Abstract
Background
The canonical Wnt signaling pathway has been considered as a potent oncogenic signaling in the initiation and progression of hematological malignancies. As a key regulator of the Wnt signaling pathway, the role of β-catenin in mantle cell lymphoma (MCL) pathogenesis and progression was investigated in this study.
Material/Methods
A total of 30 MCL samples were collected from patients and were examined for the expression of β-catenin and p-GSK3β using immunohistochemical (IHC) staining. Further in vitro studies employed MTT and Western blot assays detecting proliferation and apoptosis-related proteins in MCL cell line Jeko-1, which were transfected with β-catenin shRNA or specific inhibitor XAV939.
Results
Expression of β-catenin and phosphorylated glycogen synthase kinase-3 beta (p-GSK3β) in MCL was significantly higher than those in controlled samples. In vitro studies indicated that β-catenin knockdown significantly inhibited cell proliferation and induced apoptosis in Jeko-1 cells. Furthermore, XAV939 induced apoptosis and growth arrest in Jeko-1 cells. Both inhibitory agents increased Bax and caspase 3 proteins, and decreased Bcl-2, c-Myc, and Cyclin D1 proteins.
Conclusions
The specific inhibition of β-catenin induces apoptosis and growth arrest, making it a potential therapeutic target against MCL.
MeSH Keywords: Host Cell Factor C1; Lymphoma, Mantle-Cell; Wnt Signaling Pathway
Background
Mantle cell lymphoma (MCL) is a subtype of B cell non-Hodgkin lymphoma (NHL), and accounts for about 3–10% of total NHL cases [1]. Due to its resistance to chemotherapy and its invasive behaviors, most MCL is incurable. In clinical practice, most MCL cases are at stage III/IV after primary diagnosis. As a result, MCL is commonly considered as a refractory malignant lymphoma [2].
Wnt signaling consists of 3 types: the canonical Wnt pathway, non-canonical planar cell polarity pathway, and non-canonical Wnt/calcium pathway. Mainly regulating gene transcription, the canonical Wnt signaling pathway has been suggested in carcinogenesis. In the canonical Wnt signaling pathway, β-catenin exerts its cell-to-cell adhesion function due to its expression on both apical and basal sides of membranes and lower amounts in the cytoplasm [3]. The low intracellular concentration of β-catenin is maintained by the so called “destruction box”, which consists of a scaffold protein Axin, adenomatous polyposis coli (APC) protein, casein kinase (CK) 1, and glycogen synthase kinase (GSK) 3β. CK1 can phosphorylate β-catenin on Ser45 and GSK3β leads to the phosphorylation of β-catenin on Thr41, Ser37, and Ser33. This phosphorylated β-catenin is then recognized and targeted for ubiquitination by β-transducin repeat-containing protein (β-TrCP), followed by degradation in proteasomes.
Due to its low concentration under normal circumstances, β-catenin cannot enter the nucleus to initiate gene expression [4,5]. Under the direction of an upstream signal, Wnt protein binds to specific cell surface receptors, such as Frizzled (Fz) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), and induces the translocation of the negative Wnt regulator, Axin. Thus, the “destruction box” disintegrates, suppressing the phosphorylation level of GSK3β. These events all prevent β-catenin from phosphorylation and ubiquitination, leading to the protein accumulation. Excess β-catenin then enters the nucleus, where it binds with transcription factors to regulate the expression of target genes, such as c-Myc, Cyclin D1, and matrix metalloproteinases-2 (MMP-7) [6]. Therefore, it is the fold-change, not the absolute concentration of β-catenin, that regulates the canonical Wnt signaling pathway.
Canonical Wnt signaling has been found to be related to the pathogenesis and development of hematological malignant tumors. Previous studies found the expression of Wnt-related genes in the bone marrow of B cell acute lymphoblastic leukemia (B-ALL) patients. Other studies found that the addition of exogenous Wnt3a led to the accumulation of β-catenin and elevated cell proliferation, in addition to cell cycle and apoptosis-related genes. These data collectively suggest that Wnt pathway activation may be involved in the pathogenesis of hematological malignancies [7].
