Abstract
Dasatinib is a kinase inhibitor indicated for the treatment of newly diagnosed adults with Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in chronic phase and accelerated (myeloid or lymphoid blast) phase, and CML with resistance or intolerance to prior therapy including imatinib and in adults with Ph+ acute lymphoblastic leukemia1 The most common adverse reactions (≥15%) in patients with newly diagnosed chronic-phase (CP) CML include myelosuppression, fluid retention, and diarrhea, whereas in patients with resistance or intolerance to prior imatinib therapy, side effects include myelosuppression, fluid retention, diarrhea, headache, dyspnea, skin rash, fatigue, nausea, and hemorrhage. We report a 39-year-old Ethiopian female patient who received dasatinib as upfront therapy for the treatment of CP-CML who experienced chronic diarrhea for two months, which progressed to hemorrhagic colitis due to cytomegalovirus (CMV) infection of the colon. To our knowledge, this is the first case of CMV colitis in a patient receiving dasatinib as upfront therapy.
Keywords: CML, dasatinib, colitis
Background
Imatinib has shown unprecedented efficacy in the treatment of newly diagnosed chronic-phase chronic myeloid leukemia (CP-CML). Approximately two-thirds of patients could benefit from imatinib as upfront therapy. Several trials of next-generation agents are in progress, with the aim of improving standard imatinib 400-mg-per-day therapy. In the post-imatinib era, a range of assessments are available for evaluating novel strategies.2 Based on existing evidence, achieving a complete cytogenetic response (CCyR) within 12 months provides the best prediction for longer term benefit, although durable response and freedom from disease progression are the main treatment goals. In phase II studies, first-line treatment with dasatinib 100 mg once daily or Nilotinib 300 mg twice daily resulted in high rates of CCyR and major molecular response (MMR) compared with historical data.3 The production and analysis of emerging data from next-generation agents and modified imatinib-based strategies are likely to be an important area of debate during the next few years. Therefore, it is of paramount importance to report the events associated with the use of these medications since it may affect treatment decision by the patient or the prescribing physician.4 Cytomegalovirus (CMV) is a double-stranded DNA virus in the herpes virus family that can cause disseminated or localized end-organ disease in patients with advanced immunosuppression. Most clinical disease occurs in previously infected (seropositive) individuals and therefore represents either reactivation of latent infection or reinfection with a novel strain.
End-organ disease caused by CMV occurs in patients with advanced immunosuppression, typically in those with CD4 T-lymphocyte cell (CD4) counts <50 cells/mm3, who are either not receiving or have failed to respond to antiretroviral therapy. Other risk factors include previous opportunistic infections and a high level of CMV viremia (most often measured by polymerase chain reaction [PCR]).
Previous case reports have indicated that CD8-positive T-lymphocytes infiltrate the colonic mucosa in dasatinib-related hemorrhagic colitis, and the same pathological findings were seen in our case. Dasatinib may cause hemorrhagic colitis via immunological mechanisms in CML.5
Primary CMV infection typically runs an undifferentiated viral syndrome or is manifested by a mononucleosis-like syndrome. Infections in the immunocompetent and immunosuppressed are not rare; seroprevalence for CMV worldwide ranges from ~60% to 100%/1 Symptomatic CMV infection in nonimmunocompromised hosts has traditionally been considered to have a benign, self-limited course. However, in the medical literature, there are a considerable number of reports of severe clinical manifestations of CMV infection in immunocompetent patients.
The diagnosis of CMV infection for this review required at least one of the following laboratory methods: serology, specific intrathecal antibody production, virus isolation, direct detection of CMV pp65 antigen in blood, CMV culture, biopsy, positive specific immunohistochemical staining, PCR assay (mainly quantitative results examined together with clinical findings as false-positive results are a possibility), confocal microscopy of the eyes (to detect the “owl’s eye” morphology in the corneal endothelium), or in situ hybridization. Serological studies indicating an acute CMV infection included the presence of positive IgM anti-CMV antibodies or a significant increase in the titer of IgG anti-CMV antibodies in paired samples obtained during the infection.
The symptoms observed in patients with CMV involvement of the Gastro intestinal tract (GIT) included fever, diffuse abdominal pain, or pain located in the lower abdominal quadrants, anorexia, nausea, vomiting, weight loss, watery or bloody diarrhea, hematochezia, and melena. The signs recorded in the physical examination of these patients included abdominal tenderness or rebound tenderness and abnormal bowel sounds. The diagnostic investigations performed in these patients included abdominal ultrasound, computed tomography of the abdomen, GIT endoscopy (gastroscopy, colonoscopy, and sigmoidoscopy) with biopsy, and stool cultures.
