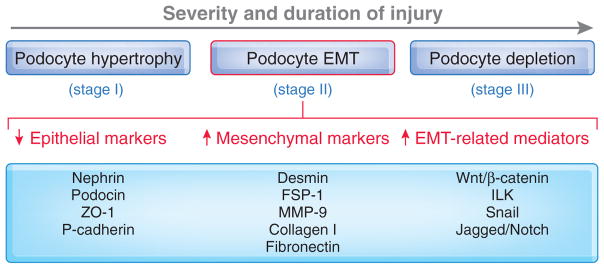
Schematic presentation of the spectrum of podocyte responses after injury. Depending on the severity and duration of the injury, podocytes may respond to injurious stimuli in different ways, including hypertrophy, dedifferentiation and mesenchymal transition (EMT), detachment and apoptosis (depletion). EMT could be a primary pathway leading to podocyte dysfunction, proteinuria, and glomerulosclerosis in many common forms of proteinuric kidney diseases.
At this stage, the notion of podocyte EMT remains controversial because these cells possess sophisticated foot processes in vivo and already express a low level of vimentin at baseline.94 Unlike tubular EMT, in which the transformed cells invade into the interstitial space and become matrix-producing cells, podocytes become motile after EMT, resulting in detachment from the GBM and leading to washout in urine or inclusion in glomerular crescent formation.83,86 Despite these differences, tubular and podocyte EMT likely share similar hallmarks and could operate through communal mechanisms.
INTRACELLULAR SIGNALING AND MECHANISM OF EMT
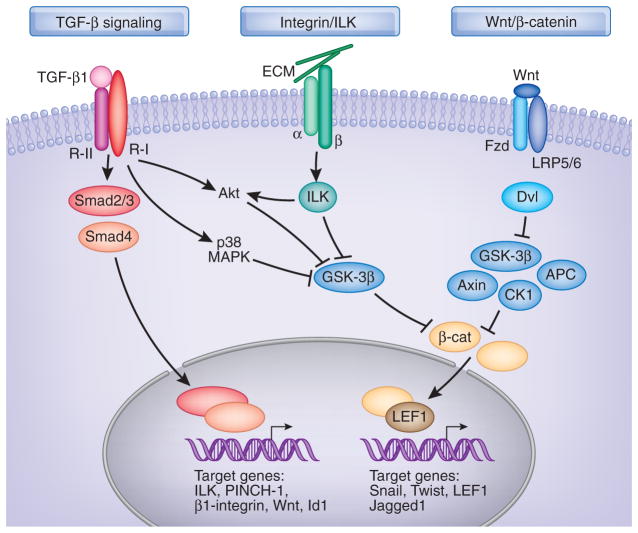
The mechanism governing EMT has been studied in great detail in recent years. Because EMT can be induced by a wide variety of stimuli (Table 1), it is not difficult to imagine that a diverse array of intracellular signal pathways and mediators is potentially involved in regulating this process.6,11 Depending on the specific pathophysiologic circumstances, these different signaling networks and mediators likely cooperate to induce a set of phenotypic changes that are consistent with EMT. In the setting of CKD, it is conceivable that three major signaling pathways (i.e., TGFβ/Smad, integrin/ILK, and Wnt/β-catenin signaling) are essential for conferring tubular and podocyte EMT, although little is known about EndoMT. These pathways are intricately connected and integrated at different levels, and together they control a host of transcription regulators and signaling mediators that are imperative for EMT (Figure 2).
Figure 2.
Simplified schematic shows major intracellular signaling networks and mediators involved in the regulation of EMT in the fibrotic kidney. Although EMT can be induced by a wide variety of stimuli and potentially involves a diverse array of intracellular mediators, three major signaling pathways (i.e., TGFβ/Smad, integrin/ILK, and Wnt/β-catenin signaling) are essential for conferring tubular and podocyte EMT. These pathways are intricately connected and integrated at different levels. See text for details.