In order to investigate the relationship between β-catenin activity and MCL pathogenesis, we measured the expression level of β-catenin and p-GSK3β protein in MCL cell lines using immunohistochemical staining (IHC) technique. RNA interference technique was used to develop a β-catenin-specific inhibitor, XAV939, and was performed in the Jeko-1 mantle cell lymphoma cell line. MTT assay and flow cytometry were used for detecting cell proliferation and apoptosis, respectively.
Material and Methods
Clinical samples
A total of 30 MCL patients, including 21 males and 9 females, were collected for lymphoma cells. All patients have been diagnosed and been hospitalized in the Department of Hematology of our hospital between January 2005 and December 2013. The diagnostic criteria followed the guideline stipulated by the Chinese Medical Association (CMA). Another 30 samples were collected from reactive hyperplastic lymphadenitis and were used as a control due to their normal expression level of β-catenin and GSK3 in a pilot study. The experimental protocols have been pre-approved by the Ethics Committee of Fujian Medical University in adherence to the Helsinki Declaration (as revised in Edinburgh 2000). Written consent was obtained from patients or their guardians before the study.
Immunohistochemical (IHC) staining
ICH staining was performed as previously reported [8]. Lymph node samples were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned. After dewaxing, tissues sides were treated with 1% hot citric acid buffer for antigen retrieval. Rabbit anti-human β-catenin (Cell Signaling, MA, USA) (1:100) and p-GSK3β (Cell Signaling, MA, USA) antibodies were used to incubate tissue slides for 1 h at room temperature, followed by 30-min secondary antibody incubation and DAB chromogenic development. After counter-staining using hematoxylin and dehydration, images were captured under light-field microscope at 100× magnification. The percentage of positive tumor cells was graded as previously described [3].
Cell lines and transfection
The human MCL Jeko-1 cell (ATCC, Manassas, VA, USA) was cultured in complete Dulbecco’s modified Eagle medium (DMEM, Gibco, NY, USA) containing 10% fetal bovine serum (FBS, Gibco, NY, USA) with 100 units/mL penicillin and 100 μg/mL streptomycin (Sigma, MO, USA) in a humidified chamber containing 5% CO2 at 37°C.
β-catenin shRNA (sense: 5′-AAACU ACUGU GGACC ACAAG CCCUG UCUC-3′; antisense: 5′-AAGCU UGUGG UCCAC AGUAG UCCUG UCUC-3′) and negative control shRNA (sense: 5′-AAUUC UCCGA ACGUG UCACG UCCUG UCUC-3′; antisense: 5′-AAACG UGACA CGUUC GGAGA ACCUG UCUC-3′) were designed and synthesized by GenePharma Co., Ltd (Shanghai, China). Cells were transfected using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, CA, USA).
Western blot
Antibodies of β-catenin (1:1000), c-Myc (1:1000), Cyclin D1 (1:1000), Bax (1:1000), Bcl-2 (1:1000), caspase-3 (1:1000), and β-Actin (1:5000) (Cell Signaling, MA, USA) were used for Western blotting assay as previously described [9]. Horseradish peroxidase (HRP)-conjugated sheep anti-mouse or duck anti-rabbit secondary antibody (Bio-Rad, CA, USA) were used to amplify the signal, which was detected by Western Blotting Luminol Reagent (Santa Cruz, CA, USA)
MTT assay and apoptosis detection
In the proliferation assay, Jeko-1 cells transfected with β-catenin shRNA or treated with β-catenin-specific inhibitor XAV939 (Sigma, LA, USA) were seeded into 96-well plates at 5000 cells/well, followed by 24-h incubation. Cell proliferation level was evaluated using 5-bromodeoxyuridine (BrdU) (Roche, IN, USA) as the chemiluminescent). The Annexin-V-FLUOS Staining Kit (Roche, IN, USA) was further used to analyze apoptosis levels as previously described [9].
