Abstract
Copper sulfide nanoparticles, effective absorbers of near-infrared light, are recently attracting broad interest as a photothermal coupling agent for cancer therapy. Lipophilic copper sulfide nanoparticles are preferred for high performance biomedical applications due to high tissue affinity. Synthesis of lipophilic copper sulfide nanoparticles requires complicated multi-step processes under severe conditions. Here, we describe a new synthetic process, developed by direct dry-grinding of copper(II) acetylacetonate with sulfur under ambient environment at low temperature. The formed CuS nanoparticles are of uniform size, ~10 nm in diameter, and are monodispersed in chloroform. Each covellite CuS nanocrystal surface is modified with oleylamine through hydrogen bonding between sulfur atoms and amine groups of oleylamine. The nanoparticles demonstrate near-infrared light absorption for photothermal applications. The synthetic methodology described here is more convenient and less extreme than previous methods, and should thus greatly facilitate the preparation of photothermal lipophilic copper sulfide nanomaterials for cancer therapy.
Keywords: biomaterials, inorganic compounds, chemical synthesis, thermal properties
1. INTRODUCTION
Copper sulfide (CuS) nanocrystals with determined vacancies are capable of absorbing near-infrared (NIR) light irradiation (650–900 nm) and instantaneously converting into local heat. [1] This property has attracted broad interest for a variety of scientific and technological applications such as solar cells, electroconducting electrodes, sensors, and therapeutics. [2–4] Notably, the NIR light is able to penetrate through normal tissues without causing significant tissue injury. [5] The photothermal conversion effect of the CuS nanoparticles is independent on the surrounding environment. [6,7] These features are especially useful for controlled drug delivery and photothermal cancer therapy. [8–10]
A series of approaches were developed to synthesize CuS nanoparticles with desired structures, such as hydrothermal [11] or solvothermal methods, [12] solid-state reaction, [13] microemulsion, [14] and reflux condensations [15]. In order to endow the CuS nanoparticles with NIR absorption characteristics, they are usually further oxidized to produce vacancies in the crystalline structures. [16] One of the most commonly used methods is based on the reaction of water-soluble copper (II) salt and sodium sulfide as the precursor at 90 °C through wet chemistry [1]. The formed citric acid-capped CuS nanoparticles are applied as a photothermal coupling agent for photothermal ablation (PTA) of cancer cells in vitro and in vivo under laser irradiation. [1] Alternatively, spherical copper (I) oxide nanoparticle aggregation can be used as an sacrificial template, hydrothermally treated in the presence of polyvinylpyrrolidone (PVP) as a capping agent. Through the Kirkendall effect, vacancies are introduced to CuS, forming hollow CuS nanospheres with surface plasmonic performance. [6] In addition, the controllable hydrothermal approach is employed to develop hydrophilic flower-like CuS superstructures with the assistance of PVP (K30, 0.2 g/mL) at 180 °C for 48 h. The resulting nanostructured CuS can be used for ablation of cancer cells upon 980 nm laser irradiation. [8]
Recently, lipophilic nanomaterials were developed for their drug delivery into hydrophobic tissues such as brain and vascular tissues. [17, 18] Hot injection, [19] cation exchange [20], and solventless approach [21] are used to retain CuS nanoparticles that are dispersible in the organic phase. Among them, the hot injection method is the most likely used. It is based on high temperature reactions of copper (II) acetylacetonate and elemental sulfur or a sulfur provider (e.g., dodecanethiol). However, lipophilic CuS nanoparticles synthesized by these methods are not able to absorb NIR light. Thus, they require additional complex oxidization treatment to show photothermal performance.
For this report, lipophilic CuS nanoparticles were synthesized by directly grinding copper (II) acetylacetonate with sulfur in oleylamine. Within a few minutes of grinding in the ambient environment followed by mild heating, the CuS nanoparticles were obtained. The reaction temperature, time, concentration and molar ratio were tuned to achieve high yield and controlled size. The resulting CuS nanoparticles were of uniform particle size, (~10 nm in diameter). Each nanoparticle had fine CuS nanocrystal core, which was capped with oleylamine through hydrogen bonding between sulfur atoms and amine groups of oleylamine. These nanoparticles were readily dispersible in chloroform without aggregation. While these CuS nanoparticle were almost identical as those synthesized by the traditional solution-based solvothermal approach, they demonstrated a unique ability to absorb NIR light, which rendered them useful for photothermal applications. Compared with the traditional solvothermal method, this synthetic approach did not need excessive quantities of toxic chemicals. And this process can be scaled up easily. The method presented here markedly facilitates the synthesis of high-performance lipophilic CuS nanoparticles for photothermal therapy.
2. EXPERIMENTAL METHODS
Materials
Chloroform (>99%), cyclohexane (>99%) and ethanol (>99%) were purchased from Fisher Scientific. Oleylamine, Sulfur, and Cu(acac)2 (copper(II) acetylacetonate) were bought from Sigma-Aldrich. All chemicals were used as received.
Synthesis of CuS Nanoparticles
For dry-grinding synthesis of CuS nanoparticles, 0.016 g sulfur was fully dissolved in 3 mL oleylamine by grinding for 2 minutes. Then, 0.131 g of copper (II) acetylacetonate was gently grinded in using a mortar and a pestle for 30 sec. During the grinding process, the mixture gradually became brown translucent liquid. Then, the liquid was transferred into a round bottom flask and stirred at 70°C for 30 min, upon which the mixture color further turned from brown to green. Subsequently, the resulting mixture was dispersed in 20 mL chloroform and centrifuge for 30 min at 15,000 rpm. The collected precipitation was dispersed in 10 mL chloroform, and 50 mL ethanol was added to precipitate the formed nanoparticles. These nanoparticles were collected by centrifugation and washed by excess ethanol repeatedly to remove the remaining surfactant. After vacuum drying at room temperature, lipophilic CuS nanoparticles were obtained. The reaction temperature, heating time, oleylamine volume and Cu(acac)2-sulfur ratio were varied to investigate the effect on nanoparticle size and yield.
As a comparison, CuS nanoparticle were prepared by traditional solution based hot injection approach. Copper (II) acetylacetonate (0.131 g) was dissolved in a mixture of 1 mL oleylamine and 3 mL chloroform, and 0.016 g sulfur was dissolved in 3 mL oleylamine. The sulfur solution was dispersed in 10 mL cyclohexane and stirred at 70 °C for 10 min. After the copper (II) solution was slowly injected into the cyclohexane solution and stirred at 1,000 rpm at 70 °C for 30 min, the mixture solution gradually transformed from brown to green. The powder collected by centrifugation at 15,000 rpm for 30 min was then dispersed in 10 mL chloroform and mixed with 50 mL ethanol to purify the resultant CuS nanoparticle. These purified nanoparticles were further washed with ethanol for several cycles to exclude the excess surfactant and dried in vacuum oven overnight.