TGFβ Signaling
Mounting evidence establishes a crucial role for TGFβ signaling in mediating EMT.6,38 TGFβ is the prototypic inducer of tubular and podocyte EMT,13,80 whereas the effects of other mediators are often context-dependent, variable, and incomplete. Given the universal upregulation of its expression in the fibrotic kidney, TGFβ-induced EMT is particularly relevant to the pathogenesis of kidney fibrosis. Smad proteins mainly mediate the signals of TGFβ. Upon stimulation by TGFβ, transmembrane type II TGFβ receptor forms tight complexes with the type I receptor, leading to phosphorylation and activation of Smad2 and Smad3. Phosphorylated Smads then heteroligomerize with the common partner Smad4 and translocate into the nucleus, where they control the transcription of TGFβ-responsive genes through interaction with specific cis-acting elements in the regulatory regions.95,96 Of interest, various EMT related genes are the targets of TGFβ/Smad signaling, such as connective tissue growth factor, ILK, PINCH-1, β1-integrin, Wnt, Snail, Id1, α-SMA, collagen IA2, and MMP-2.97–100
The necessity of Smad signaling in EMT is clearly illustrated in vivo in Smad3 knockout mice after obstructive injury. Mice lacking Smad3 are protected from renal interstitial fibrosis and show reduced EMT and collagen accumulation after unilateral ureteral obstruction.101 Consistent with this, primary tubular epithelial cells from the Smad3 null mice are resistant to induction of EMT and key EMT regulatory genes.101,102 Targeting Smad signaling by inhibitory Smad7 also blocks tubular EMT and reduces renal fibrotic lesions.103,104 Of note, Smad signaling in diseased kidney appears drastically hyperactive, not only because of TGFβ upregulation but also the dysregulation of Smad co-repressors and their regulators.105–108 Blockade of Smad signaling is also mechanistically linked to the inhibition of EMT by hepatocyte growth factor and bone morphogenic protein-7.23,109,110
Smad-independent signaling of TGFβ apparently also plays a role in regulating EMT. Non-Smad pathways of TGFβ signaling involved in EMT include RhoA, p38 mitogen-activated protein kinase (MAPK), and phopshati-dylinositol-3-kinase/Akt. In most circumstances, activation of these non-Smad pathways provides the context for induction and specification of EMT and is necessary for some aspects of EMT. For instance, the small GTPase, RhoA, is important for morphologic changes, activation of α-SMA promoter, and cytoskeletal rearrangements during TGFβ-induced EMT.111,112 TGFβ also activates p38 MAPK. However, studies show that TGFβ-mediated p38 MAPK activation is dependent on functional β1-integrin, and p38 MAPK activity is required but is not sufficient to induce EMT.113 Recent studies identify a novel pathway in which p38 MAPK can inactivate glycogen synthase kinase-3β (GSK-3β) by direct phosphorylation at its C-terminus, leading to an accumulation of β-catenin (Figure 2).114 Evidence indicates that the phopshatidylinositol-3-kinase/Akt pathway is also implicated in tubular EMT.65,115 Although the underlying mechanism remains elusive, Akt-mediated cell survival and β-catenin accumulation through inhibition of GSK-3β could play an important role.
ILK Signaling
ILK is an intracellular serine/threonine protein kinase that interacts with the cytoplasmic domains of the β-integrins and mediates the integrin signaling in diverse types of cells. ILK elicits its biologic activities through two principal properties: as a scaffolding protein and as a protein kinase.116,117 As a scaffolding protein, ILK interacts with integrins and numerous intracellular proteins, such as α-parvin and PINCH.116,118 We recently discovered that ILK also interacts with nephrin in normal glomerular podocytes, thereby building a molecular bridge that connects the cell-matrix integrin signaling with the cell-cell slit diaphragm signaling.119 Not surprisingly, conditional knockout of ILK in a podocyte-specific manner results in massive proteinuria, glomerulosclerosis, and premature death in mice.119,120 As a protein kinase, the catalytic activity of ILK renders it to directly phosphorylate several physiologically important downstream effector kinases including Akt and GSK-3β, leading to the stabilization of β-catenin (Figure 2).117 This in turn controls the expression of an array of genes that are required for the EMT program.