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Specific primers for β-catenin (forward: 5′-TGGGG CGCCC CAGGC ACCA-3′; reverse: 5′-TCCTT AATGT CACGC ACGAT TTC-3′) were synthesized. Total RNA was extracted from cultured cells using TRIzol (Life Technologies, CA, USA) according to the manufacturer’s instructions. First-strand cDNA was synthesized using the Revertid cDNA Synthesis Kit (Fermentas, ON, Canada). PCR amplification of β-catenin mRNA and β-Actin (internal reference) mRNA was performed on the ABI PRISM 7300 Sequence Detection System (Applied Biosystems, CA, USA) and SYBR Premix Ex Taq™ Kit (Takara Bio, Shiga, Japan) as previously reported.
Statistical analysis
Results are presented as mean ±SEM. Statistical analysis was performed with GraphPad Prism 5 software (GraphPad Software, CA, USA), including chi-squared test, 2-tailed t test, or analysis of variance (ANOVA), as appropriate. Statistical significance was defined at P<0.05.
Results
The expression of β-catenin and p-GSK3β in MCL
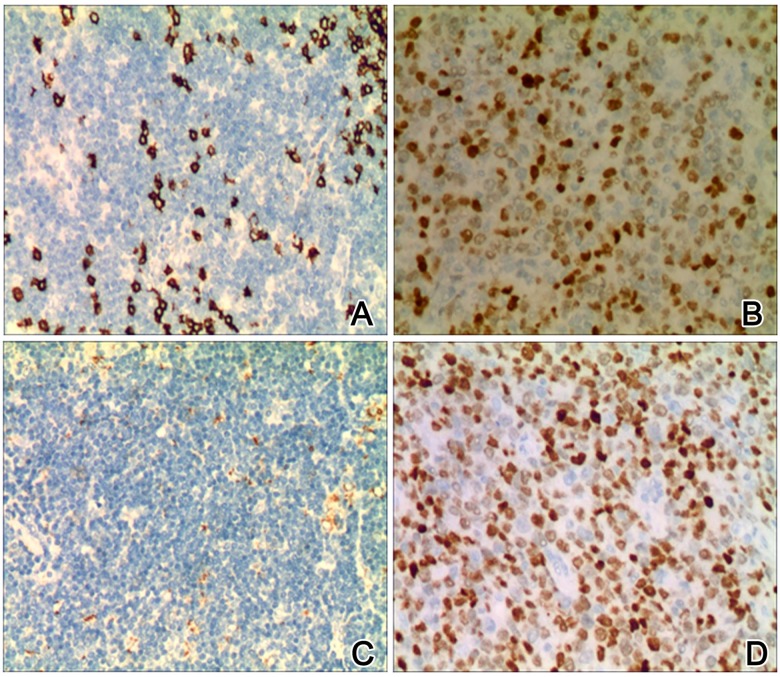
We measured the expression level of β-catenin and p-GSK3β protein in 30 MCL samples using IHC staining. β-catenin protein was found to be expressed in both nucleus and cytoplasm of MCL cells. In controlled samples, however, it was located only on the membrane s (Figure 1A, 1B). In total, we found 73.33% (22/30) β-catenin positive expression in MCL samples but only 6.67% (2/30) positive rates in controlled ones (chi-squared test, P<0.05). p-GSK3β protein was expressed in the nucleus (Figure 1C, 1D). About 66.67% (20/30) of MCL samples showed p-GSK3β-positive expression, while only 16.67% (5/30) of controlled samples showed p-GSK3β signals (P<0.05). Our results collectively indicate that the expression of β-catenin and p-GSK3β is up-regulated in MCL.
Figure 1.
