Abstract
Background
Recent reports have suggested that miR-30a plays a tumor-suppressive role in various cancers. However, miR-30a has not been completely studied in non-small lung cancer (NSCLC). Thus, the aim of the present study was to clarify the association between the expression of miR-30a and the clinicopathological features in NSCLC patients.
Material/Methods
Total RNA of miR-30a was extracted from 125 pairs of NSCLC patients (male 75, female 50) and their matching normal tissues. The miR-30a level was detected by using quantitative real-time polymerase chain reaction (qRT-PCR). Simultaneously, the 2−ΔCq method was used to calculate the correlation between miR-30a expression and the clinicopathological parameters and prognosis of NSCLC patients.
Results
MiR-30a expression was significantly down-regulated in NSCLC tissues (4.0696±2.4178) compared to their non-tumor lung tissues (7.4530±3.0561, P<0.001). Level of miR-30a was negatively correlated to tumor size (r=−0.197, P=0.028), lymphatic metastasis (r=−0.312, P<0.001), clinical TNM stage (r=−0.299, P=0.001), pathological grading (I/II vs. III, r=−0.224, P=0.001), and histological classification (r=−0.299, P=0.001). Survival time was 3.23±2.18 months in the low miR-30a expression group, remarkably shorter than that of the high expression group (20.72±11.63 months, P<0.001).
Conclusions
MiR-30a may be regarded as a tumor suppressor in NSCLC, and it could become a prognostic marker and potential therapeutic target for NSCLC.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; MicroRNAs; Prognosis; Real-Time Polymerase Chain Reaction; Survival
Background
Lung cancer, especially non-small cell lung cancer (NSCLC), accounts for the most cancer-associated deaths globally, with the annual death rate of about 1.4 million [1]. A better apprehension of the potential molecular mechanism of tumor progression is crucial for evolving novel therapeutics for NSCLC [2]. Thus, identification of better underlying molecular makers for NSCLC is essential for more accurate early diagnosis and more effective therapeutic strategies.
MicroRNAs (miRNAs) are small, non-coding RNAs consisting of 19–25 nucleotides. MiRNAs can modulate target genes expression negatively by binding to the 3′-untranslated region (3′-UTR) [3]. It is widely realized that miRNAs may act as tumor suppressors or oncogenes in all steps of tumorigenesis [4]. MiR-30a is situated on chromosome 6q.13 and is produced by an intronic transcriptional unit [5]. Two mature forms of miR-30a exist – miR-30a-3p and miR-30a-5p. MiR-30a is deregulated in several malignant tumors, such as breast cancer [6], hepatocellular cancer [7], colon cancer [8], nasopharyngeal carcinoma [9], prostatic cancer [10], endometrial cancer [11], and cutaneous squamous cell carcinoma [12]. Similarly, a few studies reported the down-regulation of miR-30a in lung cancer tissues or cultured cells [13–18]. Three of the studies investigated the molecular mechanism of miR-30 [19–21], including epithelial-mesenchymal transition (EMT). However, no clinically relevant statistical analysis has been performed in studies of lung cancer.
Hence, in the present study, we examined miR-30a expression in 125 cases of NSCLC tissues and found that miR-30a was significantly down-regulated in NSCLC, and then we explored the relationship between expression of miR-30a in NSCLC tissues and the clinicopathological parameters, as well as survival.
Material and Methods
Tissue samples
This study enrolled 125 formalin-fixed, paraffin-embedded (FFPE) NSCLC tissues and their paired non-tumor tissues (male=75, female=50), obtained from the First Affiliated Hospital of Guangxi Medical University (Nanning, Guangxi, China) after pneumonectomy performed between January 2012 and February 2014. The mean age was 61.10 years, range 23–90 years. None of the patients received any cancer-related treatment before the operation. Fifty-seven patients had complete follow-up information. Written informed consent was obtained from the participants. The diagnosis was confirmed by 2 independent pathologists. The clinicopathological information, collected from medical records, is listed in Table 1. EGFR data were obtained as previously reported [22–27].
Table 1.