Central nervous system (CNS) disorders constituted the second most frequent manifestations of CMV infection in immunocompetent patients. Specifically, patients with involvement of the CNS by CMV presented with various combinations of the following symptoms and signs: fever, chills, fatigue, myalgia, motor deficits (localized weakness, paraplegia), sensory abnormalities (numbness, hypoesthesia, paresthesia, dysesthesia, anesthesia), disorientation, confusion, unilateral or bilateral visual loss, urinary retention, constipation, or coma.
Case Presentation
A 39-year-old Ethiopian female discovered incidentally to have leukocytosis on routine checkup was referred to the Hematology Clinic at the National Center for Cancer Care and Research where her repeated Complete Blood Count (CBC) and peripheral smears were suggestive of the diagnosis of CML. Her clinical examination was not remarkable, with no hepatosplenomegaly. Bone marrow aspiration as well as cytogenetic studies and BCR/ABL by PCR confirmed the diagnosis of CML in a chronic phase with a low Sokal score. Treatment options were discussed with the patient and both pros and cons for each therapy were explained. The patient opted for dasatinib as upfront therapy, which was started as 100 mg orally once daily and the patient was followed up as per European leukemia net (ELN) guidelines. She achieved CHR, CCyR, and MMR after receiving dasatinib for 30 month. However, during her last clinic visit, she complained of diarrhea, which she described as having been watery for the past 2 months. During the last week, she described bloody diarrhea. The patient was admitted to the hospital for further evaluation and management. Her workup showed normal hemogram and normal serum concentrations of creatinine, urea, hepatic enzymes, and electrolytes on admission. Colonoscopy showed multiple, linear, polypoidal-like lesions all over the colon up to the hepatic flexure. Endoscopic diagnosis revealed a picture of infectious colitis suggestive of CMV, Clostridium difficile, and opportunistic parasitic infections less likely to be leukemic deposits or chronic colitis (Figs. 1–4).
Figure 1.
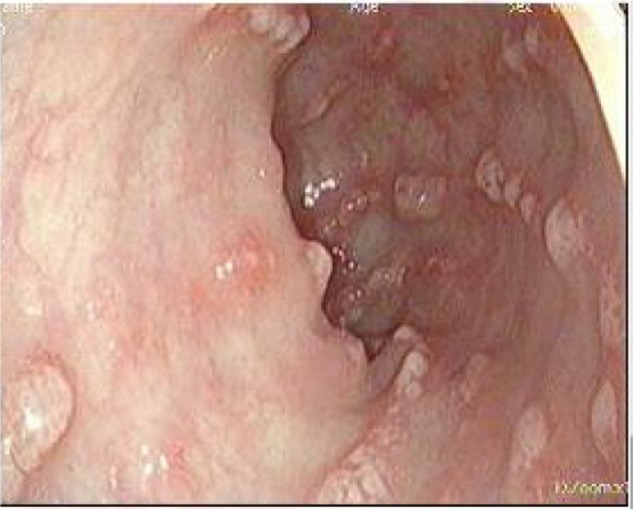
Colonic liner polypoidal lesion, which consists of multiple polypoidal tan brown soft tissue.
Figure 2.
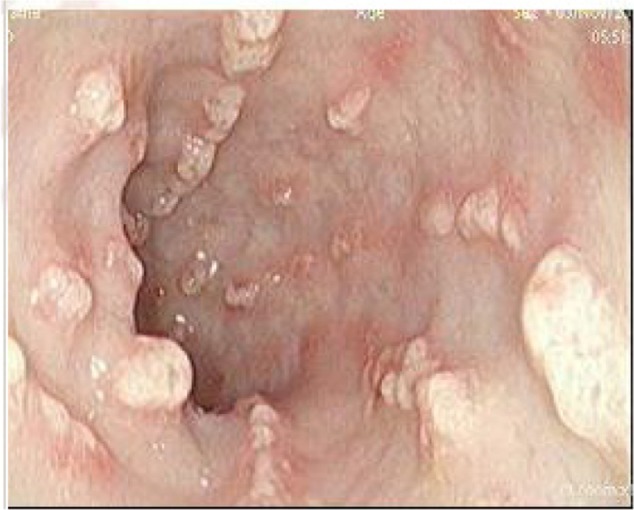
2nd picture of polypoid lesion.
Figure 3.