Transmission electron microscope (TEM)
To prepare samples for transmission electron microscope (TEM) observations, the corresponding materials were suspended in chloroform and then dropped onto a carbon coated nickel micro grid, followed by drying in air in fume hood. TEM observations were performed on a JEOL 2100EX microscope operating at an accelerating voltage of 100 kV.
Fourier transform infrared (FT-IR) spectra were measured on a Nicolet Nexus 670 spectrometer using KBr pellets.
Powder X-ray diffraction (XRD) patterns of the synthesized nanoparticles were recorded on Rigaku Ultima IV multipurpose X-ray diffractometer with a CuKa (λ = 0.15405 nm) radiation source. The X-ray tube current was 100 mA with a tube voltage of 40 kV. Each sample was scanned at a scan rate of 0.5° with resolution of ~0.02° from 2θ of 20° to 70°.
Dynamic light scattering (DLS)
DLS analysis was proceeded with the Malvern® nanoseries Nano-ZS90 nanoparticle size analyzer using a 1.0 cm path length 4-way glass cuvette.
UV–Vis–NIR Spectroscopy
Extinction spectra of all nanoparticles were recorded with a PerkinElmer Lambda 1050 UV–visible–NIR spectrophotometer with a quartz cuvette of 1.0 cm optical path length in the transmission mode employing pure chloroform as the reference standard.
X-ray Photoelectron Spectroscopy (XPS)
A Measurement was carried out on a PHI 5500 system and Al Kα radiation. Multipak versions 6.1 as well as XPS Peak 4.0 software were utilized for analysis and curve fitting respectively. A combination of Lorentzian and Gaussian functions was used for the least squares curve fitting.
3. RESULTS AND DISCUSSION
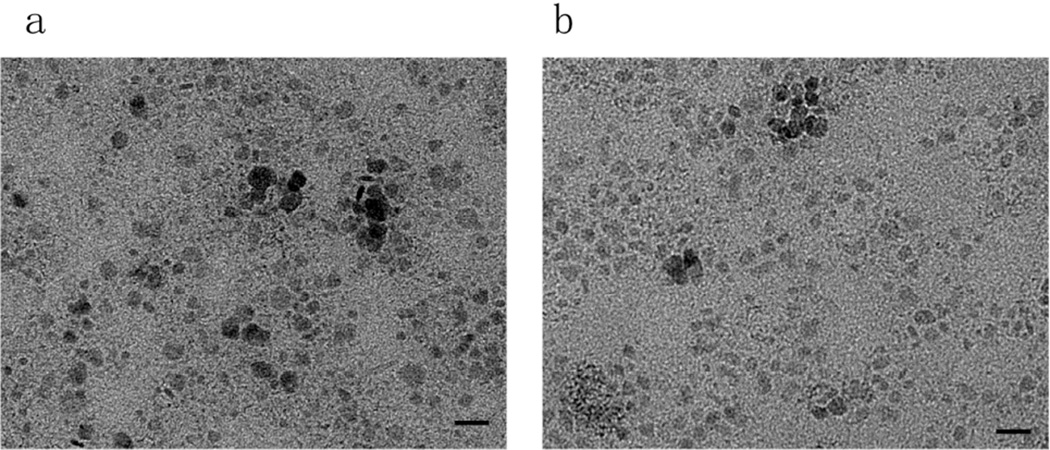
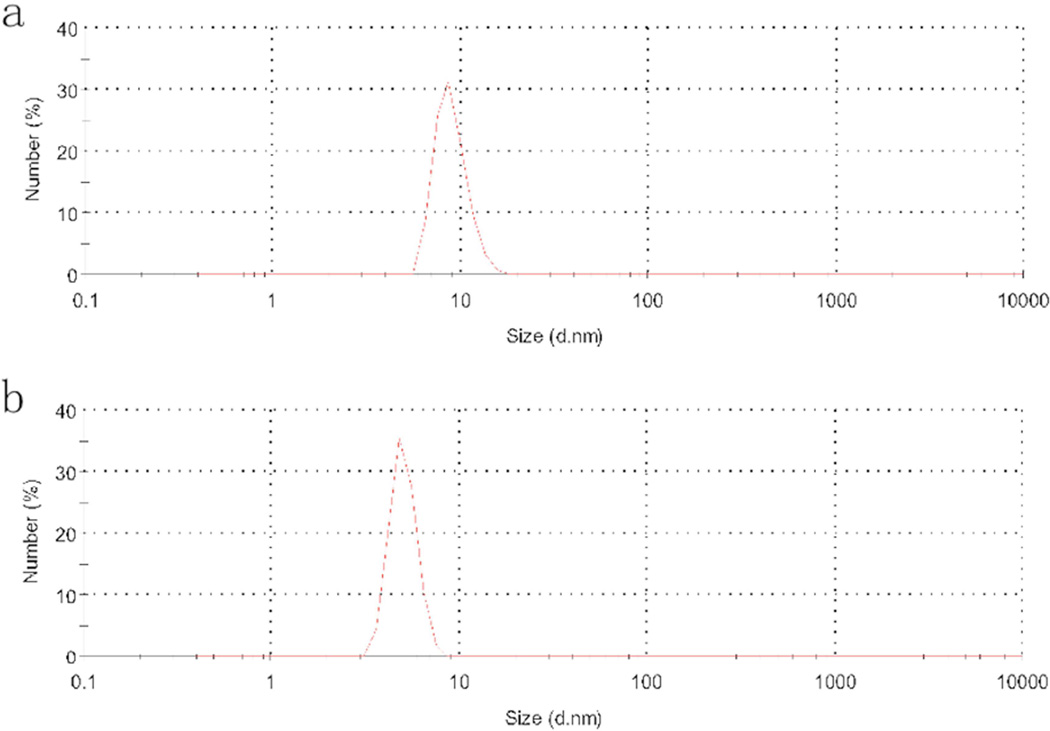
The as-prepared CuS nanoparticles were green in color and formed clear and stable colloid in chloroform for over 3 months. The dry powder was well dispersed in chloroform. The TEM image of the CuS nanoparticles synthesized by the dry grinding process is shown in Fig. 1a. The metallic nanoparticles were mainly in cubic geometry and monodispersed. Some minor aggregation was caused by the evaporation of the chloroform component during the TEM sample preparation process. Based on at least 300 particles, the average diameter for these CuS nanoparticles was calculated to be ~10 nm. This result matched well with the hydrodynamic particle diameter of the DLS analysis (Fig. 2), indicating excess surfactant was effectively removed and predominantly monodispersed fine nanoparticles remained. As a comparison, CuS nanoparticles were prepared by the previously reported solution-based technique. The average size of the formed CuS nanoparticle yielded by that method was ~9 nm (Fig. 1b). They were similar to the above nanoparticles obtained by the dry-grinding process, but more spherical because the liquid environment inhibited directional crystal growth of the nanocrystals. Overall, the dry-grinding synthesis approach achieved fine nanocrystals, which were almost identical to those produced by the traditional solution-based method.