The involvement of ILK in tubular EMT has been established by several lines of evidence.97 Intriguingly, many components of ILK signaling, including ILK, PINCH-1, and β1-integrin are induced simultaneously by TGFβ in a Smad-dependent manner.97,98 ILK expression is also upregulated in a wide variety of CKDs in experimental and clinical settings.84,97,121,122 Furthermore, ILK is independently identified as a key mediator of podocyte dysfunction and proteinuria in many forms of proteinuric kidney diseases84 in which podocyte EMT is a predominant pathologic feature.80 The action of ILK in regulating EMT is mediated primarily by its protein kinase activity, because a kinase-dead mutant and small molecule inhibitor of ILK blocks TGFβ-mediated EMT in vitro, prevents podocyte dysfunction and albuminuria after adriamycin administration, and inhibits renal interstitial fibrosis in obstructive nephropathy.88,97,123 In this context, it is plausible that hyperactive ILK, a downstream signaling of TGFβ, plays a crucial role in mediating tubular and podocyte EMT and targeting its signaling could be a rational strategy for the treatment of fibrotic kidney disorders.
Wnt/β-Catenin Signaling
The role of Wnt/β-catenin signaling in regulating EMT during organ development and tumor metastasis is well established.3,124 However, its implication in tubular and podocyte EMT in the setting of CKD has remained uncertain until recently.87,125–127 Wnt proteins belong to a highly conserved family of secreted growth factors that play an essential role in organogenesis, tissue homeostasis, and tumor formation.128,129 Wnt proteins transmit their signal across the plasma membrane through interacting with the Frizzled receptors and co-receptors LDL receptor-related protein-5/6. Upon binding to their receptors, Wnt proteins induce a series of downstream signaling events involving Disheveled, axin, adenomatosis polyposis coli, casein kinase-1, and GSK-3β, resulting in dephosphorylation of β-catenin. This leads to stabilization of β-catenin by escaping from ubiquitin-mediated degradation, allowing it to accumulate in the cytoplasm and to translocate into the nuclei, where it binds to T cell factor/lymphoid enhancer-binding factor-1 (LEF1) to stimulate the transcription of Wnt target genes.129–131 In addition to this canonical pathway, Wnt proteins may also exert their activities through numerous β-catenin-independent, noncanonical intracellular signaling routes.
Multiple distinct genes in mammals encode Wnt proteins, creating a complex network of signaling systems. Interestingly, the vast majority of 19 mouse Wnt genes are induced concurrently in the fibrotic kidney after obstructive injury.127 Induction of Wnts leads to the stabilization of β-catenin, resulting in its localization in the cytoplasm and nuclei of tubular epithelial cells, indicating a prevailed Wnt/β-catenin signaling in that model. Inhibition of Wnt signaling by Dickkopf-1, an endogenous Wnt antagonist that specifically inhibits the canonical Wnt/β-catenin signal pathway by binding to the LDL receptor-related protein-5/6 component of the receptor complex,132 blocks the expression of Wnt target genes such as Twist, LEF1, c-myc, and fibronectin and ameliorates renal fibrosis after obstructive injury in vivo.127 Likewise, activation of Wnt/β-catenin signaling is apparently involved in mediating podocyte EMT by inducing Snail and suppression of nephrin, and mice with conditional ablation of β-catenin in glomerular podocytes are protected from proteinuria and podocyte dysfunction after administration of adriamycin.87,89
TGFβ, ILK, and Wnt signals are interconnected and converge at the activation of β-catenin (Figure 2), which leads to the activation of EMT transcriptional programs. In this context, it is of interest to note that many β-catenin target genes (e.g., Snail, Twist, LEF1, and Jaggad1) are key EMT regulatory transcription factors and mediators. For instance, Snail is a zinc-finger protein that acts as transcription repressor by recognizing E-box elements in its target gene promoters.133 Overexpression of Snail suppresses E-cadherin in tubular epithelial cells and inhibits nephrin and P-cadherin in podocytes.75,80,89 In vivo, Snail activation is sufficient to induce EMT and kidney fibrosis in adult transgenic mice.134 Twist is a basic helix-loop-helix transcription factor that is implicated in tubular EMT and kidney fibrosis.70,135 Ectopic expression of Twist not only suppresses E-cadherin but induces mesenchymal markers such as fibronectin, vimentin, α-SMA, and N-cadherin.136 Therefore, to some extent, β-catenin could function as a master switch that can integrate signal inputs from multiple pathways and control the EMT-related transcriptome.