Immunohistochemical (IHC) images of β-catenin p-GSK3β in MCL and control samples (×200). A and B show representative images of β-catenin in control and MCL tissues; C and D show representative staining images of p-GSK3β in control and MCL tissues. Higher expression level of β-catenin and p-GSK3β existed in MCL tissues (B, D) when compared to control samples (A, C).
The correlation between β-catenin and p-GSK3β expressions in MCL
We further analyzed the correlation between β-catenin expression and p-GSK3β in MCL and found that β-catenin expression existed in 90.0% (18/20) of all MCL samples with positive p-GSK3β expression, whereas only 40.0% (4/10) of MCL specimens with negative p-GSK3β expression showed positive β-catenin signals, indicating a significantly (r=0.852, P<0.05, Table 1) positive correlation between β-catenin and p-GSK3β expression in MCL.
Table 1.
The correlation between β-catenin and p-GSK3β in MCL cases (n=30).
| β-catenin | |||
|---|---|---|---|
| Positive | Negative | ||
| p-GSK3β | Positive | 18 | 2 |
| Negative | 4 | 6 | |
β-catenin knockdown and MCL cells proliferation and apoptosis
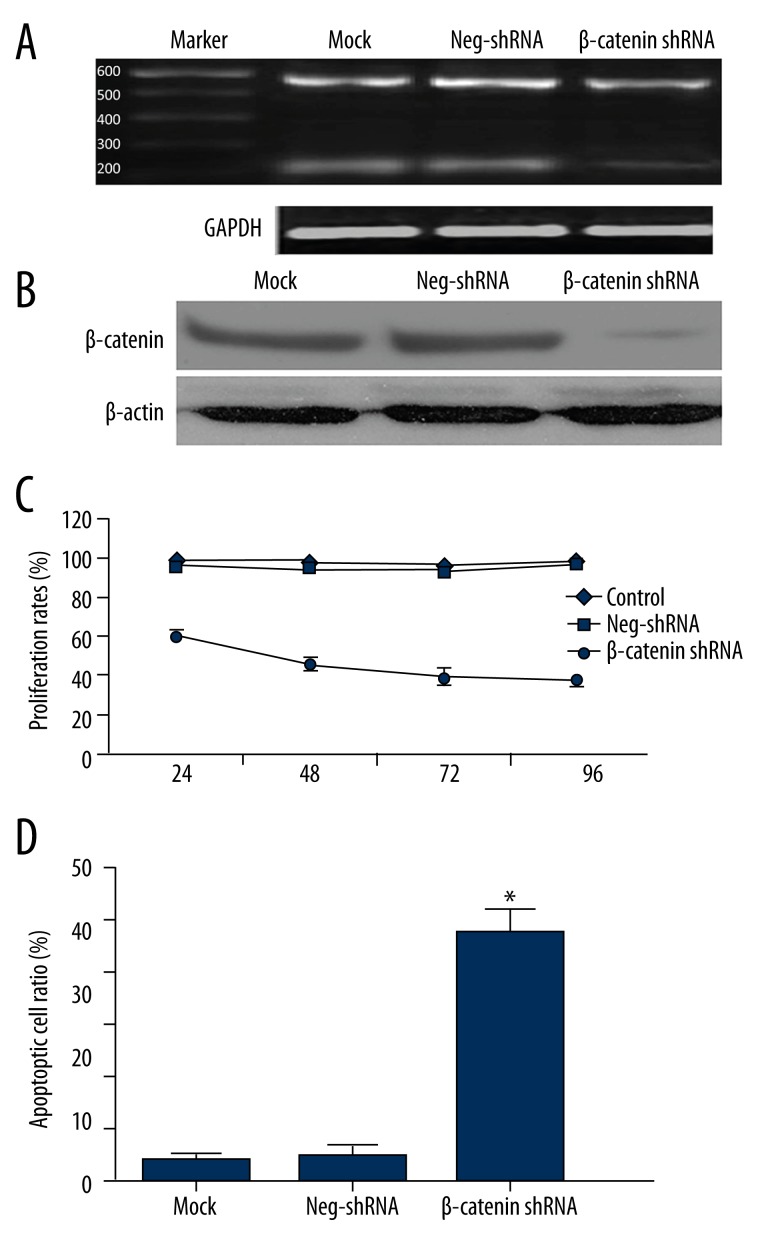
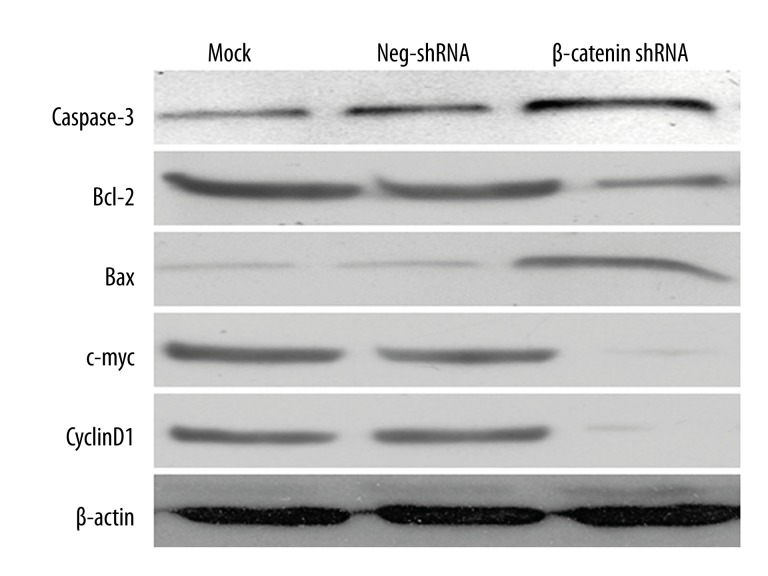
To further elucidate the role of β-catenin in MCL, we transfected Jeko-1 cells with non-targeting (NT) shRNA or β-catenin shRNA. As measured by RT-PCR and Western blot, β-catenin mRNA and protein levels were significantly suppressed by ectopically expressing β-catenin shRNA (P<0.05, Figure 2A, 2B, respectively). MTT assays were performed to test the