Abstract
Background
Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung carcinoma cases, which becomes more and more important in the field of lung carcinoma as well as primary lung carcinoma in females.
Material/Methods
We analyzed the medical history of 62 female NSCLC patients. Immunohistochemistry was used to observe and compare the expression of EGFR. The chi-square test was conducted to analyze associations between EGFR expression and the different variables. The cumulative survival rate was determined by the Kaplan-Meier product-limit method. The prognosis of female patients with NSCLC was examined by using a multivariate Cox proportional hazard regression model.
Results
The expression proportion of EGFR in Chinese female NSCLC patients was 70.97%, and it was remarkably higher in adenocarcinoma than in squamous cell carcinoma and bronchioloalveolar carcinoma. A positive correlation was observed between EGFR expression and tumor-node metastasis staging or lymph node metastasis. The Cox proportional risk model analysis showed a correlation between postoperative survival time of the patients and pathology of the tumor type and lymph node metastasis.
Conclusions
Expression of EGFR was closely related to pathology of the tumor type, tumor-node metastasis staging, and lymph node metastasis, which could be used as a promising indicator of NSCLC in Chinese female patients.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Female; Immunohistochemistry; Receptors, Vascular Endothelial Growth Factor
Background
Lung carcinoma is the mostly frequently diagnosed cancer in the world and is also the most common cause of cancer deaths in males and females all over the world [1,2]. Female lung cancer, with mortality higher than that of the combined mortality from breast and colon/rectum cancer, accounted for 26% of the estimated cancer deaths in 2012. Smoking remains the largest risk factor for lung cancer in women [3]. A recently study, in which never smokers with lung cancer were frequently women, demonstrated an increased prevalence of EGFR mutations in never-smoking lung cancer patients with familial cancer history [4]. Hsieh et al. also reported a much higher mutation of loci related to NSCLC in females than in males, as detected by sequencing [5]. This trend of rapidly increasing incidence of lung carcinoma in females has recently drawn much attention.
Lung cancer is broadly classified as small cell lung cancer originating from neuroendocrine cells versus non-small cell lung cancer (NSCLC) originating from bronchial epithelial cell precursors. NSCLC can be divided into 3 types: squamous cell carcinoma, adenocarcinoma, and large cell carcinoma [6]. Among all types of lung carcinoma, non-small cell lung cancer (NSCLC) accounts for approximately 85% of cases. The 5-year survival rate is still about 17%, despite improvements in cancer therapies over the past few decades [7]. Other effective therapies without adverse effects are badly needed. Although many indicators of lung carcinoma have been found [8], there has been limited work in developing indicators from the perspective of potentially unique or vulnerable subpopulations, such as females. As the understanding of lung carcinoma evolves, researchers found that a kinase, epidermal growth factor receptor (EGFR), is overexpressed in 40–80% of NSCLC patients, and is therefore an important target of interest for therapy in this disease. EGFR is the expression product of the proto-oncogene c-erbB-1 and possesses tyrosine kinase (TK) activity. In general, EGFR is seldom detected in NSCLC. However, Hirsch et al. reported that the expression rate of EGFR was 50% in female NSCLC cases, which revealed the indicator potential of EGFR with the increasing incidence of female NSCLC [9]. Recent studies have shown that the EGFR receptor is overexpressed in NSCLC and several other types of cancers [10]. Clinical research has demonstrated that EGFR inhibitors have a relatively good therapeutic effect on female lung carcinoma, particularly adenocarcinoma. According to a report [11], use of EGFR mutations-specific tyrosine kinase inhibitors (TKI), such as erlotinib, gefitinib, or afatinib, is the most effective treatment strategy of NSCLC that is targeted to EGFR. Gefitinib and erlotinib significantly increased overall survival (OS) and progression-free survival (PFS) compared with placebo or best support care (BSC) [12].
In this study, we investigated the expression of EGFR in Chinese female NSCLC patients, trying to explore the relationship between EGFR expression and the clinical and pathological characteristics in prognosis of female NSCLC patients. Our results showed that high expression of EGFR was observed in female NSCLC and its expression was closely related to pathology of the tumor type, TNM staging, and LNM. Our study shows that EGFR is an important indicator of disease progression and prognosis in female NSCLC, and that it can be used to investigate the target therapy in female NSCLC.