CONCLUSIONS AND PERSPECTIVE
EMT has become one of the most fascinating topics in the studies of embryonic development, tumor metastasis, and organ fibrosis in recent years. The idea that epithelial cells after stress/injury can undergo conversion to give rise to fibroblasts, and thereby contribute to the pathogenesis of kidney fibrosis, is quite attractive and is receiving increasing attention. Undoubtedly, EMT prompts one to appreciate the role of epithelia and endothelia in the evolution of kidney fibrosis, thereby representing a paradigm shift in the field. A growing list of the extracellular factors and intracellular mediators that control EMT has been identified and could be exploited in developing future antifibrotic therapeutics.
Despite these progresses, many open questions remain. One fundamental issue is to what extent EMT contributes to renal fibrosis in vivo. A definitive answer has to rely on the cell lineage tracing technique in vivo by using a genetic model.14,86 Because many cell types other than epithelial and endothelial cells (including interstitial fibroblasts, circulating fibrocytes, and vascular pericytes) also participate in matrix production in the fibrotic kidney,14,15,37,137,138 dissection of the relative contribution of EMT to kidney fibrosis remains extremely difficult, if possible at all. Another challenge is to completely elucidate the key molecular mechanism controlling EMT. Given the diversity of known EMT regulatory factors, the underlying signal pathways could be immensely complex, with almost immeasurable cross-talks and feedbacks. What one needs may be to identify a converging “master switch” that integrates various signal inputs and controls the EMT transcriptional program. Finally, perhaps the most difficult challenge ahead is to develop a plan to translate many experimental innovations into clinically effective regimes. Several strategies targeting key EMT signaling appear to work in animal models.5,110,123 It is hoped that well designed clinical trials will be carried out in the years to come.
Acknowledgments
I am grateful to all members of my laboratory for their hard work and dedication. This work was supported by the National Institutes of Health grants DK061408, DK064005, and DK071040.
Footnotes
DISCLOSURES
None.
References
- 1.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin. 2008;40:643–650. doi: 10.1111/j.1745-7270.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavadil J, Bottinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 7.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strutz FM. EMT and proteinuria as progression factors. Kidney Int. 2009;75:475–481. doi: 10.1038/ki.2008.425. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs. 2007;185:222–231. doi: 10.1159/000101323. [DOI] [PubMed] [Google Scholar]
- 11.Neilson EG. Mechanisms of disease: Fibroblasts—A new look at an old problem. Nat Clin Pract Nephrol. 2006;2:101–108. doi: 10.1038/ncpneph0093. [DOI] [PubMed] [Google Scholar]
- 12.Grande MT, Lopez-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol. 2009;5:319–328. doi: 10.1038/nrneph.2009.74. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: Implications for diabetic renal disease. J Am Soc Nephrol. 2006;17:2484–2494. doi: 10.1681/ASN.2006050525. [DOI] [PubMed] [Google Scholar]
- 18.Holian J, Qi W, Kelly DJ, Zhang Y, Mreich E, Pollock CA, Chen XM. Role of Kruppel-like factor 6 in transforming growth factor-β1-induced epithelial-mesenchymal transition of proximal tubule cells. Am J Physiol Renal Physiol. 2008;295:F1388–F1396. doi: 10.1152/ajprenal.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Rajur K, Tolbert E, Dworkin LD. Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int. 2000;58:2028–2043. doi: 10.1111/j.1523-1755.2000.00375.x. [DOI] [PubMed] [Google Scholar]
- 21.Aresu L, Rastaldi MP, Scanziani E, Baily J, Radaelli E, Pregel P, Valenza F. Epithelial-mesenchymal transition (EMT) of renal tubular cells in canine glomerulonephritis. Virchows Arch. 2007;451:937–942. doi: 10.1007/s00428-007-0482-8. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu M, Kondo S, Urushihara M, Takamatsu M, Kanemoto K, Nagata M, Kagami S. Role of integrin-linked kinase in epithelial-mesenchymal transition in crescent formation of experimental glomerulonephritis. Nephrol Dial Transplant. 2006;21:2380–2390. doi: 10.1093/ndt/gfl243. [DOI] [PubMed] [Google Scholar]