Fig. 1.
TEM micrograph for CuS nanoparticles synthesized by the dry grinding approach (a) and the traditional hot-injection method (b). Bars: 20 nm.
Fig. 2.
DLS analysis of the oleylamine coated CuS nanoparticle developed by the dry grinding approach (a) and the traditional hot-injection method (b).
In order to establish a reliable synthesis method with maximum yield, the effect of reaction time, temperature, and amount of oleylamine on the CuS nanoparticle size distribution and yield was studied. As shown in Table 1, increasing the temperature from 70 °C to 100 °C did not contribute much to the yield rate or size change. However, prolonging reaction time effectively promoted the yield without affecting the particle size. The yield rate almost doubled when the reaction time was elongated from 15 min to 30 min. Whereas, increasing reaction time to 60 min did not significantly enhance the yield rate in comparison to that of 30-min of reaction, indicating that 30 min was sufficient to complete the reaction. In the presence of low amount of oleylamine, large particles were formed. However, the hydrodynamic size of CuS particles decreased significantly by increasing the addition of oleylamine due to oleylamine functioning as a capping agent to prevent CuS particles from agglomeration. The CuS nanoparticles prepared with 3 mL oleylamine at 70°C for 30 min remained as fine nanoparticles in chloroform stably for at least 3 months.
Table 1.
The effect of temperature, reaction time and oleylamine amount on yield rate and particle size of the as-prepared copper sulfide nanoparticles with Cu(acac)2:S = 0.5 mmol/0.5 mmol as precursor.
| Controlled parameter | Yield rate (%) * |
Particle size (nm) |
||
|---|---|---|---|---|
| Temperature (°C) |
Reaction time (min) |
Oleylamine amount (mL) |
||
| 70 | 30 | 3.0 | 88 ± 5 | 12.9 ± 6.1 |
| 85 | 30 | 3.0 | 90 ± 4 | 9.5 ± 5.1 |
| 100 | 30 | 3.0 | 91 ± 5 | 12.4 ± 6.2 |
| 70 | 15 | 3.0 | 44 ± 4 | 10.2± 6 |
| 70 | 30 | 3.0 | 89 ± 5 | 11.7 ± 5 |
| 70 | 60 | 3.0 | 90 ± 4 | 12.1 ±6 |
| 70 | 30 | 0.3 | 73 ± 5 | 346.9 ± 102 |
| 70 | 30 | 1.0 | 81 ± 6 | 121.2 ± 51.2 |
| 70 | 30 | 3.0 | 89 ± 6 | 11.9 ± 5.3 |
Yield rate (%) = (actual yield) / (theoretical yield) × 100%.
Although it was reported that higher S concentration promoted the growth of larger CuS flakes, [22] no obvious change in particle size or yield was found by adjusting Cu(acac)2/S ratio from 1:1 to 1:3 in our study (Table 2). It was considered that the grinding process in dry state and presence of oleylamine capping agent effectively prevented aggregation of CuS from growing larger flakes. Thus, the reaction parameters were optimized as follows to prepare fine and stable CuS nanoparticles: Cu/S molar ratio 1:1, oleylamine 3 mL, temperature 70 °C, reaction time 30 min. Repeated experiment revealed that the percent yield was around 90%.
Table 2.
The impact of precursors’ molar ratio on yield rate and particle size. The reactions were carried out in 3 mL oleylamine at 70 °C for 30 min.
| Cu (mmol) | S (mmol) | Yield rate (%) | DLS particle size (nm) |
|---|---|---|---|
| 0.50 | 0.50 | 88 ± 4 | 13.0 ± 6.1 |
| 0.50 | 0.75 | 88 ± 5 | 12.9 ± 6.2 |
| 0.50 | 1.00 | 90 ± 5 | 13.7 ± 7.4 |
| 0.50 | 1.50 | 91 ± 5 | 12.2 ± 6.0 |
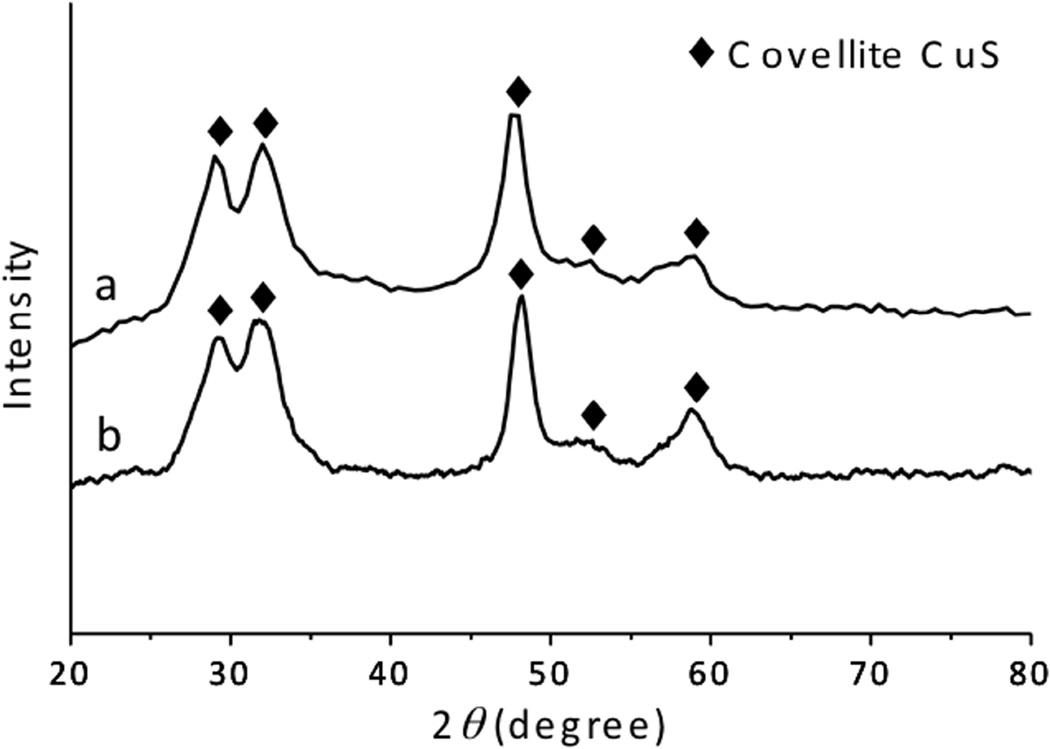
The XRD pattern of the powder sample, prepared by the dry-grinding method, presented clear peaks at 29.3°, 31.8°, 47.9 °, 52.7 °, and 59.3° (Fig. 3a), which were in fair agreement with (102), (103), (110), (108), and (116) plane of covellite phase CuS (JCPDS Card File No. 06-0464). The broad peaks inferred the nanoscale nature of the sample. [23] The crystal size, calculated assuming a (110) plane, was ~ 7.73 nm. This size was relatively smaller than the particle size measured in TEM images (10 nm), because the minor amorphous oleylamine layer was modified on the nanocrystal surface. These characteristic peaks were identical to those prepared by the solution-based method. Therefore, the current CuS nanoparticles prepared by the dry-grinding process formed high-quality, fine covellite CuS nanoparticles.