Material and Methods
Patients and data collection
We enrolled 62 female patients diagnosed with pathologic stages I to IV NSCLC from January 2000 to June 2002 at the archives of the Changzheng Hospital. Of the 62 specimens from patients aged 32–75 years (mean, 56 years), none had received chemotherapy, radiotherapy, or any other anticancer therapies; 46 had adenocarcinoma, 15 had bronchioloalveolar carcinoma (BAC), and 16 had squamous cell carcinoma (SCC). Poorly-differentiated, moderately-differentiated, and well-differentiated carcinoma was observed in 22, 25, and 15 cases, respectively. Lymph node metastasis was observed in 37 cases and non-lymph node metastasis was observed in 25 cases. Stage I, stage II, stage III, and stage IV were observed in 7, 25, 28, and 2 cases, respectively. Sixty were non-smokers and 2 were smokers (Table 1). The survival period was expressed in months until death or the last recorded follow-up; this period was determined through telephone or mail inquiries until Oct 31, 2005. Thirty-four patients survived less than or equal to 3 years and 28 patients survived more than 3 years. Ten control samples were randomly selected from the nonmalignant lung tissue of female patients during the study period. This investigation was approved by the Research Ethics Committee and the written informed consent was obtained from all individuals. According to personal medical records and individual interviews from research group and control group, a uniform epidemiology questionnaire was designed to obtain patients’ information, including sex, age, smoking, and alcohol consumption.
Table 1.
Patients’ characteristics.
| Groups | Cases |
|---|---|
| Tumor type | |
| Adenocarcinoma | 46 |
| Bronchioloalveolar carcinoma (BAC) | 15 |
| Squamous cell carcinoma (SCC) | 16 |
| Tumor differentiation degree | |
| Poorly-differentiated carcinoma | 22 |
| Moderately-differentiated carcinoma | 25 |
| Well-differentiated carcinoma | 15 |
| LNM | |
| Lymph node metastasis | 37 |
| Non-lymph node metastasis | 25 |
| TNM staging | |
| Stage I | 7 |
| Stage II | 25 |
| Stage III | 28 |
| Stage IV | 2 |
| Smoking history | |
| Non-smokers | 60 |
| Smokers | 2 |
Immunohistochemical staining
A biotin-free immunohistochemical staining technique was used for detecting EGFR expression according to the manufacturer’s instructions (Dako). Serial sections were placed on l-lysine coated slides, deparaffinized in xylene, and rehydrated through an ethanol gradient. Antigen retrieval was achieved by slide immersion in pepsin solution for 15 min at 37°C. Endogenous peroxidase activity was blocked by incubating the sections in 3% hydrogen peroxide for 15 min, and nonspecific binding was blocked with 10% goat serum for 15 min. The sections were then incubated with anti-EGFR monoclonal antibody (1:1000) at 4°C overnight in a humid chamber. Subsequently, the sections were incubated with EnVision™ reagent for 30 min at room temperature. The sections were visualized with the application of diaminobenzidine substrate chromogen solution and hematoxylin counterstain. After dehydrating, the sections were mounted in Paramount (Dako).
Evaluation of EGFR immunostaining
The positive cells were determined as described previously [13]. The staining index (SI) was based on the percentage of positive cells and staining intensity. The positive cell percentage was scored as follows: 0–5% (score 1), 5%–25% (score 2), 51–75% (score 3), and >76% (score 4). The staining intensity was scored as follows: no staining (score 1), brown-yellow (score 2), and chocolate brown (score 3). The SI score was the sum of the positive cell percentage and staining intensity scores. An SI score of 0 to 2 indicated negative expression, and a score of 3 to 7 indicated positive expression.
