Abstract
Background
Many epidemiology studies have indicated that several functional polymorphisms of the IL-27 gene may contribute to individual susceptibility to cancer. Nevertheless, the data arising from these studies were inconclusive. Therefore, we conducted the current meta-analysis aiming to elucidate the effects of IL-27 polymorphisms (rs153109, rs17855750, and rs181206) on cancer susceptibility.
Material/Methods
We searched the CNKI (Chinese National Knowledge Infrastructure), Wanfang database, PubMed, Web of Science, and Google Scholar for all eligible publications. We used odds ratios (ORs) corresponding with 95% confidence intervals (CIs) by using the random/fixed-effects model to evaluate the association. Finally, a total of 12 publications, including 27 case-control studies comprising of 7570 patients and 9839 controls, were enrolled in our meta-analysis.
Results
Our work demonstrates that IL-27 rs17855750 polymorphism is significantly associated with cancer susceptibility, particularly for bladder cancer. However, no association between IL-27 rs153109 and rs181206 polymorphisms and cancer susceptibility was identified. When a stratification analysis was performed by cancer type, we identified an increased susceptibility of bladder cancer in rs153109 polymorphism. Moreover, in the stratification analysis by genotyping method, we identified an increased susceptibility for PCR-RFLP group in rs17855750 polymorphism, whereas a decreased susceptibility was identified in rs153109 polymorphism.
Conclusions
Our study shows that IL-27 rs17855750 polymorphism is significantly associated with increased susceptibility to cancer in Chinese.
MeSH Keywords: Interleukin-27; Meta-Analysis; Polymorphism, Single-Stranded Conformational
Background
With the obvious increasing prevalence and mortality rate, cancer has become one of the primary causes of morbidity and mortality [1]. The underlying mechanisms of the tumorigenesis are obscure because of the involvement of multiple risk factors containing complicated gene-gene and gene-environment interactions [2]. Many studies have demonstrated that the occurrence of cancers may be related to inflammation, and that cytokines are associated with individual susceptibility to cancers [3].
Cytokine-mediated immunity plays a critical role in the tumorigenesis [4]. IL-27 is included in the cytokines of IL-12 family. It is located on chromosome 16 (16p11) and comprises 2 subunits-Epstein-Barr virus-induced gene 3 (EBI3) and p28, which is a recently discovered IL-12p35-related polypeptide [5]. IL-27 is mainly secreted by antigen-presenting cells. It was described as a pro-inflammatory cytokine that enhances T helper (Th) 1 responses, cytotoxic T lymphocytes (CTLs) maturation, natural killer (NK) cells stimulation, and secretion of interferon-γ (IFN-γ) [5]. Research shows that IL-27 can impair tumor progression by CD8+ T cells with promoted CTL reaction, regardless of tumor immunogenicity [6]. Additionally, IL-12 inhibits neoangiogenesis in tumors by stimulation of IFN-γ-inducible protein-10 (IP-10) [7].
In view of the association of IL-27 polymorphisms and susceptibility to cancer, some previous case-control studies were conducted among Chinese population [8–13]. However, the conclusions were inconsistent and inconclusive. In 2014, Hu et al. [14] conducted a meta-analysis of 6 case-control studies and concluded that IL-27 rs153109 polymorphism was associated with cancer susceptibility in Chinese, a result consistent with another meta-analysis of 8 case-control studies by Xu et al. [15] that IL-27-964A/G (rs153109) polymorphism might enhance cancer susceptibility. Using more recent studies, we conducted an updated meta-analysis to obtain a more precise evaluation of the relevance of IL-27 polymorphisms (rs153109, rs17855750, and rs181206) and cancer susceptibility.
Material and Methods
Literature search strategy
We conducted a comprehensive literature search in PubMed, Web of Science, Google Scholar, CNKI (Chinese National Knowledge Infrastructure), and Wanfang databases to find all eligible case-control studies up to June 11, 2015 on the association between polymorphisms of IL-27 (rs153109, rs17855750 and rs181206) and cancer susceptibility by applying the searching terms: (“interleukin-27” OR “IL-27”) and (“mutation” OR “polymorphism” OR “variant”) and (“cancer” OR “malignancy” OR “carcinoma” OR “tumour” OR “neoplasm”) without language restriction. In addition, we checked the reference lists of all the eligible publications or the relevant reviews for additional studies.
Selection criteria
The studies enrolled in the current meta-analysis had to satisfy the following criteria: (a) studies that assessed the relevance between the polymorphisms in IL-27 and cancer susceptibility; (b) studies designed as case-control; (c) we can obtain specific genotype frequency of all the cases and controls or they can be obtained from the available data. We excluded studies which were: (a) case-only studies, reviews, comments, and case reports; (b) studies without the raw statistics of the polymorphisms of IL-27; (c) publications that were repetitive; (d) studies focused on animals.
Data extraction
The following details were recorded from each study by 3 investigators (Meng Zhang, Xiuxiu Tan, and Junjie Huang): the name of the first author, year published, ethnicity of the case-control studies, source of controls, genotyping method, the number of cases and controls, and the P value of the HWE in control groups. Any disagreements were discussed by the 3 authors until a consensus was reached.