- 23.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 24.Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev. 2008;22:1–5. doi: 10.1016/j.trre.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvajal G, Droguett A, Burgos ME, Aros C, Ardiles L, Flores C, Carpio D, Ruiz-Ortega M, Egido J, Mezzano S. Gremlin: A novel mediator of epithelial mesenchymal transition and fibrosis in chronic allograft nephropathy. Transplant Proc. 2008;40:734–739. doi: 10.1016/j.transproceed.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 26.Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC. Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant. 2006;6:2937–2946. doi: 10.1111/j.1600-6143.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 27.Djamali A, Reese S, Yracheta J, Oberley T, Hullett D, Becker B. Epithelial-to-mesenchymal transition and oxidative stress in chronic allograft nephropathy. Am J Transplant. 2005;5:500–509. doi: 10.1111/j.1600-6143.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 28.Rastaldi MP, Ferrario F, Giardino L, Dell’Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D’Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 29.Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 2007;71:846–854. doi: 10.1038/sj.ki.5002180. [DOI] [PubMed] [Google Scholar]
- 30.Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, Fogo AB. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int. 2005;68:2621–2628. doi: 10.1111/j.1523-1755.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- 31.Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, Bruneval P. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant. 2003;18:1777–1784. doi: 10.1093/ndt/gfg231. [DOI] [PubMed] [Google Scholar]
- 32.Nishitani Y, Iwano M, Yamaguchi Y, Harada K, Nakatani K, Akai Y, Nishino T, Shiiki H, Kanauchi M, Saito Y, Neilson EG. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int. 2005;68:1078–1085. doi: 10.1111/j.1523-1755.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 33.Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: The role of tubular cells in fibrogenesis. Am J Transplant. 2005;5:1367–1374. doi: 10.1111/j.1600-6143.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 34.Hertig A, Anglicheau D, Verine J, Pallet N, Touzot M, Ancel PY, Mesnard L, Brousse N, Baugey E, Glotz D, Legendre C, Rondeau E, Xu-Dubois YC. Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol. 2008;19:1584–1591. doi: 10.1681/ASN.2007101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol. 2008;130:141–155. doi: 10.1007/s00418-008-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitalone MJ, O’Connell PJ, Jimenez-Vera E, Yuksel A, Wavamunno M, Fung CL, Chapman JR, Nankivell BJ. Epithelial-to-mesenchymal transition in early transplant tubulointerstitial damage. J Am Soc Nephrol. 2008;19:1571–1583. doi: 10.1681/ASN.2007050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the Kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willis BC, Borok Z. TGF-β-induced EMT: Mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 40.Strutz F, Neilson EG. New insights into mechanisms of fibrosis in immune renal injury. Springer Semin Immunopathol. 2003;24:459–476. doi: 10.1007/s00281-003-0123-5. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Alvarez C, Blanco MJ, Perez R, Rabadan MA, Aparicio M, Resel E, Martinez T, Nieto MA. Snail family members and cell survival in physiological and pathological cleft palates. Dev Biol. 2004;265:207–218. doi: 10.1016/j.ydbio.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nightingale J, Patel S, Suzuki N, Buxton R, Takagi KI, Suzuki J, Sumi Y, Imaizumi A, Mason RM, Zhang Z. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J Am Soc Nephrol. 2004;15:21–32. doi: 10.1097/01.asn.0000102479.92582.43. [DOI] [PubMed] [Google Scholar]
- 44.Pollack V, Sarkozi R, Banki Z, Feifel E, Wehn S, Gstraunthaler G, Stoiber H, Mayer G, Montesano R, Strutz F, Schramek H. Oncostatin M-induced effects on EMT in human proximal tubular cells: Differential role of ERK signaling. Am J Physiol Renal Physiol. 2007;293:F1714–F1726. doi: 10.1152/ajprenal.00130.2007. [DOI] [PubMed] [Google Scholar]
- 45.Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Kidney Dis. 2001;37:820–831. doi: 10.1016/s0272-6386(01)80132-3. [DOI] [PubMed] [Google Scholar]