Statistical analysis
We looked for associations between EGFR expression and various patient characteristics, including age, smoking history, pathology of the tumor type, TNM staging, and LNM. For statistical analysis, the chi-square test was conducted to analyze associations between EGFR expression and the different variables. The cumulative survival rate was determined by the Kaplan-Meier product-limit method. The prognosis of female patients with NSCLC was examined by using a multivariate Cox proportional hazard regression model. Statistical analysis was carried out using a commercially available computer program (SPSS version 10.0; SPSS; Chicago, IL). Significance was defined at p<0.05.
Results
Expression of EGFR in female NSCLC and its relationship with clinical and pathological characteristics of NSCLC
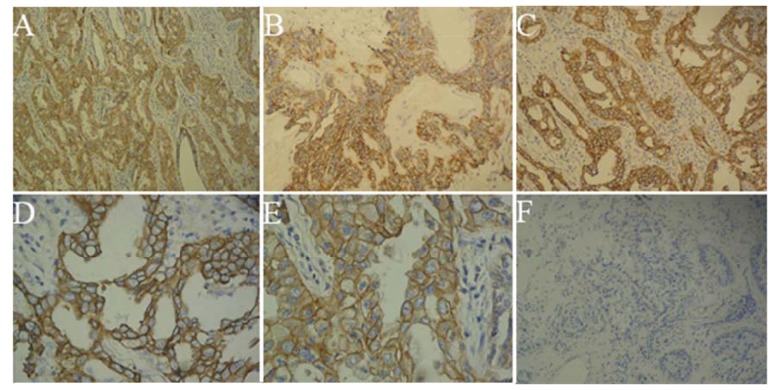
Positive staining of EGFR was observed in the cytoplasm and cytomembrane of tumor cells, which was characterized by brownish-yellow particles. Staining efficacy varied among different tissues, as indicated by the pathological images (Figure 1). EGFR expression was positive in 70.97% (44/62) of female NSCLC cases, while it was significantly negative in the control group with nonmalignant lung tissues (p=0.000014).
Figure 1.
Expression of EGFR in sample tissues (A–F). (A) Positive expression of EGFR in female adenocarcinoma (×100); (B) Positive expression of EGFR in female squamous cell carcinoma (×100); (C) Positive expression of EGFR in female bronchioloalveolar carcinoma (×100); (D) Positive expression of EGFR in female bronchioloalveolar carcinoma (×400); (E) Positive overexpression of EGFR in female adenocarcinoma (×400); (F) Negative expression of EGFR in female non-malignant lung tissues cases (×100).
Detail information of EGFR expression is shown in Table 2. The expression of EGFR in female NSCLC showed dramatic differences in different pathology of the tumor type, with or without LNM, and various TNM staging (p<0.05), indicating the various expression types of EGFR with different pathological characteristics. However, no remarkable correlations between EGFR expression and age, smoking history, tumor size, tumor site, tumor differentiation degree, or tumor distal metastases were found (p>0.05).
Table 2.
Relationships between expression of EGFR and clinical and pathological characteristics of female NSCLC patients.
| Parameter | Total cases | EGFR | χ2 | P value | ||
|---|---|---|---|---|---|---|
| + | − | Positive rate (%) | ||||
| Ages | ||||||
| ≤60 | 35 | 23 | 12 | 65.71% | ||
| <60 | 27 | 21 | 6 | 77.78% | 1.0766 | 0.2995 |
| Smoking history | ||||||
| Yes | 60 | 43 | 17 | 71.67% | ||
| No | 2 | 1 | 1 | 50.00% | 0.4410 | 0.5066 |
| Pathological type | ||||||
| Squamous cell | 16 | 8 | 8 | 50.00% | ||
| Adenocarcinoma | 46 | 36 | 10 | 78.26% | 4.6017 | 0.0319a |
| Bronchioloalveolar carcinoma | 15 | 13 | 2 | 86.67% | 4.7676 | 0.0290b |
| Tumor size | ||||||
| ≤3 cm | 34 | 25 | 9 | 73.53% | ||
| <3 cm | 28 | 19 | 9 | 67.86% | 0.2398 | 0.6244 |
| Tumor position | ||||||
| Central | 22 | 18 | 4 | 81.82% | ||
| Peripheral | 40 | 26 | 14 | 65.00% | 1.9485 | 0.1627 |
| Lymph nodes metastasis | ||||||
| No | 25 | 14 | 11 | 56.00% | ||
| Yes | 37 | 30 | 7 | 81.08% | 4.5551 | 0.0328 |
| TNM stage | ||||||
| I–II | 32 | 19 | 13 | 59.38% | ||
| III–IV | 30 | 25 | 5 | 83.33% | 4.3137 | 0.0378 |
| Tumor grade | ||||||
| High | 22 | 15 | 7 | 68.18% | ||
| Middle | 25 | 19 | 6 | 76.00% | ||
| Low | 15 | 10 | 5 | 66.67% | 0.5208 | 0.7707 |
| Distant metastases | ||||||
| M0 | 59 | 42 | 17 | 71.19% | ||
| M1 | 3 | 2 | 1 | 66.67% | 0.0283 | 0.8664 |
– adenocarcinoma compared squamous cell;
– bronchioloalveolar carcinoma compared squamous cell;
bronchioloalveolar carcinoma (total cases: 15) is divided from adenocarcinoma (total cases: 46).