Relationship between the expression of miR-30a and clinicopathological parameters in NSCLC.
| Clinicopathological features | n | miR-30a relevant expression(2−ΔCq) | |||
|---|---|---|---|---|---|
| Mean ± SD | t | P | |||
| Tissue | Adjacent non-cancerous lung | 125 | 7.4530±3.0561 | −9.707 | <0.001 |
| NSCLC | 125 | 4.0696±2.4178 | |||
| Age (years) | <60 | 57 | 3.4649±1.9437 | −2.692 | 0.008 |
| ≥60 | 68 | 4.5765±2.6626 | |||
| Gender | Male | 75 | 3.7280±1.9226 | −1.957 | 0.053 |
| Female | 50 | 4.5820±2.9605 | |||
| Smoking | No | 38 | 3.6553±2.6169 | 0.009 | 0.993 |
| Yes | 30 | 3.6500±2.2325 | |||
| Tumor size (cm) | ≤3 | 60 | 4.6417±2.7824 | 2.562 | 0.012 |
| >3 | 65 | 3.5415±1.8972 | |||
| Lymphatic metastasis | No | 56 | 4.8946±2.6320 | 3.599 | <0.001 |
| Yes | 69 | 3.4000±2.0103 | |||
| Vascular invasion | No | 90 | 3.9622±2.5287 | −0.795 | 0.428 |
| Yes | 35 | 4.3457±2.1150 | |||
| Clinical TNM stage | I–II | 54 | 4.9074±2.6750 | 3.396 | 0.001 |
| III–IV | 71 | 3.4324±1.9961 | |||
| Pathological grade | I | 17 | 4.2529±2.9140 | *F=1.497 | 0.228 |
| II | 78 | 4.2846±2.2760 | |||
| III | 30 | 3.4067±2.4410 | |||
| Histological classification | Adenocarcinoma | 101 | 4.4109±2.5160 | *F=5.662 | 0.004 |
| Squamous carcinoma | 23 | 2.6565±1.1739 | |||
| Large cell carcinoma | 1 | 2.1000 | |||
| EGFR amplification | No | 39 | 3.3231±1.8688 | 0.177 | 0.860 |
| Yes | 18 | 3.2278±1.9235 | |||
| EGFR protein expression | low | 40 | 3.3625±1.7624 | 0.427 | 0.671 |
| high | 17 | 3.1294±2.1496 | |||
| EGFR mutation | Wild type | 44 | 3.1068±1.8520 | −1.395 | 0.169 |
| Mutation** | 13 | 3.9231±1.8606 | |||
One-way analysis of variance (ANOVA) test was performed;
EGFR mutation included short in-frame deletions in exon 19 and point mutations that result in a substitution of arginine for leucine at codon 858 (L858R) in exon 21.
qRT-PCR
RNA extraction and normalization, and quantitative real-time polymerase chain reaction (qRT-PCR) were performed as described previously [22,26–28]. MiR-191 and miR-103 were selected as endogenous controls as previously reported [27,29]. The sequences of miRNAs were: miR-30a (TaqMan® MicroRNA Assays, Applied Biosystems Cat. No. 4427975-000416, Life Technologies Grand Island, NY 14072 USA): 000417, UGUAAACAUCCUCGACUGGAAG; miR-191 (Applied Biosystems Cat. No. 4427975-000490): CAACGGAAUCCCAAAAGCAGCU; miR-103 (Applied Biosystems Cat. No. 4427975-000439): AGCAGCAUUGUACAGGGCUAUGA. The 10 μl RT reactions were performed using TaqMan® MicroRNA Reverse Transcription Kit (4366596, Applied Biosystems, Life Technologies Grand Island, NY 14072 USA). The PCR reactions were performed on an Applied Biosystems PCR7900. All the experiments were performed in triplicate, including no-pattern controls. Then 2−Δcq method was used to calculate the relevant expression values of miR-30 in NSCLC and corresponding normal tissues.
Statistical analysis
SPSS 20.0 was used in all statistical analysis. Student’s t-test was performed to analyze the significance of differential expression between 2 groups. One-way analysis of variance (ANOVA) was used to separate data into 3 groups based on pathological grade. Spearman correlation analysis was utilized to study the relationship between miR-30a expression and clinical parameters. Receiver operating characteristic (ROC) curves were drawn to examine the effectiveness of miR-30a in distinguishing NSCLC from their non-tumor lung tissues and to predict disease progression. Survival curves were drawn using Kaplan-Meier and log-rank test. P<0.05 was considered as a statistically significant difference.