effect on tumor cell proliferation. Results showed that β-catenin knockdown led to a significant reduction of cell proliferation (P<0.05, Figure 2C). Furthermore, as determined by flow cytometry, the apoptosis level was significantly elevated after β-catenin knockdown (P<0.05, Figure 2D). Moreover, β-catenin knockdown increased Bax and caspase 3 protein expression and decreased Bcl-2, c-Myc, and Cyclin D1 in Jeko-1 cells (Figure 3).
Figure 2.
β-catenin regulates proliferation and apoptosis in Jeko-1 cells. (A) Jeko-1 cells transfected with non-targeting (NT) shRNA or β-catenin shRNA were tested for β-catenin mRNA levels using RT-PCR. β-catenin mRNA was significantly suppressed by β-catenin shRNA (rightmost lane); (B) Western blotting analysis indicated that β-catenin protein was largely eliminated by β-catenin shRNA transfection; (C) MTT assays showed that β-catenin knockdown reduced the viability of Jeko-1 cells. (P<0.01 by two-way ANOVA; n=3); (D) Quantification of apoptotic cell percentage by flow cytometry. β-catenin knockdown cells were composed of a larger subset of apoptotic cells compared to the control cells. (P<0.01 by t test; n=3). Data presented as mean ±SD.
Figure 3.
β-catenin regulates apoptosis-related proteins in Jeko-1 cells. Jeko-1 cells transfected with NT shRNA or β-catenin shRNA were quantified for caspase-3, c-Myc, Cyclin D1, Bcl-2, and Bax protein expression under Western blotting. β-catenin knockdown decreased c-Myc, Cyclin D1, and Bcl-2 protein levels, and elevated caspase-3 and Bax expressions in Jeko-1 cells.