Relationship between the expression of EGFR and survival time of the patients
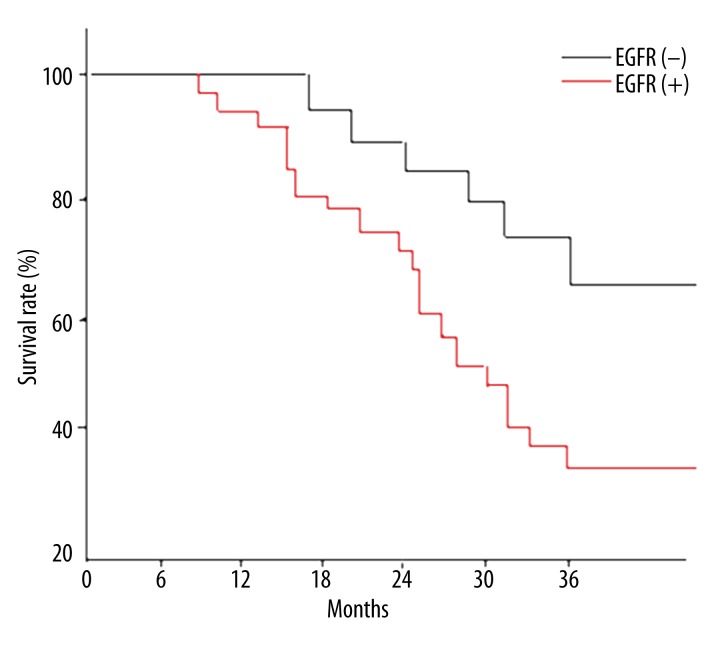
Of the 44 cases with positive EGFR expression, survival time was less than or equal to 3 years in 28 cases and greater than 3 years in 16 cases. In the 18 cases with negative EGFR expression, the survival time was less than or equal to 3 years in 6 cases and greater than 3 years in 12 cases. The 3-year survival rate of the group with positive EGFR expression was significantly lower than that of the group with negative EGFR expression (p 0.0295). The Kaplan-Meier survival curve based on the relationship between the different levels of expression of EGFR and survival time is shown in Figure 2.
Figure 2.
Survival curve of positive expression group and negative expression group of EGFR in female NSCLC.
Cox proportional risk model analysis
A Cox proportional risk regression model was constructed according to patient’s age, pathology of the tumor type, tumor size, tumor site, LNM, surgical method, EGFR expression, and survival time. The analysis of the Cox proportional risk model indicated that the postoperative survival time was significantly associated with pathology of the tumor type and LNM (p<0.05). Adenocarcinoma with LNM was an independent poor prognostic factor (i.e., positive Cox proportional risk regression model) (Table 3); however, patient age, tumor size, tumor site, surgical method, and EGFR expression were not independent prognostic factors.
Table 3.