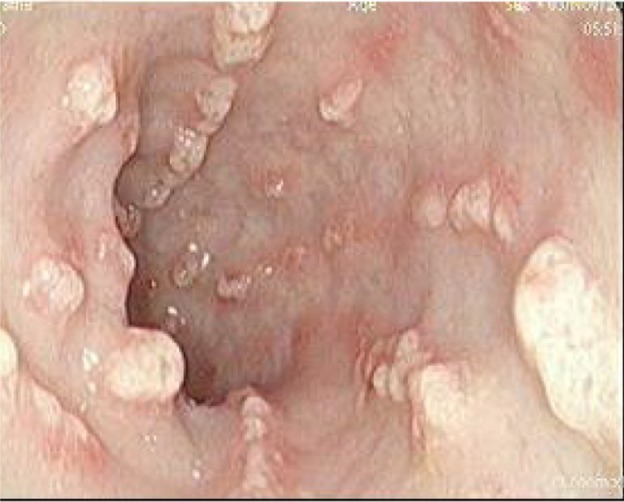
Third view of polypoid lesions.
Figure 4.
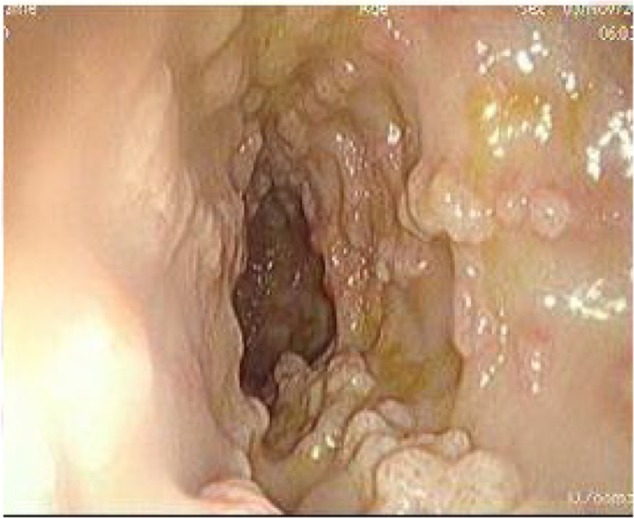
Another view of polypoid lesion.
Histopathology report showed colonic liner polypoidal lesions that consisted of multiple polypoidal, tan brown soft tissue fragments in aggregate, which measured 1 × 0.5 cm (all embedded with one cassette). CMV colitis is diagnosed by the presence of CMV inclusions, using immunohistochemistry stain, with mixed inflammatory cell infiltrate into the kamiapropria with numerous neutrophils associated with cryptitis and focal ulceration. The biopsy was negative for leukemic/lymphoma infiltrate.
Stool microbiology as well as the clostridium toxin essay were negative. On admission, dasatinib was stopped immediately and diarrhea improved spontaneously after 5 days. The patient received Ganciclovir for a total of 6 weeks, and her follow-up colonoscopy proved normalization of the colonic mucosa.
Discussion
CMV is a herpes-type virus, which is related to the virus that causes chickenpox. Infection with CMV is very common. It spreads by saliva, urine, respiratory droplets, sexual contact, and blood transfusions. Most people are exposed at some point of their life. However, most of the time the virus produces mild or no symptoms in healthy people. Between 50% and 80% of the world’s population is seropositive for CMV. Initial CMV infection in the immunocompetent host typically is mild and goes undetected clinically.6 This is followed by a chronic latent state, during which the virus remains present within host cells, but viral proliferation is prevented by host cell–mediated immunity. Failure of immune containment may lead to reactivation with viral proliferation and severe systemic illness. Systemic CMV disease is characterized by fever, pancytopenia, and inflammatory changes in multiple organs including the liver and lungs and in the retina. Colitis is a frequent manifestation of this acute systemic illness. Serious CMV infections can occur in people with weakened immune systems due to chemotherapy treatment for cancer, immune-suppressing medicines following an organ transplant, and inflammatory bowel disease (ulcerative colitis [UC] or Crohn’s disease). Rarely, serious CMV infection involving the GI tract has occurred in people with a healthy immune system.7
Histological diagnosis is considered the “gold standard” for diagnosing CMV disease in the gastrointestinal tract8–13 and includes many methods. Histological staining using hematoxylin and eosin (HE) typically reveals cytomegalic cells that are two- or fourfold larger than normal cells, containing basophilic intranuclear inclusions in eccentric locations surrounded by a clear halo, giving it an “owl’s eye” appearance. Cells show a thickened nuclear membrane and smaller granular intracytoplasmic inclusions. Colonic biopsies should be taken from inflamed mucosa near or within the ulcer. This method has very high specificity (92%–100%), but its main problem is a poor sensitivity (10%–87%), making the diagnosis require many samples and a trained pathologist.8,9 Anecdotally, cytomegalic cells have been described in colon biopsies from normal mucosa in healthy individuals.10,11 Another method is immunohistochemical (IHC) detection of CMV antigensin-infected cells from colon biopsies. It has a higher sensitivity than HE (78%–93%).12,13 Other techniques for CMV detection do not rely on histology. PCR DNA amplification assay in colon mucosa has the greatest accuracy for virus detection14,15 and may be used as a qualitative or quantitative method. PCR DNA levels in the colon are not related to viremia levels measured in blood.16,17 Some studies detected prevalence greater than 30% in Inflammatory bowel disease patients (IBD) patients.15 However, the significance of a positive result in the absence of histological signs of CMV infection is unclear. Very few studies have shown a correlation between histology (HE/IHC) and PCR results.18 This suggests that detection of low DNA levels could determine latent infection and requires a cut-off level of viremia to distinguish infection from disease. In other words, a positive result in the colon does not necessarily reflect the involvement of CMV in UC flare-ups. A recent study from France suggests a cut-off of >250 copies/mg for diagnosing CMV disease14 with a sensitivity of 100% and a specificity of 66%. To avoid false-positive results, and awaiting further studies, this determination should be performed only in patients with active UC refractory to conventional treatment. Viral culture was previously regarded as the gold standard in CMV detection with a sensitivity of 45%–78% and a very high specificity (89%–100%). The virus is placed in a fibroblast tissue culture, and diagnosis is made once the virus causes cytopathic changes in fibroblasts. The problem is that the result takes days to weeks,11 so this method is not used in clinical practice.15