Results
MiR-30a was significantly down-regulated in NSCLC tissues
MiR-30a was significantly down-regulated in NSCLC tissues (4.0696±2.4178) compared with their paired adjacent non-cancerous tissues (7.4530±3.0561, P<0.001, Figure 1A, Table 1). In addition, an ROC curve was drawn to show the diagnostic role of miR-30a. The area under the curve (AUC) of miR-30a was 0.818 (95% CI: 0.766–0.870, P<0.001, Figure 1B).
Figure 1.
(A) Expression of miR-30a in peritumoral tissues and non-small cell lung cancer tissues. Quantitative real-time RT-PCR (RT-qPCR) was performed to detect the expression of miR-30a. The difference in relevant miR-30a expression between non-small cell lung cancer tissues and paired non-tumor tissues. *** P<0.001. (B) ROC curve of miR-30a expression to distinguish non-small cell lung cancer from peritumoral tissues liver. The area under the curve (AUC) of miR-30a was 0.818 (95% CI: 0.766–0.870, P<0.001).
Correlation of miR-30a expression and clinical parameters in NSCLC
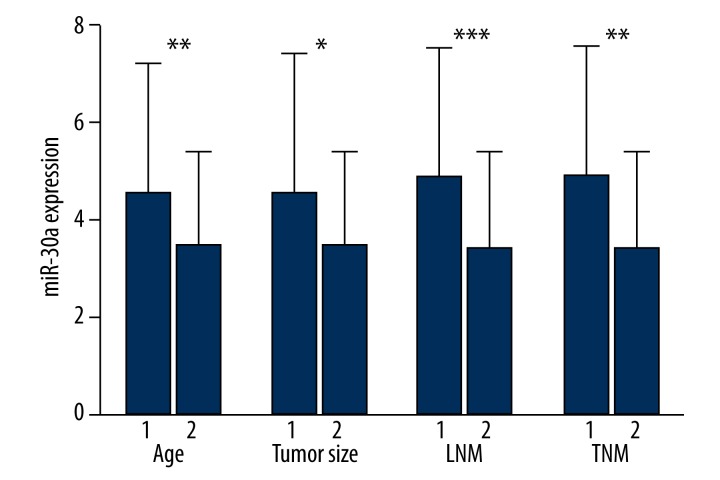
Down-regulation of miR-30a was correlated with a series of clinicopathological parameters, including patient age, tumor size, lymph node status, clinical TNM stage, and tumor histological grade. The older patients (4.5765±2.6626) had higher levels of miR-30a than the younger ones (3.4649±1.9437, P=0.008). Compared with the group with smaller tumors (≤3 cm, 4.6417±2.7824), miR-30a in the group of large tumors was markedly decreased (>3 cm, 3.5415±1.8972, P=0.012). MiR-30a level in patients with lymph node metastasis (3.4000±2.0103) was down-regulated in comparison with those without lymphatic metastasis (4.8946±2.6320, P<0.001). In comparison to early stages (I & II, 4.9074±2.6750), the relevant level of miR-30a in advanced stages was markedly decreased (III and IV, 3.4324±1.9961, P=0.001) (Figure 2).
Figure 2.
The relationship between miR-30a and clinical features. Age: 1. >60; 2. ≤60. Tumor size: 1. ≤3 cm; 2. >3 cm. Lymph node metastasis (LNM): 1. No; 2. Yes. Clinical TNM stage (TNM): 1. I–II; 2. III–IV. * P<0.05; ** P<0.01; *** P<0.001.
Spearman correlation analysis demonstrated significant negative correlations between the low expression of miR-30a and a series of parameters, such as tumor size (r=−0.197, P=0.028), lymphatic metastasis (r=−0.312, P<0.001), clinical TNM stage (r=−0.299, P=0.001), pathological grading (I/II vs. III, r=−0.224, P=0.001), and histological classification (r=−0.299, P=0.001). Nevertheless, the relative expression of miR-30a had no correlation with other characteristics.