Positive result of Cox’s proportional hazard model analysis.
| Parameter Estimate | Standard error | Wald Chi-square | Pr >Chi-square | Risk ratio | χ2 | P value | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Pathological type | 2.301599 | 1.14890 | 4.01326 | 0.0451 | 9.990 | 10.4377 | 0.0012 | 1.384~5.676 |
| Lymph nodes metastasis | 1.742543 | 0.84438 | 4.25886 | 0.0390 | 5.712 | 4.7899 | 0.0286 | 1.137~4.531 |
| Age | 0.165329 | 0.19372 | 1.367014 | 0.8741 | 4.410 | 0.1964 | 0.6576 | 0.678~2.653 |
| Tumor size | 0.193175 | 0.32408 | 0.67936 | 0.6024 | 2.579 | 2.3063 | 0.1288 | 0.512~1.607 |
| Tumor site | 0.731946 | 0.39814 | 1.78639 | 0.1728 | 3.287 | 1.0593 | 0.3034 | 0.926~2.083 |
| Surgical method | 0.835932 | 0.29681 | 1.21642 | 0.4351 | 1.726 | 1.6197 | 0.2031 | 0.546~2.347 |
| EGFR expression | 0.148724 | 0.91075 | 2.01348 | 0.1376 | 2.405 | 0.4385 | 0.5078 | 1.084~2.513 |
Discussion
The expression of EGFR in tumor tissues is closely related to pathology of the tumor types, differentiation degree, LNM, and tumor invasion; moreover, a correlation has been observed between EGFR expression and patient prognosis [13,14]. In this study, we recruited Chinese female NSCLC patients as subjects to explore the expression of EGFR in female NSCLC. The expression rate of EGFR was 70.97% in Chinese female NSCLC cases, which was higher than that reported in previous studies [15]. With more detail classification of pathology in our research, we found that the expression of EGFR in female adenocarcinoma was higher than that in SCC and even higher than that in female BAC; the expression of EGFR was positively correlated with LNM and TNM staging, and it was not associated with patient age, smoking history, tumor size, tumor site, differentiation degree, and distal metastases.
Some studies confirmed that female adenocarcinoma and BAC were also associated with EGFR gene mutations; therefore, the high level of expression of EGFR in female NSCLC might be related to female EGFR gene mutation [16–18], which corresponded with our immunohistochemical results. The relationship between EGFR expression level and NSCLC prognosis is disputable. Some studies have indicated that the expression of EGFR is associated with a reduction in disease-free survival time and total survival time, poor prognosis, easy recurrence, late staging, and high metastasis rate, but other studies have reported contradictory findings [19,20].
Our study showed that the 3-year survival rate of female NSCLC patients with positive EGFR expression was clearly lower than that of patients with negative EGFR expression. The Cox proportional risk model analysis demonstrated that the postoperative survival time of the patients was significantly associated with pathology of the tumor type and LNM (P<0.05), and that adenocarcinoma with LNM was the independent poor prognostic factor. Currently, in the treatment of tumors, target therapy of lung cancer has drawn much attention. EGFR is an important target for tumor inhibition, which affects the signal transduction system of tumor cells and suppresses tumor proliferation, invasion, metastases, and angiogenesis. Clinical research has validated the therapeutic effects of the EGFR-TK inhibitor in female patients with adenocarcinoma. This conforms to the cohort with EGFR expression in the study. Therefore, the study of EGFR expression in Chinese female NSCLC can predict the pathogenesis, development, and prognosis of the disease in Chinese female NSCLC patients and can also be a good choice for the target therapy of female NSCLC.
Conclusions
The expression of EGFR is high (70.97%) in female NSCLC and the positive EGFR expression exhibited a reduction in 3-year survival rate compared with those of the negative EGFR expression. Our results also showed that EGFR expression was closely related to pathology of the tumor type, tumor-node metastasis staging, and lymph node metastasis, which could be used as a promising indicator of NSCLC in Chinese female patients. The points above could be applied to Chinese female NSCLC patients. A more comprehensive study that includes male patients and different races is needed to determine if our results have universal applicability.
Footnotes
Declaration of interests
The authors declare that they have no competing interests.