Fig. 3.
XRD spectra of the CuS nanoparticles synthesized through the dry grinding approach (a) and the traditional hot-injection method (b).
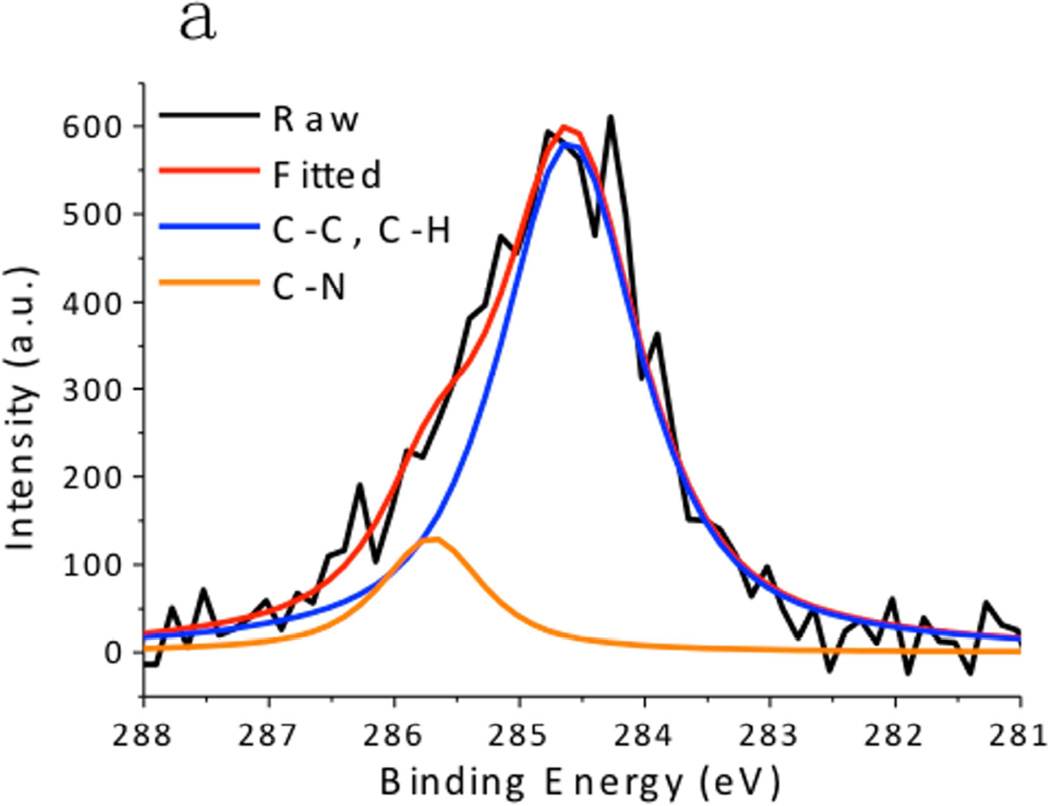
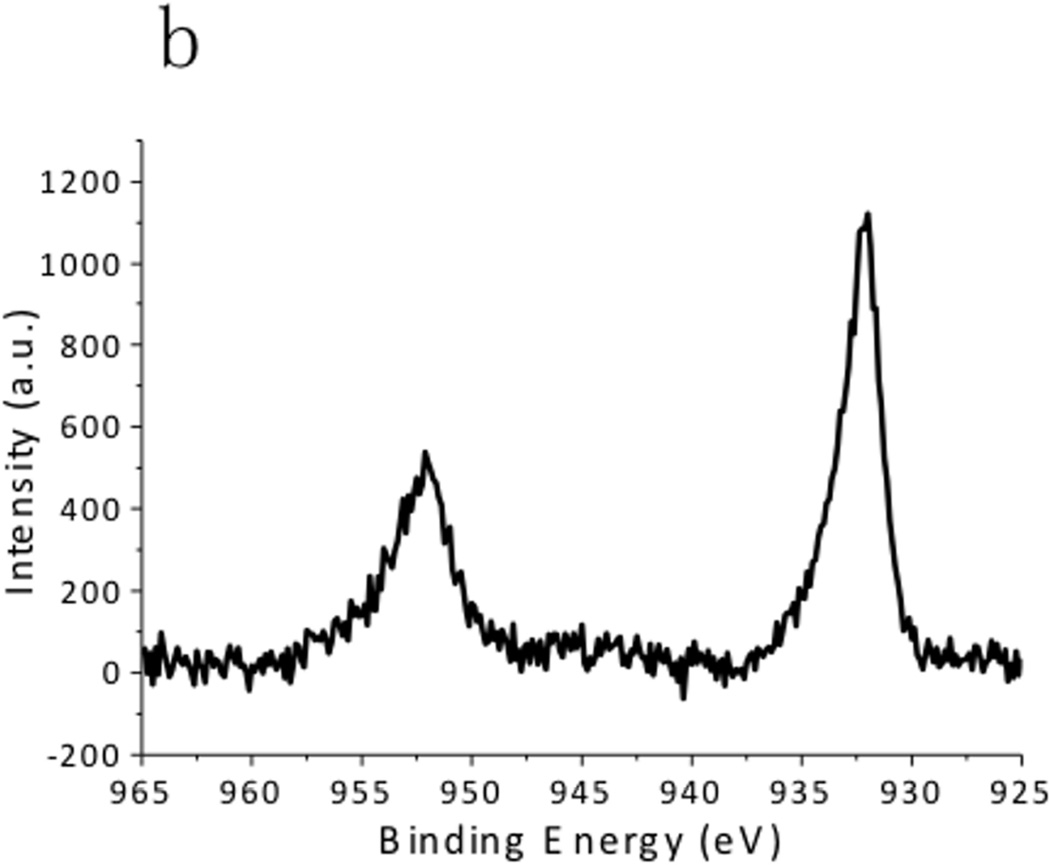
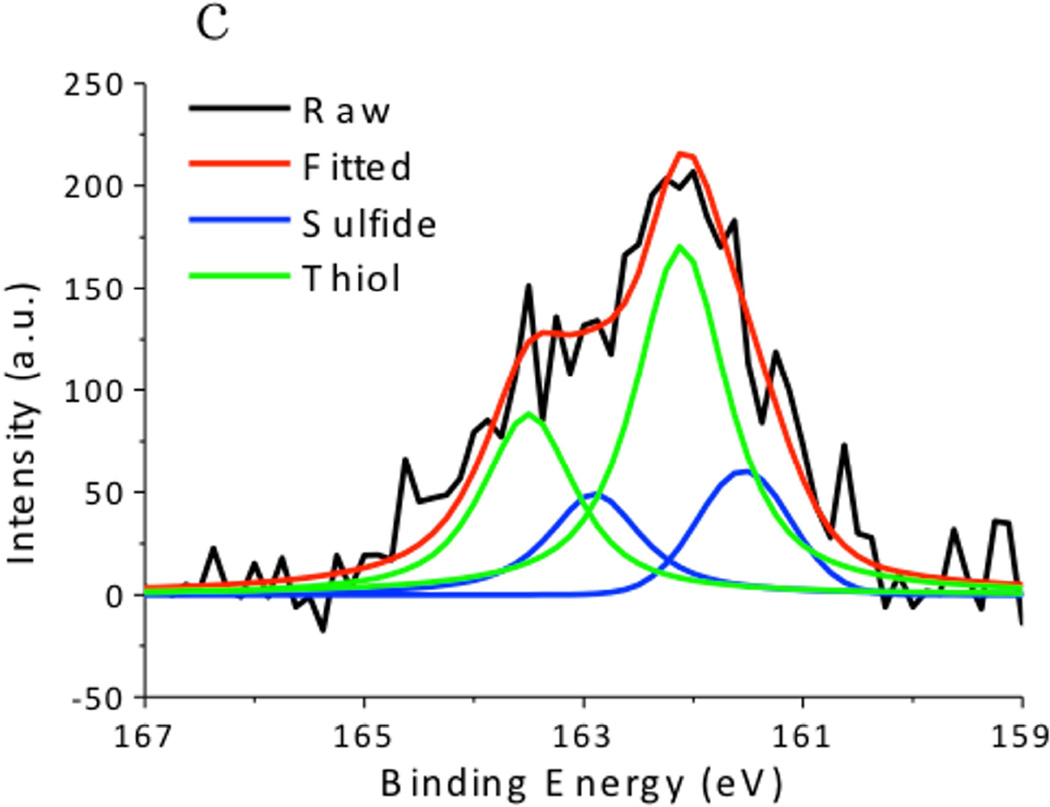
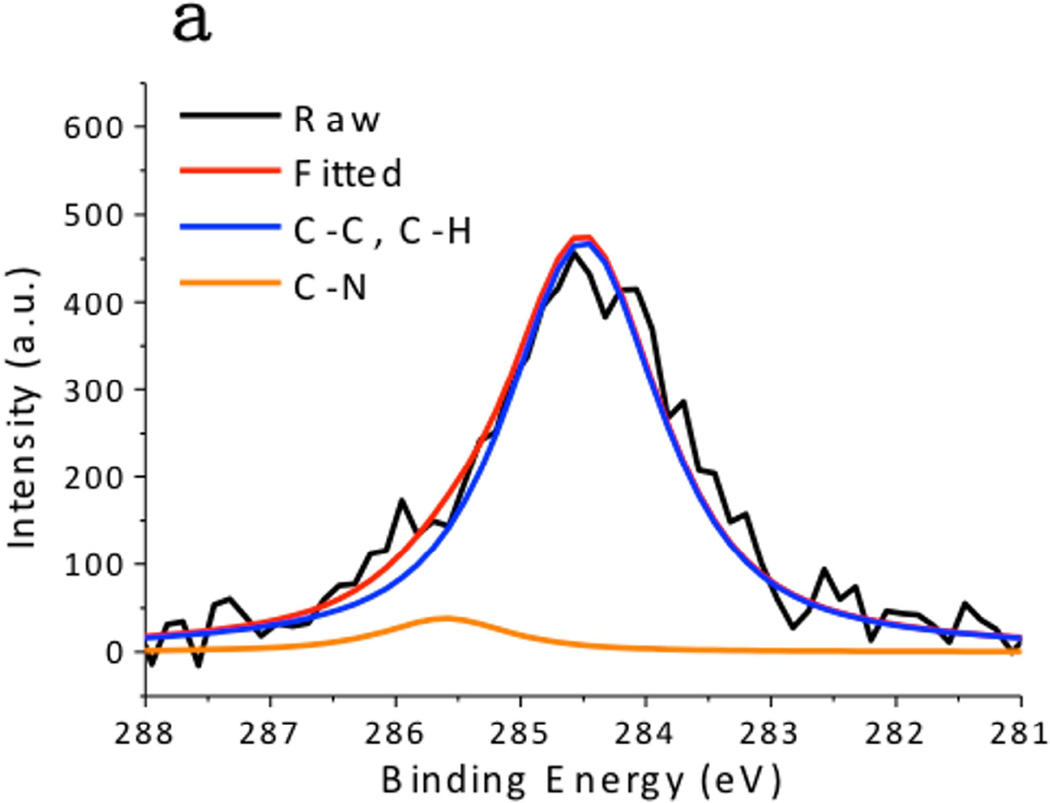
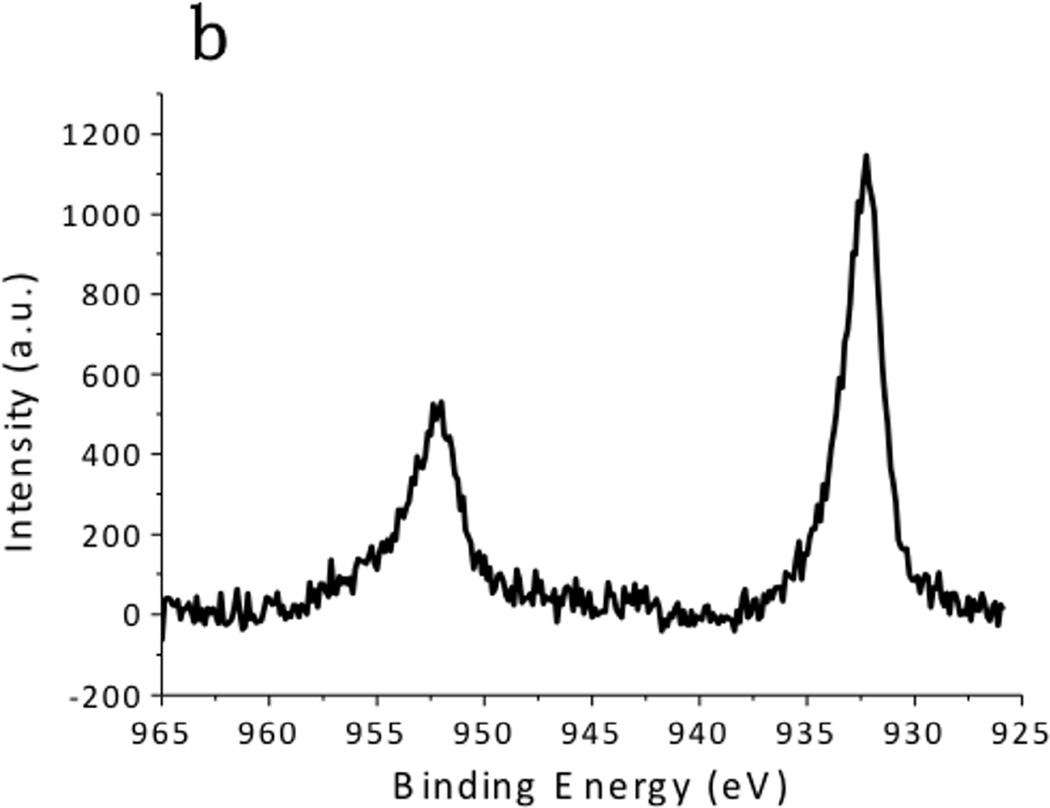
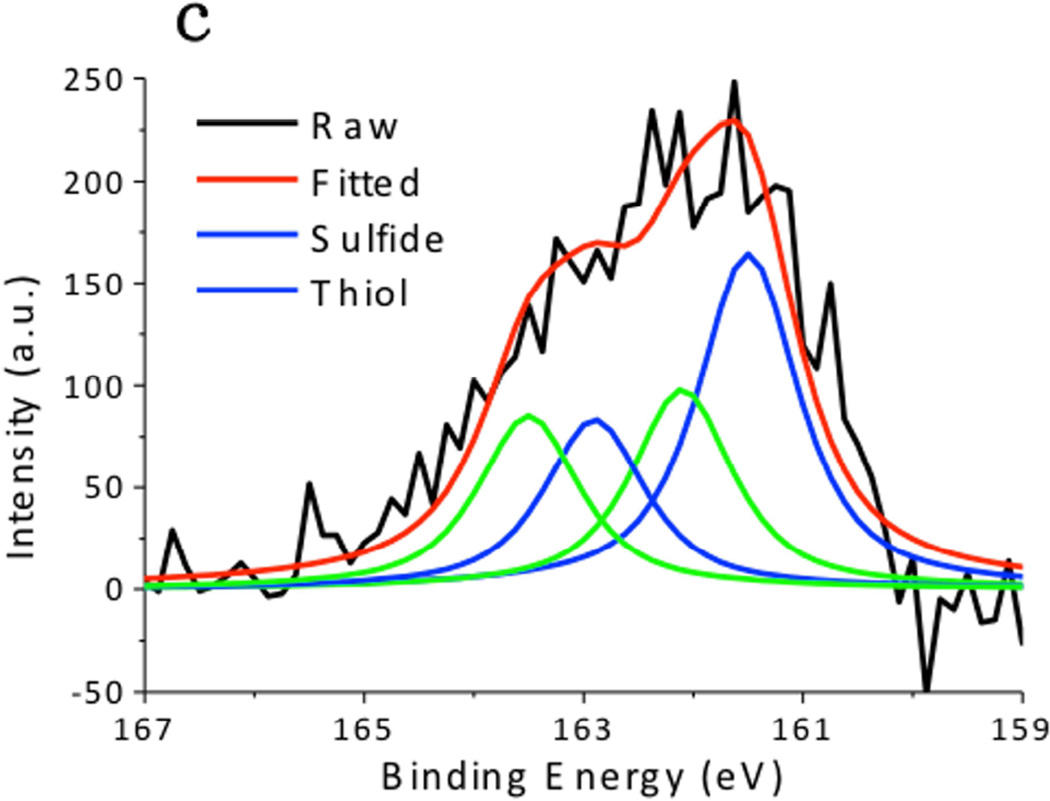
XPS spectra of the CuS nanoparticles are summarized in Fig. 4. The Cu 2p spectrum exhibited 2p3/2 peak at 932.0 eV and 2p1/2 peak at 952.2 eV, which were typical peaks for Cu(II) in copper sulfide. [24] The C 1s peak was resolved as two peaks located at 284.6 eV and 285.7 eV, which respectively corresponded to the hydrocarbon (C-C, C-H) in oleylamine and the C-N bond in oleylamine. [25] The S 2p peak of the CuS nanoparticles consisted of two distinct peaks. The one at 161.5 eV originated from the typical sulfide bond, and the doublets at 162 eV and 163.5 eV demonstrated the formation of S–H bonds. [26] These peaks matched well with the XPS spectra obtained from the CuS nanoparticle synthesized via the hot-injection method (Fig. 5), supporting the observation that the CuS nanoparticles were capped with oleylamine. Hydrophilic Sulfur atoms in CuS are electron acceptors. [16] Although they hardly interacted with the hydrophobic alkyl terminals of the oleylamine, they readily accepted electrons from the amine group in oleylamine, forming S–H bonds.
Fig. 4.
X-ray Photoelectron Spectroscopy (XPS) spectra of CuS synthesized by the dry grinding approach. (a) C 1s, (b),Cu 2p, and (c) S 2p regions.
Fig. 5.
XPS spectra of CuS synthesized by the traditional hot-injection approach. (a) C 1s, (b) Cu 2p, and (c) S 2p regions.