ROC analyses of clinical data
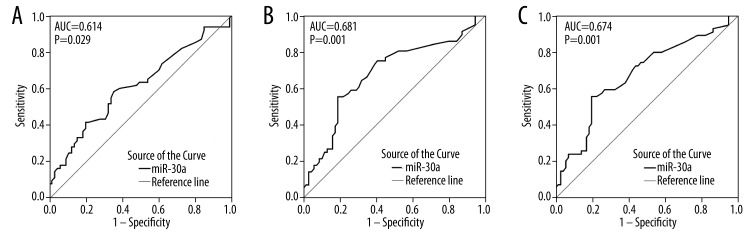
ROC curves were utilized to ascertain the predictive value of miR-30a expression in NSCLC patients for disease progression. The AUC of tumor size was 0.614 (95% CI: 0.514–0.713, P=0.029). The AUC of lymphatic metastasis was 0.681 (95% CI: 0.584–0.778, P=0.001). The ROC curve showed an AUC of 0.674 (95% CI: 0.578–0.771, P=0.001) to predict clinical TNM stage (Figure 3).
Figure 3.
ROC curve of miR-30a expression of clinicopathological features. (A). ROC curve of tumor size. The area under curve (AUC) was 0.614 (95% CI: 0.514–0.713, P=0.029). (B). ROC curve of lymph node metastasis. The AUC was 0.681 (95% CI: 0.584–0.778, P=0.001). (C). ROC curve of TNM. The AUC was 0.674 (95% CI: 0.578–0.771, P=0.001).
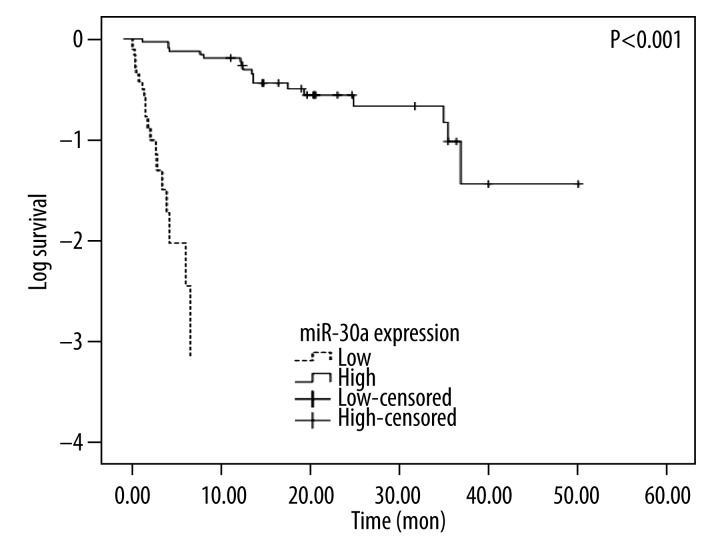
Role of miR-30a level in NSCLC survival
Fifty-seven patients obtained complete follow-up, including 21 cases with down-regulated miR-30a level and 36 with up-regulated miR-30a. Compared to the survival in the high-level group (20.72±11.63 months), the low-level group had a significantly poorer prognosis (3.23±2.18 months, P<0.001, Figure 4).
Figure 4.
The Kaplan-Meier curve of survival between high-expression and low-expression group of miR-30a (P<0.001).
Targets prediction of miR-30a
We then searched in on-line databases for the predictive potential target genes of miR-30a, including MIRBD, TARGETSCAN, PICTAR, MICRORNA.ORG, TARGETMINER, and RNA22-HAS. Two qualified target genes, CELSR3 and TNRC6A, were found in at least 4 databases.
Discussion
Lung cancer, especially NSCLC, is the most common cause of cancer death in the world, and its incidence is steadily increasing [1]. NSCLC always spreads to further sites through hematologic or lymphatic means like adrenal glands and most lung cancer patients with adrenal glands are incurable [35]. The poor prognosis of lung cancer is principally due to diagnosis made at advanced clinical stages. Thus, recognition of more early biomarkers is urgently required [30]. Although several studies have suggested miR-30a as a suppressor gene for inhibiting the development of lung cancer [13–18], no correlation between miR-30a and clinicopathological parameters has been shown.