Typical endoscopic findings of CMV colitis include microerosions, deep ulcers, and pseudotumoral lesions.16 Most studies in patients with IBD, specifically in active UC, have not found specific endoscopic features.9 Anecdotal studies describe some characteristic endoscopic ulcers (large, irregular, punched-out, or longitudinal ulcers) caused by virus.17 Discrepancy can be explained by the different criteria used to define CMV infection or disease.
In summary, different methods of detection of CMV have different sensitivities and specificities. In the setting of a severe UC flare-up refractory to conventional treatment, colonic IHC or PCR in blood should be performed; PCR presents a good correlation with the results of HE and IHC. Serology and antigenemia only are useful as negative predictors for CMV infection, since they do not correlate with actual CMV colitis. The role of PCR positivity in colon remains to be determined; it is not known how to interpret its positivity in the absence of histological changes. However, recent studies suggest positivity of this method in the disease prognosis.16 Current European guidelines18 recommend the use of tissue PCR or IHC to detect CMV in patients with UC resistant to immunomodulators, whereas older American guidelines19 recommend performing sigmoidoscopic biopsy and CMV viral culture in refractory cases of UC.
Dasatinib treatment of CML has been associated with CMV infections in very few cases. One case was reported by Nishant Patodi et al in a 59-year-old male with CP-CML, who received dasatinib as a second-line treatment and the event happened 3 years after the initiation of dasatinib. The second case was reported by Zahra Karima et al in a 36-year-old male with CML transforming to blast crisis acute myeloid leukemia (AML) who received dasatinib at a dose of 140 mg orally once daily after Doxorubicin Adriamycin (DA) or 3-plus-7 protocol on day 34 of treatment. Our patient is the third case who received dasatinib as upfront treatment of CML chronic phase and colitis was associated with CMV so it seems that dasatinib triggered the colitis and CMV infection superimposed. To the best of our knowledge, this is the first described case of CMV colitis in patients with CML who progressed to hemorrhagic colitis with improvement in few days after stopping dasatinib treatment.
Colitis appears to have a relation with the phase of CML. It has been described during the blast crisis of CML after imatinib failure and in CP-CML when used as upfront treatment. However, it was not reported during the accelerated phase or blast lymphoid phase of the disease. A recent report looking at bleeding diathesis in CML patients receiving dasatinib therapy noted that 81% of all bleeding episodes were confined to the gastrointestinal tract.20 Gastrointestinal bleeding is consistent with the oral route of dasatinib, which is eliminated in the feces. Therefore, the lower gastrointestinal tract may be particularly vulnerable after being exposed to dasatinib during its elimination and this may explain the occurrence of hemorrhagic colitis in our patient.
Mustjoki et al described an association between lymphocytosis secondary to significant expansion of clonal large granular lymphocytes (LGL) and patients receiving dasatinib.21 Dasatinib-induced complications such as colitis and pleural effusions have developed in a marked proportion of patients who have had LGL expansion, and therefore, clonal LGL expansion may play a potential pathogenic role in bleeding induced by dasatinib.21 In conclusion, prolonged diarrhea in patients with CML receiving dasatinib for chronic or blast phase should be taken seriously since colitis due to CMV is a possibility.