Effects of XAV939 on cell proliferation and apoptosis
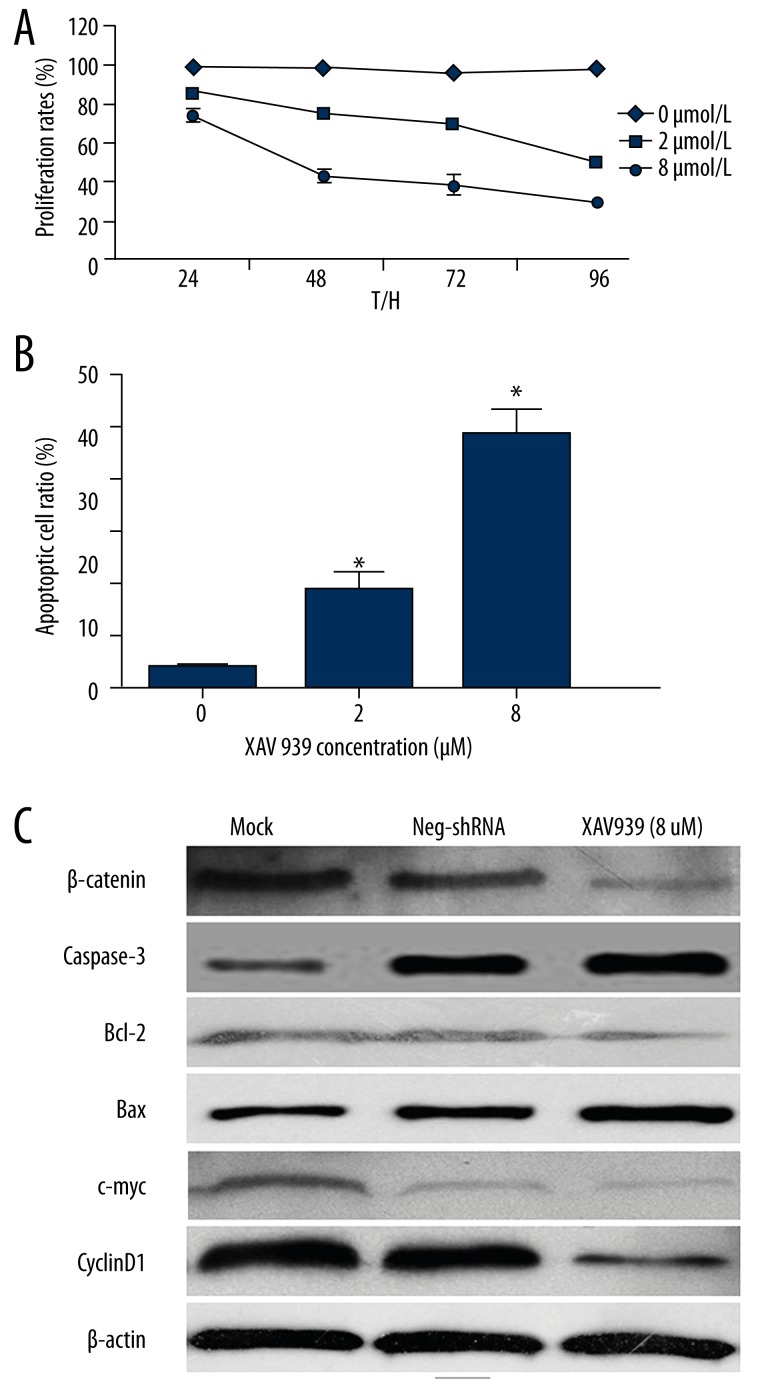
We next aimed to determine whether the β-catenin-specific inhibitor, XAV939, influenced Jeko-1 cell proliferation and apoptosis. Under MTT assays, we found that β-catenin inactivation led to a significant reduction of cell proliferation in a dose-dependent manner (2-way ANOVA, P<0.05, Figure 4A). Furthermore, as determined by flow cytometry, the percentage of apoptotic Jeko-1 cells was positively correlated with XAV939 dosage (P<0.05, Figure 4B). Moreover, β-catenin inactivation also increased Bax and caspase 3 expression levels, and decreased Bcl-2, c-Myc, and Cyclin D1 proteins in Jeko-1 cells (Figure 4C). These results indicate that β-catenin may promote cell proliferation and inhibit apoptosis in MCL.