Among the studies, 3 covered related molecular mechanisms, validated via in vitro experiments. Kumarswamy et al. [20] found that miR-30a was down-regulated in NSCLC tissues (N=64), and Snai1, which was known as a transcriptional repressor of E-cadherin, was a target of miR-30a. EMT represses E-cadherin level and can enhance cell motility, so decontrol EMT is an essential component of tumor metastasis [31]. Kumarswamy et al. [20] reported that miR-30a inhibited EMT in NSCLC cell lines through targeting Snai1. In contrast, Kumarswamy et al. [20] proved in vitro that over-expression of miR-30a inhibited migration, invasion, and distant metastasis by using 5 cell lines (A549, Calu-1, Calu-3, H1299, and H1395). The results supported the function of miR-30a as a tumor-suppressor in NSCLC. Nevertheless, no correlation analysis was performed between miR-30a expression and patient features in this report. Liu et al. [21] recognized a competing endogenous RNA regulation network among miR-30a, AEG-1, Snai1, and Vimentin in the EMT progress and metastasis of NSCLC cells (A549 cells). In the network, AEG-1, Snai1, and Vimentin could affect each other via competition for their shared common miRNA-miR-30a. In other words, they were all target genes of miR-30a. Further, Liu discovered that the level of miR-30a was decreased, and the levels of AEG-1, Snai1, and Vimentin were increased; then, AEG-1 could bind to the miR-30a, resulting in less free miR-30a available. The repression effects of miR-30a on the Snail and Vimentin level were weakened, thus inducing EMT and cell metastasis, consistent with the report of Kumarswamy et al. [20]. The results of functional assays showed that over-expression of miR-30a restrained migration and invasion in A549 cells. Notably, except cell cultures, the expression of miR-30a was not assessed in lung cancer tissues in this study. Jiang et al. [19] verified the decline of miR-30a in 22 paired NSCLC patients by using TaqMan real-time PCR, and reported that B-cell lymphoma/leukemia 11A (BCL11A), a known proto-oncogene, was negatively regulated by miR-30a. It was found that forceful high BCL11A expression was associated with no lymphatic metastasis, early clinical TNM stage, and longer survival time of patients with NSCLC, but the mechanism is still unknown.
In the current study, we analyzed the level of miR-30a in 125 NSCLC patients and demonstrated that miR-30a was remarkably lower, in accordance with previous studies [19–21]. The present study included more tissue cases (N=125) compared with the study of Kumarswamy et al. (N=64) and Jiang et al. (N=22). Notably, our data show that miR-30a was repressed in NSCLC tissues with large tumor size compared to those with smaller ones (P=0.012). As NSCLC developed and metastasized, miR-30a was found to be down-regulated (P<0.001), in accordance with the results reported by Kumarswamy et al. [20] and Liu et al. [21] that miR-30a suppressed metastasis and invasion in NSCLC. Similarly, miR-30a was expressed more weakly in advanced TMN stage (P=0.001). In addition, there was statistical significance in histological classification (P=0.004). There were also negative correlations between miR-30a and the aforementioned clinical characteristics, as detected by Spearman test. Furthermore, low miR-30a level predicted shorter overall survival. We are the first to analyze the statistical differences between miR-30a and clinical parameters, including survival time of patients. In contrast to the reports of Kumarswamy et al. [20] and Liu et al. [21], we found that over-expression of miR-30a directly targeted AEG-1, Snai1, and Vimentin, attenuated the EMT progress, and then inhibited proliferation, invasion, and metastasis. Thus, the miR-30a low-expression, to some extent, accelerated the deterioration in NSCLC.
On account of the unknown mechanism of miR-30a, we then attempted to predict the potential target genes of miR-30a. We detected 2 eligible genes after searching in 6 bioinformatics databases: MIRBD, TARGETSCAN, PICTAR, MICRORNA.ORG, TARGETMINER, and RNA22-HSA. TNRC6A,. The proteins, encoded by TNRC6A (also called GW1/GW182) assemble to miRNA targets via direct interactions with certain proteins and facilitate target silencing [32]. Then, CELSR3, a member of the cadherin superfamily with a role in cell contact-mediated communication, was detected to be over-regulated in pancreatic satellite cells (PSC) in pancreatic ductal adenocarcinoma (PDAC) [33]. It is unclear whether the activated PSC plays a significant role in promoting the occurrence and metastasis of pancreatic cancer [34]. However, no study has been aimed at the expression and mechanism of the 2 prediction targets in NSCLC. The genes mentioned above were just suggested based on theory. More experiments need to be carried out to explore the contribution of miR-30a in NSCLC through various targeting genes and molecular mechanisms.