Source of support: Departmental sources
References
- 1.Travis WD, Lubin J, Ries L, et al. Epidermal growth factor receptor-tyrosine kinase inhibitors for non-small-cell lung cancer patients aged 80 years or older: A retrospective analysis. Mol Clin Oncol. 2015;3:403–7. doi: 10.3892/mco.2014.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel JD. Lung cancer in women. J Clin Oncol. 2005;23:3212–18. doi: 10.1200/JCO.2005.11.486. [DOI] [PubMed] [Google Scholar]
- 3.North CM, Christiani DC. Women and lung cancer: what is new? Semin Thorac Cardiovasc Surg. 2013;25:87–94. doi: 10.1053/j.semtcvs.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng PC, Cheng YC. Correlation between familial cancer history and epidermal growth factor receptor mutations in Taiwanese never smokers with non-small cell lung cancer: a case-control study. J Thorac Dis. 2015;7:281–87. doi: 10.3978/j.issn.2072-1439.2015.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh RK, Lim KH, Kuo HT, et al. Female sex and bronchioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest. 2005;128:317–31. doi: 10.1378/chest.128.1.317. [DOI] [PubMed] [Google Scholar]
- 6.Marshall AL, Christiani DC. Genetic susceptibility to lung cancer – light at the end of the tunnel? Carcinogenesis. 2013;34:487–502. doi: 10.1093/carcin/bgt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Luo J, Zhai X, et al. Prognostic value of phospho-Akt in patients with non-small cell lung carcinoma: a meta-analysis. Int J Cancer. 2014;135:1417–24. doi: 10.1002/ijc.28788. [DOI] [PubMed] [Google Scholar]
- 8.Krzyzanowska MK, Barbera L, Elit L, et al. Identifying population-level indicators to measure the quality of cancer care for women. Int J Qual Health C. 2011;23:554–64. doi: 10.1093/intqhc/mzr043. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch FR, Varella-Garcia M, McCoy J, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;23:6838–68. doi: 10.1200/JCO.2005.01.2823. [DOI] [PubMed] [Google Scholar]
- 10.Delaney C, Frank S, Huang RS. Pharmacogenomics of EGFR-targeted therapies in non-small cell lung cancer: EGFR and beyond. Chin J Cancer. 2015;34(1):7. doi: 10.1186/s40880-015-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steuer CE, Ramalingam SS. Targeting EGFR in lung cancer: Lessons learned and future perspectives. Mol Aspects Med. 2015 doi: 10.1016/j.mam.2015.05.004. pii: S0098-2997(15)00034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Yang K, Kuang K. The efficacy and safety of EGFR inhibitor monotherapy in non-small cell lung cancer: a systematic review. Curr Oncol Rep. 2014;16:390. doi: 10.1007/s11912-014-0390-4. [DOI] [PubMed] [Google Scholar]
- 13.Wu P, Zhang J, Li R. Expressions of epidermal growth factor receptor (EGFR) and Ki67 in non-small cell lung cancer and correlative research. Chinese Journal of Cancer Prevention. 2004;11:913–18. [Google Scholar]
- 14.Ohsaki Y, Tanno S, Fujita Y, et al. Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol Rep. 2000;7:603–60. doi: 10.3892/or.7.3.603. [DOI] [PubMed] [Google Scholar]
- 15.Feng ZY, Hu HC. Expressions of epidermal growth factor receptor (EGFR) in lung cancer tissues. Suzhou University Journal of Medical Science. 2003;23:692–94. [Google Scholar]
- 16.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Shigematsu H, Hiroshima K, et al. Epidermal growth factor receptor expression status in lung cancer correlates with its mutation. Hum Pathol. 2005;36:1127–34. doi: 10.1016/j.humpath.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhou CC, Zhao YM, Tang L. Clinical significance of epidermal growth factor receptor mutation in chinese patients with non-small cell lung cancer. Tumor. 2005;25:458–61. [Google Scholar]
- 19.Suda K, Mitsudomi T. Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch Toxicol. 2015 doi: 10.1007/s00204-015-1524-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Ahn JH, Kim SW, Hong SM. Epidermal growth factor receptor (EGFR) expression in operable non-small cell lung carcinoma. J Korean Med Sci. 2004;19:529–35. doi: 10.3346/jkms.2004.19.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]