Figure 4.
β-catenin inactivation inhibits cell proliferation and induces apoptosis in Jeko-1 cells. (A) Jeko-1 cells were treated with 0, 2, or 8 μmol/L XAV939 were tested for cell proliferation by MTT assay. XAV939 treatment reduced the viability of Jeko-1 cells in a dose-dependent manner (P<0.01 by two-way ANOVA; n=3). Data presented as mean ±SEM; (B) Quantification of apoptotic cell percentage by flow cytometry. XAV939 treated Jeko-1 cells composed of a larger subset of apoptotic cells compared to control cells (P<0.01 by t test; n=3) (C) XAV939 treatment decreased β-catenin, c-Myc, Cyclin D1, and Bcl-2 protein levels, and increased caspase-3 and Bax expression in Jeko-1 cells.
Discussion
β-catenin accumulation in cytoplasm and nucleus is the key point for the oncogenic Wnt signaling pathway. It is well known that β-catenin is a positive regulator in he Wnt pathway, while APC, GSK3β, and Axin are negative regulators. Due to the role of β-catenin in cell-to-cell adhesion and cell proliferation, it has become a research focus in the pathogenesis and progression of cancer. High β-catenin expression is observed in colon cancer cells with APC gene mutation because wild-type APC alleles negatively regulate β-catenin expression. Thus, the APC gene acts as a tumor suppressor by decreasing the expression of β-catenin [10,11]. The canonical Wnt/β-catenin signaling pathway plays a critical role in the development of lymphocytes and hematopoietic stem cells, in addition to various hematologic malignancies [12,13]. Elevated β-catenin expression was observed in diffused large B cell lymphoma, which also showed aberrant nuclear accumulation of β-catenin. In our study, 73.33% of MCL samples showed β-catenin nuclear accumulation, in contrast to 6.67% in control samples.
As a key regulator for the Wnt signaling pathway, GSK-3β binds to Axin, APC, and β-catenin in the absence of Wnt ligands and leads to phosphorylation, ubiquitination, and degradation of β-catenin [14]. As a result, β-catenin cannot be degraded after the inactivation of GSK-3β. As active β-catenin moves into the nucleus and binds to transcription factors to initiate gene expression, including Cyclin D1 and c-Myc, the inactive form of p-GSK-3β inhibits degradation of β-catenin and promotes tumor development [15]. In this study, p-GSK-3β was expressed in nuclei of MCL cells at a significantly higher level than in control tissues. Furthermore, p-GSK-3β expression was positively correlated with β-catenin in MCL. This supports the conclusion of a previous study in which high expression of p-GSK-3β predicted unfavorable prognosis of MCL patients [16]. These results suggest that β-catenin and GSK-3β may play an important role in the pathogenesis and progression of MCL.
In the second part of this study, β-catenin expressions were knocked down by specific shRNA transfection. Such gene knockdown inhibited cell proliferation and induced apoptosis in Jeko-1 cells. Verma et al. reported that β-catenin siRNA consistent inhibited colon cancer cell proliferation and decreased tumorigenesis in vivo [17]. As an inhibitor of β-catenin [18,19], XAV939 has been demonstrated to significantly decrease clone formation rate in human neuroblastoma cells in vitro. We treated Jeko-1 cells with various concentrations of XAV939 and found that Jeko-1 cell proliferation was inhibited, but its apoptosis was promoted in a dose-dependent manner. This can be explained by the cellular mechanism of β-catenin, as either the gene knockdown or XAV939 inhibition suppressed the downstream effectors of the Wnt signaling pathway, including c-Myc and Cyclin D1, resulting in apoptosis.