Acknowledgments
This research is conducted as part of Qatar National Research Funds (QNRF)-sponsored project (Novel approach in Myeloproliferative Neoplasms what determines the Pathophysiology NPRP No: 4-471-3-148).
Footnotes
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
FUNDING: This research is part of proposal sponsored by Qatar National Research Funds NPRP 4-471-3-148. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MAY. Analyzed the data: MAY. Wrote the first draft of the manuscript: MAY. Contributed to the writing of the manuscript: MAY. Agree with manuscript results and conclusions: MAY, AJN, ATS, AY, AM, AA, SFM, DSM, SE, D-RA, H-LGG, MA, RMH, AHM, HED, NA, SB, SK. Jointly developed the structure and arguments for the paper: MAY. Made critical revisions and approved final version: MAY, AJN, ATS, AY, AM, AA, SFM, DSM, SE, D-RA, H-LGG, MA, RMH, AHM, HED, NA, SB, SK. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29:2289–308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–50. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 3.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 4.Saglio G, Baccarani M. First-line therapy for chronic myeloid leukemia: new horizons and an update. Clin Lymphoma Myeloma Leuk. 2010;10(3):169–76. doi: 10.3816/CLML.2010.n.026. [DOI] [PubMed] [Google Scholar]
- 5.Kmira Z, et al. World J Gastrointest Pathophysiol. 2013;4(3):59–62. doi: 10.4291/wjgp.v4.i3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang LJ, Hanff P, Rutherford C, Churchill WH, Crumpacker CS. Detection of human cytomegalovirus DNA, RNA, and antibody in normal donor blood. J Infect Dis. 1995;171:1002–6. doi: 10.1093/infdis/171.4.1002. [DOI] [PubMed] [Google Scholar]
- 7.Pass RF. Epidemiology and transmission of cytomegalovirus. J Infect Dis. 1985;152:243–8. doi: 10.1093/infdis/152.2.243. [DOI] [PubMed] [Google Scholar]
- 8.Wu GD, Shintaku IP, Chien K, Geller SA. A comparison of routine light microscopy, immunohistochemistry, and in situ hybridization for the detection of cytomegalovirus in gastrointestinal biopsies. Am J Gastroenterol. 1989;84:1517–20. [PubMed] [Google Scholar]
- 9.Cotte L, Drouet E, Bissuel F, Denoyel GA, Trepo C. Diagnostic value of amplification of human cytomegalovirus DNA from gastrointestinal biopsies from human immunodeficiency virus-infected patients. J Clin Microbiol. 1993;31:2066–9. doi: 10.1128/jcm.31.8.2066-2069.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieterich DT, Rahmin M. Cytomegalovirus colitis in AIDS: presentation in 44 patients and a review of the literature. J Acquir Immune Defic Syndr. 1991;4(suppl 1):S29–35. [PubMed] [Google Scholar]
- 11.Franzin G, Muolo A, Griminelli T. Cytomegalovirus inclusions in the gastroduodenal mucosa of patients after renal transplantation. Gut. 1981;22:698–701. doi: 10.1136/gut.22.9.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaugerie L, Cywiner-Golenzer C, Monfort L, et al. Definition and diagnosis of cytomegalovirus colitis in patients infected by human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:423–9. doi: 10.1097/00042560-199704150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–65. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar A, Boström L, Lagerstedt U, Magnusson I, Söder-berg-Naucler C, Sundqvist VA. Evidence of active cytomegalovirus infection and increased production of IL-6 in tissue specimens obtained from patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2003;9:154–61. doi: 10.1097/00054725-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Robey SS, Gage WR, Kuhajda FP. Comparison of immunoperoxidase and DNA in situ hybridization techniques in the diagnosis of cytomegalovirus colitis. Am J Clin Pathol. 1988;89:666–71. doi: 10.1093/ajcp/89.5.666. [DOI] [PubMed] [Google Scholar]
- 16.Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001–8. doi: 10.1038/ajg.2011.202. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol. 2010;16:1245–9. doi: 10.3748/wjg.v16.i10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahier JF, Ben-Horin S, Chowers Y, et al. European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47–91. doi: 10.1016/j.crohns.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–85. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 20.Christopher J, Cui D, Wuetal C, et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos. 2008;36:1357–64. doi: 10.1124/dmd.107.018267. [DOI] [PubMed] [Google Scholar]
- 21.Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398–405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]