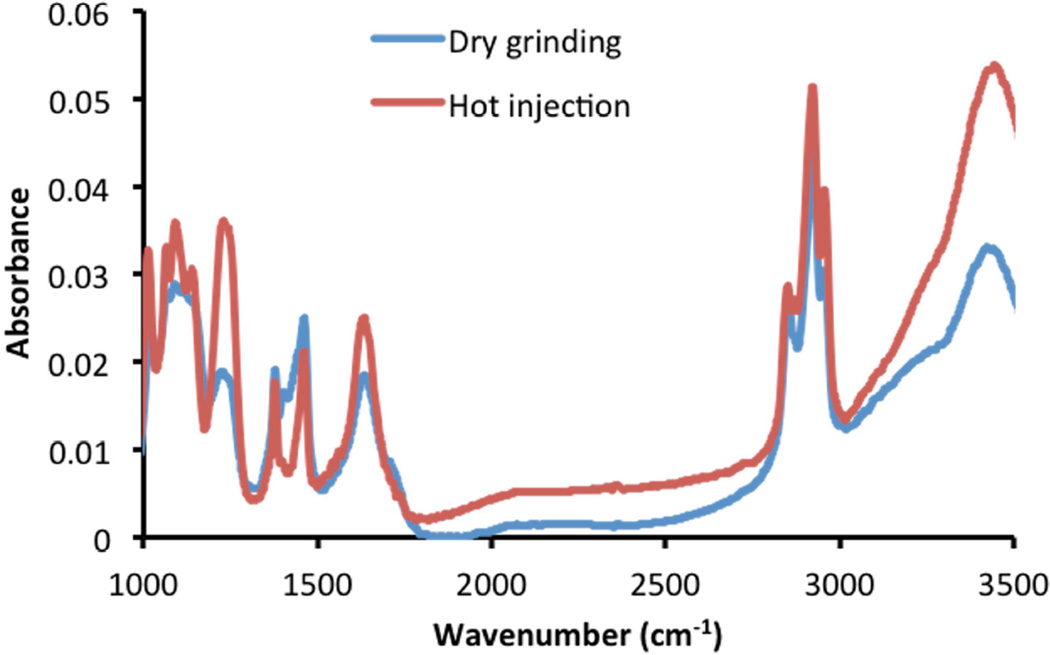
In the Fourier transform infrared spectroscopy (FTIR) spectra of both resultant CuS nanoparticles (Fig 6), the broad band at ~3450 cm−1 was assigned to N-H stretching vibrations of the amine group in oleylamine, [27] the two bands at 2922 cm−1 and 2852 cm−1 were assigned to the asymmetric (νas) and symmetric (νs) stretching vibrations of methylene (CH2=CH) in the alkyl chain of oleylamine, and the bands centered at 1634 cm−1 were attributed to N-H bending vibrations. [28–30] All of these characteristic bands were in fair agreement with the FTIR spectrum of pure oleylamine, adding for the support for capping of the CuS nanoparticles with oleylamine.
Fig. 6.
FTIR spectra of CuS synthesized by the dry grinding approach and the traditional hot-injection method.
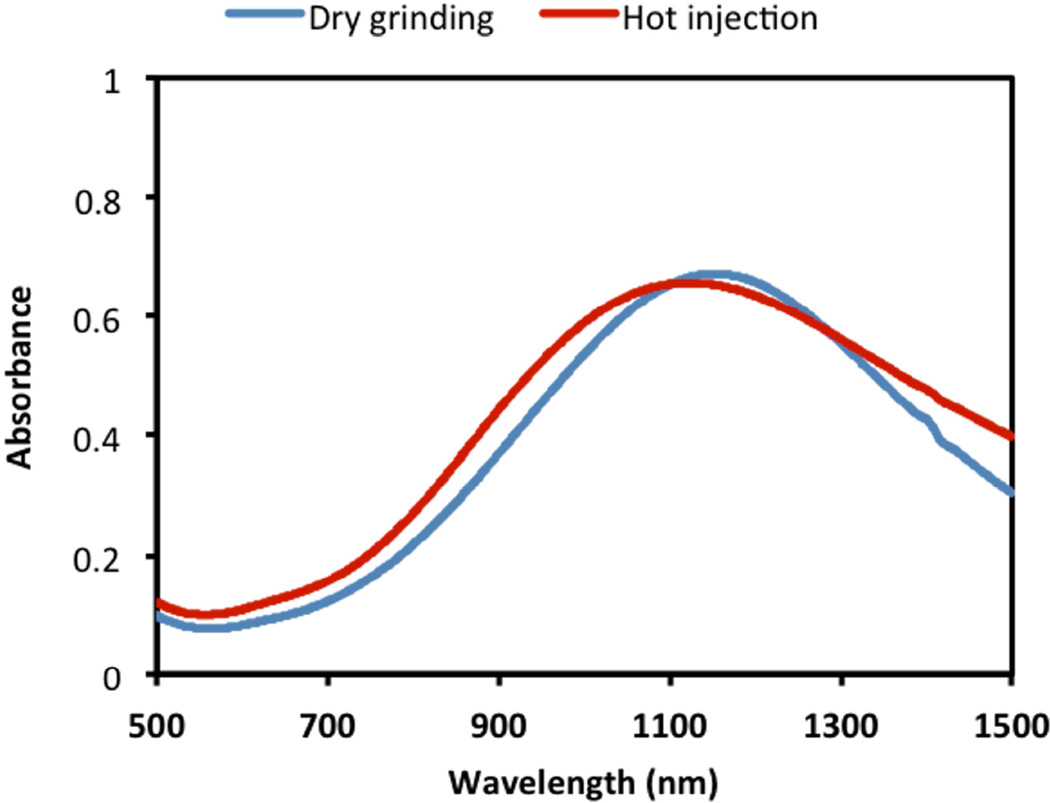
Interestingly, the dry-grinding synthesized CuS nanoparticles demonstrated broad NIR absorption peaks centered at ~ 1100 nm (Fig. 7), which was very close to the spectra of traditional CuS nanoparticles prepared by the solution-based technique. Such strong absorption suggested that the current CuS nanopaticles possessed localized surface plasmon resonances for photothermal ablation applications.
Fig. 7.
Visible-NIR spectra of CuS nanoparticle (1 mM) dispersion in chloroform synthesized by the dry grinding approach and the traditional hot-injection method.
Conventional synthetic methods of lipophilic fine CuS nanoparticles require liquid environments, high temperatures, and N2 protection. A liquid environment allows oleylamine to form micelles to direct the nucleation, promote growth of nanocrystals, and to prohibit nanoparticle agglomeration. Meanwhile, high temperatures accelerate the reaction, and inert environments prevent over-oxidation of CuS and resulting damage of NIR absorbance (peak absorbance < 1150 nm). [16] In the current study, it was proved that such conditions were not mandatory for the synthesis of monodispersive fine CuS nanoparticles. The existence of oxygen in the reaction process induced the formation of vacancies in the CuS crystals, resulting in NIR absorption in favor of prospective photothermal therapy. However, it should be noticed that low temperature (heating temperature <145 °C) might result in smaller and thinner crystal planes. [22] Moreover, the grinding process enabled full contact of the copper (II) salts with oleylamine for complexation. The copper(II) salt-complexed oleylamine consisted of hydrophilic salt terminals and long alkyl chain groups, which formed micelle structures and controlled the crystal growth. Further work will be needed to clarify the actual reaction mechanism of the dry-grinding synthesis approach.