- 46.Liu X. Inflammatory cytokines augments TGF-β1-induced epithelial-mesenchymal transition in A549 cells by up-regulating TβR-I. Cell Motil Cytoskeleton. 2008;65:935–944. doi: 10.1002/cm.20315. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krensky AM, Ahn YT. Mechanisms of disease: Regulation of RANTES (CCL5) in renal disease. Nat Clin Pract Nephrol. 2007;3:164–170. doi: 10.1038/ncpneph0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guijarro C, Egido J. Transcription factor-κB (NF-κB) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 50.Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- 51.Lange-Sperandio B, Trautmann A, Eickelberg O, Jayachandran A, Oberle S, Schmidutz F, Rodenbeck B, Homme M, Horuk R, Schaefer F. Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am J Pathol. 2007;171:861–871. doi: 10.2353/ajpath.2007.061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu BC, Li MX, Zhang JD, Liu XC, Zhang XL, Phillips AO. Inhibition of integrin-linked kinase via a siRNA expression plasmid attenuates connective tissue growth factor-induced human proximal tubular epithelial cells to mesenchymal transition. Am J Nephrol. 2008;28:143–151. doi: 10.1159/000110019. [DOI] [PubMed] [Google Scholar]
- 54.Carvajal G, Rodriguez-Vita J, Rodrigues-Diez R, Sanchez-Lopez E, Ruperez M, Cartier C, Esteban V, Ortiz A, Egido J, Mezzano SA, Ruiz-Ortega M. Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int. 2008;74:585–595. doi: 10.1038/ki.2008.213. [DOI] [PubMed] [Google Scholar]
- 55.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 56.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–365. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol. 2003;162:1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: A unifying mechanism for progressive renal injury. FASEB J. 2006;20:1898–1900. doi: 10.1096/fj.06-5898fje. [DOI] [PubMed] [Google Scholar]
- 59.Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, Succar L, Rangan GK, Hu M, Henderson BR, Alexander SI, Harris DC. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol. 2009;175:580–591. doi: 10.2353/ajpath.2009.080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang G, Kernan KA, Collins SJ, Cai X, Lopez-Guisa JM, Degen JL, Shvil Y, Eddy AA. Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol. 2007;18:846–859. doi: 10.1681/ASN.2006080886. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110:1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 63.Singh DK, Winocour P, Farrington K. Mechanisms of disease: The hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008;4:216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 64.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 65.Zeng R, Yao Y, Han M, Zhao X, Liu XC, Wei J, Luo Y, Zhang J, Zhou J, Wang S, Ma D, Xu G. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc Nephrol. 2008;19:380–387. doi: 10.1681/ASN.2006111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manotham K, Tanaka T, Matsumoto M, Ohse T, Inagi R, Miyata T, Kurokawa K, Fujita T, Ingelfinger JR, Nangaku M. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004;65:871–880. doi: 10.1111/j.1523-1755.2004.00461.x. [DOI] [PubMed] [Google Scholar]
- 67.Shi XY, Hou FF, Niu HX, Wang GB, Xie D, Guo ZJ, Zhou ZM, Yang F, Tian JW, Zhang X. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology. 2008;149:1829–1839. doi: 10.1210/en.2007-1544. [DOI] [PubMed] [Google Scholar]
- 68.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023–F1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han S, Liu L, Du R, Xia L, He L, Fan D. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75:1278–1287. doi: 10.1038/ki.2009.62. [DOI] [PubMed] [Google Scholar]
- 71.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J, Liu Y. Delayed administration of hepatocyte growth factor reduces renal fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2003;284:F349–F357. doi: 10.1152/ajprenal.00154.2002. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13:96–107. doi: 10.1681/ASN.V13196. [DOI] [PubMed] [Google Scholar]
- 75.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Dai C, Liu Y. Hepatocyte growth factor gene therapy and angiotensin II blockade synergistically attenuate renal interstitial fibrosis in mice. J Am Soc Nephrol. 2002;13:2464–2477. doi: 10.1097/01.asn.0000031827.16102.c1. [DOI] [PubMed] [Google Scholar]