Conclusions
The results of our study suggest that the specific inhibition of β-catenin reduces cell proliferation and induces apoptosis in Jeko-1 MCL cells, indicating the potential role of β-catenin as a novel therapeutic target against MCL, although its detailed mechanism needs to be elaborated.
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Source of support: This study was supported by grants from the Youth Scientific Research Subject of Fujian Province Health Department [2012-2-114] and the National Natural Science Foundation of Fujian Province [2012J0142]
References
- 1.Dreyling M, Geisler C, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2014;25(Suppl 3):iii83–92. doi: 10.1093/annonc/mdu264. [DOI] [PubMed] [Google Scholar]
- 2.Smedby KE, Sampson JN, Turner JJ, et al. Medical history, lifestyle, family history, and occupational risk factors for mantle cell lymphoma: the interlymph non-hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. 2014;(48):76–86. doi: 10.1093/jncimonographs/lgu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maruyama K, Ochiai A, Akimoto S, et al. Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncology. 2000;59(4):302–9. doi: 10.1159/000012187. [DOI] [PubMed] [Google Scholar]
- 4.Saito-Diaz K, Chen TW, Wang X, et al. The way Wnt works: components and mechanism. Growth Factors. 2013;31(1):1–31. doi: 10.3109/08977194.2012.752737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi Y, Chen Y. Wnt signaling pathway: Implications for therapy in lung cancer and bone metastasis. Cancer Lett. 2014;353(1):8–16. doi: 10.1016/j.canlet.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama NN, Shao S, Hoang BH, et al. Wnt signaling in castration-resistant prostate cancer: implications for therapy. Am J Clin Exp Urol. 2014;2(1):27–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Khan NI, Bradstock KF, Bendall LJ. Activation of Wnt/beta-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol. 2007;138(3):338–48. doi: 10.1111/j.1365-2141.2007.06667.x. [DOI] [PubMed] [Google Scholar]
- 8.Tu K, Yang W, Li C, et al. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer. 2014;13(1):110. doi: 10.1186/1476-4598-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu K, Zheng X, Zhou Z, et al. Recombinant human adenovirus-p53 injection induced apoptosis in hepatocellular carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls c-Myc and cyclin E. PLoS One. 2013;8(7):e68574. doi: 10.1371/journal.pone.0068574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Kitazaki T, Oka M, Nakamura Y, et al. Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer. 2005;49(3):337–343. doi: 10.1016/j.lungcan.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin AT, Phillips BT. The tumor suppressor APC differentially regulates multiple beta-catenins through the function of axin and CKIalpha during C. elegans asymmetric stem cell divisions. J Cell Sci. 2014;127( Pt 12):2771–81. doi: 10.1242/jcs.146514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ysebaert L, Chicanne G, Demur C, et al. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20(7):1211–16. doi: 10.1038/sj.leu.2404239. [DOI] [PubMed] [Google Scholar]
- 13.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Shakoori A, Ougolkov A, Yu ZW, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun. 2005;334(4):1365–73. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Merli M, Giusto M, Molfino A, et al. MuRF-1 and p-GSK3βeta expression in muscle atrophy of cirrhosis. Liver Int. 2013;33(5):714–21. doi: 10.1111/liv.12128. [DOI] [PubMed] [Google Scholar]
- 17.Verma UN, Surabhi RM, Schmaltieg A, et al. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9(4):1291–300. [PubMed] [Google Scholar]
- 18.Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 19.Tian XH, Hou WJ, Fang Y, et al. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/beta-catenin signaling pathway. J Exp Clin Cancer Res. 2013;32:100. doi: 10.1186/1756-9966-32-100. [DOI] [PMC free article] [PubMed] [Google Scholar]