Conclusions
Our study suggests the correlation between the expression of miR-30a and clinicopathological characteristics in NSCLC tissues. We have more NSCLC tissue samples (n=125) than any previous study in the literature. Together with previous reports, our study shows that miR-30a may act as a suppressive miRNA in tumorigenesis and progression of lung cancer In conclusion, our study suggests that miR-30a may be a new efficient biomarker for diagnosis and prognosis prediction for NSCLC patients, and it could also be a potential therapeutic target for NSCLC.
Footnotes
Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Source of support: The present study was supported by the Fund of Guangxi Provincial Health Bureau Scientific Research Project (Z2013201, Z2014055) and the Fund of National Natural Science Foundation of China (NSFC 81360327)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Soini Y. Tight junctions in lung cancer and lung metastasis: a review. Int J Clin Exp Pathol. 2012;5(2):126–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Di Leva G, Briskin D, Croce CM. MicroRNA in cancer: new hopes for antineoplastic chemotherapy. Ups J Med Sci. 2012;117(2):202–16. doi: 10.3109/03009734.2012.660551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian B, Nag SA, Su Y, et al. miRNAs in cancer prevention and treatment and as molecular targets for natural product anticancer agents. Curr Cancer Drug Targets. 2013;13(5):519–41. doi: 10.2174/15680096113139990031. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10A):1902–10. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu J, Xu X, Kang L, et al. miR-30a suppresses breast cancer cell proliferation and migration by targeting Eya2. Biochem Biophys Res Commun. 2014;445(2):314–19. doi: 10.1016/j.bbrc.2014.01.174. [DOI] [PubMed] [Google Scholar]
- 7.Dai H, Kang B, Zuo D, Zuo G. [Effect of miR-30a-5p on the proliferation, apoptosis, invasion and migration of SMCC-7721 human hepatocellular carcinoma cells]. Zhonghua Gan Zang Bing Za Zhi. 2014;22(12):915–20. doi: 10.3760/cma.j.issn.1007-3418.2014.12.008. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Tang Q, Qin D, et al. Role of microRNA 30a targeting insulin receptor substrate 2 in colorectal tumorigenesis. Mol Cell Biol. 2015;35(6):988–1000. doi: 10.1128/MCB.01242-14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Wang HY, Li YY, Fu S, et al. MicroRNA-30a promotes invasiveness and metastasis in vitro and in vivo through epithelial-mesenchymal transition and results in poor survival of nasopharyngeal carcinoma patients. Exp Biol Med (Maywood) 2014;239(7):891–98. doi: 10.1177/1535370214532758. [DOI] [PubMed] [Google Scholar]
- 10.Katz B, Reis ST, Viana NI, et al. Comprehensive study of gene and microRNA expression related to epithelial-mesenchymal transition in prostate cancer. PLoS One. 2014;9(11):e113700. doi: 10.1371/journal.pone.0113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukamoto O, Miura K, Mishima H, et al. Identification of endometrioid endometrial carcinoma-associated microRNAs in tissue and plasma. Gynecol Oncol. 2014;132(3):715–21. doi: 10.1016/j.ygyno.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Sand M, Skrygan M, Georgas D, et al. Microarray analysis of microRNA expression in cutaneous squamous cell carcinoma. J Dermatol Sci. 2012;68(3):119–26. doi: 10.1016/j.jdermsci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28(10):1721–26. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 14.Markou A, Tsaroucha EG, Kaklamanis L, et al. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem. 2008;54(10):1696–704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 15.Patnaik SK, Kannisto E, Knudsen S, Yendamuri S. Evaluation of microRNA expression profiles that may predict recurrence of localized stage I non-small cell lung cancer after surgical resection. Cancer Res. 2010;70(1):36–45. doi: 10.1158/0008-5472.CAN-09-3153. [DOI] [PubMed] [Google Scholar]