- 77.Patel S, Mason RM, Suzuki J, Imaizumi A, Kamimura T, Zhang Z. Inhibitory effect of statins on renal epithelial-to-mesenchymal transition. Am J Nephrol. 2006;26:381–387. doi: 10.1159/000094780. [DOI] [PubMed] [Google Scholar]
- 78.Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006;69:2029–2036. doi: 10.1038/sj.ki.5000161. [DOI] [PubMed] [Google Scholar]
- 79.Copeland JW, Beaumont BW, Merrilees MJ, Pilmore HL. Epithelial-to-mesenchymal transition of human proximal tubular epithelial cells: effects of rapamycin, mycophenolate, cyclosporin, azathioprine, and methylprednisolone. Transplantation. 2007;83:809–814. doi: 10.1097/01.tp.0000255680.71816.aa. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shankland SJ. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 82.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 83.Yamaguchi Y, Iwano M, Toyoda M, Kimura K, Harada K, Nakatani Y, Yoshimoto S, Asai O, Akai Y, Suzuki D, Kanauchi M, Neilson EG, Saito Y. Epithelial-mesenchymal transition as an explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–664. doi: 10.1053/j.ajkd.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Kretzler M, Teixeira VP, Unschuld PG, Cohen CD, Wanke R, Edenhofer I, Mundel P, Schlondorff D, Holthofer H. Integrin-linked kinase as a candidate downstream effector in proteinuria. FASEB J. 2001;15:1843–1845. doi: 10.1096/fj.00-0832fje. [DOI] [PubMed] [Google Scholar]
- 85.Zou J, Yaoita E, Watanabe Y, Yoshida Y, Nameta M, Li H, Qu Z, Yamamoto T. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006;448:485–492. doi: 10.1007/s00428-005-0134-9. [DOI] [PubMed] [Google Scholar]
- 86.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol. 2004;15:61–67. doi: 10.1097/01.asn.0000102468.37809.c6. [DOI] [PubMed] [Google Scholar]
- 87.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang YS, Dai C, Li Y, Dedhar S, Liu Y. Upregulation of integrin-linked kinase is a convergent pathway leading to podocyte injury and proteinuria. J Am Soc Nephrol. 2006;17:539A–540A. [Google Scholar]
- 89.Matsui I, Ito T, Kurihara H, Imai E, Ogihara T, Hori M. Snail, a transcriptional regulator, represses nephrin expression in glomerular epithelial cells of nephrotic rats. Lab Invest. 2007;87:273–283. doi: 10.1038/labinvest.3700518. [DOI] [PubMed] [Google Scholar]
- 90.Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, Quaggin SE, Piscione TD. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 92.Wiggins RC. The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 93.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 94.Reidy K, Susztak K. Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Am J Kidney Dis. 2009;54:590–593. doi: 10.1053/j.ajkd.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Massague J, Gomis RR. The logic of TGF-β signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 96.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Dai C, Wu C, Liu Y. PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J Am Soc Nephrol. 2007;18:2534–2543. doi: 10.1681/ASN.2007030315. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Yang J, Luo JH, Dedhar S, Liu Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J Am Soc Nephrol. 2007;18:449–460. doi: 10.1681/ASN.2006030236. [DOI] [PubMed] [Google Scholar]
- 100.Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME. The differential role of Smad2 and Smad3 in the regulation of profibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem J. 2006;393:601–607. doi: 10.1042/BJ20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signaling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lan HY. Smad7 as a therapeutic agent for chronic kidney diseases. Front Biosci. 2008;13:4984–4992. doi: 10.2741/3057. [DOI] [PubMed] [Google Scholar]
- 104.Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-β on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol. 2002;13:1464–1472. doi: 10.1097/01.asn.0000014252.37680.e4. [DOI] [PubMed] [Google Scholar]
- 105.Yang J, Zhang X, Li Y, Liu Y. Downregulation of Smad transcriptional corepressors SnoN and Ski in the fibrotic kidney: An amplification mechanism for TGF-beta1 signaling. J Am Soc Nephrol. 2003;14:3167–3177. doi: 10.1097/01.asn.0000099373.33259.b2. [DOI] [PubMed] [Google Scholar]
- 106.Tan R, Zhang J, Tan X, Zhang X, Yang J, Liu Y. Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J Am Soc Nephrol. 2006;17:2781–2791. doi: 10.1681/ASN.2005101055. [DOI] [PubMed] [Google Scholar]