4. CONCLUSIONS
We successfully developed a facile one-step dry-grinding process to synthesize monodispersed CuS nanocrystals by tuning the reaction conditions. The nanoparticles were composed of covellite phase CuS, and the particle size was finely controlled to yield a fairly uniform diameter of 10 nm. The CuS nanoparticle surface was capped with oleylamine by hydrogen bonding between sulfur atoms with the amine groups of oleylamine. The resultant CuS nanoparticles produced by the method described here were highly comparable to those prepared by the traditional solvothermal method. Notably, having the current approach could be carried out under ambient conditions and with markedly decreased requirements for toxic solvents. This method thus appears to be a significantly improved pathway for the large-scale production of photothermal nanocrystals for drug delivery.
We make lipophilic CuS nanoparticles by mechanical grinding method in large scale.
The reaction condition is studied to obtain high yield and uniform size.
The synthesis does not need nitrogen protection or high temperature.
Lipophilic CuS nanoparticles show significant near-infrared absorbance.
Acknowledgements
The authors thank R. Rodgers for editing the manuscript, and R. Kingsley for assisting in transmission electron microscopy studies. This work was supported in part by grants from the National Institutes of Health (R01EB018748, P20GM103430, and P20GM104937), and by The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No.2012-05).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li Y, Lu W, Huang Q, Huang M, Li C, Chen W. Nanomedicine. 2010;5:1161–1171. doi: 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- 2.Cai Y, Ho JC, Batabyal SK, Liu W, Sun Y, Mhaisalkar SG, Wong LH. ACS Appl. Mater. Interfaces. 2013;5:1533–1537. doi: 10.1021/am303057z. [DOI] [PubMed] [Google Scholar]
- 3.Chung JS, Sohn HJ. J. Power Sources. 2002;108:226–231. [Google Scholar]
- 4.Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, Yan B, Lu W. ACS Nano. 2014;8:5670–5681. doi: 10.1021/nn5002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weissleder RA. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Wang J, Wu D. Inorg. Chem. 2009;48:7099–7104. doi: 10.1021/ic900201p. [DOI] [PubMed] [Google Scholar]
- 7.Ku G, Zhou M, Song S, Huang Q, Hazle J, Li C. ACS Nano. 2012;6:7489–7496. doi: 10.1021/nn302782y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu M, Yang S, Wang J, Hu J. Adv. Mater. 2011;23:3542–3547. doi: 10.1002/adma.201101295. [DOI] [PubMed] [Google Scholar]
- 9.Song S, Xiong C, Zhou M, Lu W, Huang Q, Ku G, Zhao J, Flores LG, Jr, Ni Y, Li C. J. Nucl. Med. 2011;52:792–799. doi: 10.2967/jnumed.110.086116. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C. J. Am. Chem. Soc. 2010;132:15351–15358. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Z, Wang S, Si D, Geng B. J. Alloys Compd. 2010;492:L44–L49. [Google Scholar]
- 12.Shen XP, Zhao H, Shu HQ, Zhou H, Yuan AH. J. Phys. Chem. Solids. 2009;70:422–427. [Google Scholar]
- 13.Thongtem S, Wichasilp C, Thongtem T. Mater. Lett. 2009;63:2409–2412. [Google Scholar]
- 14.Chen L, Shang Y, Liu H, Hu Y. Mater. Des. 2010;31:1661–1665. [Google Scholar]
- 15.Mageshwari K, Mali SS, Hemalatha T, Sathyamoorthy R, Patil PS. Prog. Solid State Chem. 2011;39:108–113. [Google Scholar]
- 16.Saldanha PL, Brescia R, Prato M, Li H, Povia M, Manna L, Lesnyak V. Chem. Mater. 2014;26:1442–1449. [Google Scholar]
- 17.Olivier J-C. NeuroRx. 2005;2:108–119. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood E, Simone E, Wattamwar P, Dziubla T, Muzykantov V. Nanomedicine (Lond) 2011;6:1257–1272. doi: 10.2217/nnm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghezelbash A, Korgel BA. Langmuir. 2005;21:9451–9456. doi: 10.1021/la051196p. [DOI] [PubMed] [Google Scholar]
- 20.Luther JM, Zheng H, Sadtler HB, Alivisatos AP. J. Am. Chem. Soc. 2009;131:16851–16857. doi: 10.1021/ja906503w. [DOI] [PubMed] [Google Scholar]
- 21.Sigman MB, Jr, Ghezelbash A, Hanrath T, Saunders AE, Lee F, Korgel BA. J. Am. Chem. Soc. 2003;125:16050–16057. doi: 10.1021/ja037688a. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HT, Wu G, Chen XH. Mater. Chem. Phys. 2006;98:298–303. [Google Scholar]
- 23.Gu Y, Huang J. Chem. Eur. J. 2013;19:10971–10981. doi: 10.1002/chem.201300649. [DOI] [PubMed] [Google Scholar]
- 24.Briggs D, Seah MP. Second ed. New York: John Willey & Sons; 1993. [Google Scholar]
- 25.Pang DW-P, Yuan F-W, Chang Y-C, Li G-A, Tuan H-Y. Nanoscale. 2012;4:4562–4570. doi: 10.1039/c2nr30915g. [DOI] [PubMed] [Google Scholar]
- 26.Sirtl T, Lischka M, Eichhorn J, Rastgoo-Lahrood A, Strunskus T, Heckl WM, Lackinger M. J. Phys. Chem. C. 2014;118:3590–3598. [Google Scholar]
- 27.Nakaya M, Kanehara M, Teranishi T. Langmuir. 2006;22:3485–3487. doi: 10.1021/la053504p. [DOI] [PubMed] [Google Scholar]
- 28.Lu XM, Tuan HY, Chen JY, Li ZY, Korgel BA, Xia YN. J. Am. Chem. Soc. 2007;129:1733–1742. doi: 10.1021/ja067800f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bu WB, Chen ZX, Chen F, Shi JL. J. Phys. Chem. C. 2009;113:12176–12185. [Google Scholar]
- 30.Mourdikoudis S, Liz-Marzán LM. Chem. Mater. 2013;25:1465–1476. [Google Scholar]