- 16.Voortman J, Goto A, Mendiboure J, et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non-small cell lung carcinoma. Cancer Res. 2010;70(21):8288–98. doi: 10.1158/0008-5472.CAN-10-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Yu SL, Chen HY, Chang GC, et al. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008;13(1):48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Jiang BY, Zhang XC, Su J, et al. BCL11A overexpression predicts survival and relapse in non-small cell lung cancer and is modulated by microRNA-30a and gene amplification. Mol Cancer. 2013;12:61. doi: 10.1186/1476-4598-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumarswamy R, Mudduluru G, Ceppi P, et al. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int J Cancer. 2012;130(9):2044–53. doi: 10.1002/ijc.26218. [DOI] [PubMed] [Google Scholar]
- 21.Liu K, Guo L, Guo Y, et al. AEG-1 3′-untranslated region functions as a ceRNA in inducing epithelial-mesenchymal transition of human non-small cell lung cancer by regulating miR-30a activity. Eur J Cell Biol. 2015;94(1):22–31. doi: 10.1016/j.ejcb.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Kronenberger P, Teugels E, De Greve J. Influence of RT-qPCR primer position on EGFR interference efficacy in lung cancer cells. Biol Proced Online. 2011;13:1. doi: 10.1186/1480-9222-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, Kronenberger P, Teugels E, et al. Targeting the epidermal growth factor receptor in non-small cell lung cancer cells: the effect of combining RNA interference with tyrosine kinase inhibitors or cetuximab. BMC Med. 2012;10:28. doi: 10.1186/1741-7015-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Kronenberger P, Teugels E, et al. Effect of siRNAs targeting the EGFR T790M mutation in a non-small cell lung cancer cell line resistant to EGFR tyrosine kinase inhibitors and combination with various agents. Biochem Biophys Res Commun. 2013;431(3):623–29. doi: 10.1016/j.bbrc.2012.12.070. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Kronenberger P, Umelo IA, et al. Quantification of epidermal growth factor receptor T790M mutant transcripts in lung cancer cells by real-time reverse transcriptase-quantitative polymerase chain reaction. Anal Biochem. 2010;398(2):266–68. doi: 10.1016/j.ab.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Noor A, Kronenberger P, et al. Synergistic effect of afatinib with su11274 in non-small cell lung cancer cells resistant to gefitinib or erlotinib. PLoS One. 2013;8(3):e59708. doi: 10.1371/journal.pone.0059708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen G, Umelo IA, Lv S, et al. miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8(3):e60317. doi: 10.1371/journal.pone.0060317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rong M, He R, Dang Y, Chen G. Expression and clinicopathological significance of miR-146a in hepatocellular carcinoma tissues. Ups J Med Sci. 2014;119(1):19–24. doi: 10.3109/03009734.2013.856970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Li P, Rong M, et al. MicroRNA-141 is a biomarker for progression of squamous cell carcinoma and adenocarcinoma of the lung: clinical analysis of 125 patients. Tohoku J Exp Med. 2015;235(3):161–69. doi: 10.1620/tjem.235.161. [DOI] [PubMed] [Google Scholar]
- 30.Duffy MJ. Role of tumor markers in patients with solid cancers: A critical review. Eur J Intern Med. 2007;18(3):175–84. doi: 10.1016/j.ejim.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Braun JE, Huntzinger E, Izaurralde E. The role of GW182 proteins in miRNA-mediated gene silencing. Adv Exp Med Biol. 2013;768:147–63. doi: 10.1007/978-1-4614-5107-5_9. [DOI] [PubMed] [Google Scholar]
- 33.Erkan M, Weis N, Pan Z, et al. Organ-, inflammation- and cancer specific transcriptional fingerprints of pancreatic and hepatic stellate cells. Mol Cancer. 2010;9:88. doi: 10.1186/1476-4598-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni JQ, Jiang XH, Tang WH. Relationship between pancreatic stellate cells and pancreatic cancer. Shijie Huaren Xiaohua Zazhi. 2008;(33):3782–86. [Google Scholar]
- 35.Taira N, Kawabata T, Ichi T, et al. Long-term survival after surgical treatment of metachronous bilateral adrenal metastases of non-small cell lung carcinoma. Am J Case Rep. 2014;15:444–46. doi: 10.12659/AJCR.891027. [DOI] [PMC free article] [PubMed] [Google Scholar]