- 107.Tan R, He W, Lin X, Kiss LP, Liu Y. Smad ubiquitination regulatory factor-2 in the fibrotic kidney: Regulation, target specificity, and functional implication. Am J Physiol Renal Physiol. 2008;294:F1076–F1083. doi: 10.1152/ajprenal.00323.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu FY, Li XZ, Peng YM, Liu H, Liu YH. Arkadia regulates TGF-β signaling during renal tubular epithelial to mesenchymal cell transition. Kidney Int. 2008;73:588–594. doi: 10.1038/sj.ki.5002713. [DOI] [PubMed] [Google Scholar]
- 109.Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y. Hepatocyte growth factor in kidney fibrosis: Therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287:F7–F16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- 111.Fan L, Sebe A, Peterfi Z, Masszi A, Thirone AC, Rotstein OD, Nakano H, McCulloch CA, Szaszi K, Mucsi I, Kapus A. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell. 2007;18:1083–1097. doi: 10.1091/mbc.E06-07-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-β1-induced α-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 113.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin β1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 114.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3β inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Docherty NG, O’Sullivan OE, Healy DA, Murphy M, O’Neill AJ, Fitzpatrick JM, Watson RW. TGF-β1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. Am J Physiol Renal Physiol. 2006;290:F1202–F1212. doi: 10.1152/ajprenal.00406.2005. [DOI] [PubMed] [Google Scholar]
- 116.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 117.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 118.Wu C. The PINCH-ILK-parvin complexes: Assembly, functions and regulation. Biochim Biophys Acta. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 119.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17:2164–2175. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]
- 120.El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006;17:1334–1344. doi: 10.1681/ASN.2005090921. [DOI] [PubMed] [Google Scholar]
- 121.de Teixeira VP, Blattner SM, Li M, Anders HJ, Cohen CD, Edenhofer I, Calvaresi N, Merkle M, Rastaldi MP, Kretzler M. Functional consequences of integrin-linked kinase activation in podocyte damage. Kidney Int. 2005;67:514–523. doi: 10.1111/j.1523-1755.2005.67108.x. [DOI] [PubMed] [Google Scholar]
- 122.Guo L, Sanders PW, Woods A, Wu C. The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am J Pathol. 2001;159:1735–1742. doi: 10.1016/S0002-9440(10)63020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:1907–1918. doi: 10.1681/ASN.2008090930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 126.Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol. 2002;282:F431–F441. doi: 10.1152/ajprenal.0009.2001. [DOI] [PubMed] [Google Scholar]
- 127.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 129.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signaling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 130.Huang H, He X. Wnt/beta-catenin signaling: New (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.White BD, Nguyen NK, Moon RT. Wnt signaling: It gets more humorous with age. Curr Biol. 2007;17:R923–R925. doi: 10.1016/j.cub.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 132.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 133.Yoshino J, Monkawa T, Tsuji M, Inukai M, Itoh H, Hayashi M. Snail1 is involved in the renal epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2007;362:63–68. doi: 10.1016/j.bbrc.2007.07.146. [DOI] [PubMed] [Google Scholar]
- 134.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kida Y, Asahina K, Teraoka H, Gitelman I, Sato T. Twist relates to tubular epithelial-mesenchymal transition and interstitial fibrogenesis in the obstructed kidney. J Histochem Cytochem. 2007;55:661–673. doi: 10.1369/jhc.6A7157.2007. [DOI] [PubMed] [Google Scholar]
- 136.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 137.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein-1-mediated integrin signaling. J Clin Invest. 2007;117:3821–3832. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bucala R. Circulating fibrocytes: Cellular basis for NSF. J Am Coll Radiol. 2008;5:36–39. doi: 10.1016/j.jacr.2007.08.016. [DOI] [PubMed] [Google Scholar]