Statistical analysis
We assessed the association between IL-27 polymorphisms and cancer susceptibility by OR and 95% CI. A total of 5 different ORs were calculated: allele contrast model (B vs. A), dominant model (BB+AB vs. AA), recessive model (BB vs. AB+AA), heterozygote comparison (AB vs. AA), and homozygote comparison (BB vs. AB) (AA, homozygotes for the common allele; AB, heterozygotes; BB, homozygotes for the rare allele). We conducted a χ2-test-based Q statistic test to evaluate the heterogeneity within the case-control studies [16]. If the Q test (P>0.1) suggested homogeneity across studies, we selected the fixed-effects model [17]; otherwise, the random-effects model was used [18]. In addition, we further quantified the heterogeneity effect by I2 test (I2<25%: no heterogeneity; I2=25–50%: moderate heterogeneity; I2=50–75%: high heterogeneity, I2>75%: extreme high heterogeneity) [19]. P values of the HWE for control groups were tested by χ2 test. Stratification analyses on tumor type and genotyping method were conducted. If any cancer type totals no more than 2 studies, we included it into the “other cancers” group. Sensitivity analyses were used to calculate the stability of the results by removing a single case-control study from the enrolled pooled data to detect the influence of that data set on the pooled ORs. Finally, we used Begg’s funnel plot and Egger’s regression test to evaluate the potential publication bias [20, 21]. P<0.05 was considered as statistically significant. We used STATA 12.0 (Stata Corporation, College Station, Texas) to conduct all statistical analyses.
Results
Study characteristics
As presented in Figure 1, after a systematic literature search in the databases, 46 potential relevant studies on the association between IL-27 polymorphisms and cancer were identified. Nevertheless, of them, 34 were unqualified in that some were based on case-only design, some were not polymorphism studies and the others were not concerning the susceptibility of cancer. In the end, a total of 12 publications with 27 independent case-control studies comprising of 7,570 cases and 9,839 controls were concerning the associations of IL-27 polymorphisms and cancer susceptibility. The characteristics of enrolled studies were presented in Table 1 [8–13,22–27]. The genotype distributions of the three polymorphisms and the genotyping method of the enrolled studies were retrieved scrupulously, and the controls were selected from non-cancer populations. Additionally, there were three case-control studies deviated from HWE [13,23].
Figure 1.
The flow chart presenting the publications selecting procedure.
Table 1.
Characteristics of the enrolled studies.
| SNP | First author | Year | Source of Control | Country | Genotyping method | Cancer type | Case | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AB | BB | AA | AB | BB | P (HWE) | |||||||
| RS153109 | Wei et al. | 2009 | HB | Chinese | PCR-RFLP | NPC | 119 | 150 | 33 | 113 | 161 | 36 | 0.060 |
| Zhao et al. | 2009 | HB | Chinese | PCR-RFLP | Glioma | 79 | 101 | 30 | 81 | 112 | 27 | 0.215 | |
| Peng et al. | 2013 | HB | Chinese | PCR-RFLP | HCC | 38 | 48 | 21 | 40 | 46 | 19 | 0.371 | |
| Zhang et al. | 2015 | HB | Chinese | PCR-RFLP | PTC | 287 | 309 | 68 | 332 | 399 | 96 | 0.147 | |
| Pan et al. | 2012 | HB | Chinese | PCR-RFLP | NPC | 90 | 78 | 22 | 85 | 87 | 28 | 0.453 | |
| Tao et al. | 2012 | HB | Chinese | PCR-RFLP | ESC | 163 | 205 | 58 | 162 | 219 | 51 | 0.075 | |
| Zhang et al. | 2014 | HB | Chinese | PCR-RFLP | EOC | 85 | 103 | 41 | 161 | 124 | 35 | 0.139 | |
| Guo et al. | 2012 | HB | Chinese | PCR-RFLP | CRC | 53 | 84 | 33 | 75 | 66 | 19 | 0.449 | |
| Huang et al. | 2012 | HB | Chinese | PCR-RFLP | CRC | 151 | 213 | 46 | 183 | 222 | 45 | 0.059 | |
| Zhang et al. | 2014 | HB | Chinese | PCR-RFLP | BRC | 143 | 156 | 27 | 185 | 223 | 52 | 0.213 | |
| Tang et al. | 2014 | HB | Chinese | PCR | Osteosarcoma | 56 | 85 | 19 | 100 | 124 | 26 | 0.167 | |
| Zhou et al. | 2015 | HB | Chinese | PCR | BC | 66 | 87 | 23 | 229 | 204 | 66 | 0.058 | |
| Zhou et al. | 2015 | HB | Chinese | PCR | BC | 61 | 73 | 22 | 229 | 204 | 66 | 0.058 | |
| RS17855750 | Wei et al. | 2009 | HB | Chinese | PCR-RFLP | NPC | 247 | 55 | 0 | 259 | 51 | 0 | 0.115 |
| Zhao et al. | 2009 | HB | Chinese | PCR-RFLP | Glioma | 169 | 41 | 0 | 185 | 35 | 0 | 0.200 | |
| Peng et al. | 2013 | HB | Chinese | PCR-RFLP | HCC | 83 | 21 | 3 | 72 | 28 | 5 | 0.304 | |
| Zhang et al. | 2014 | HB | Chinese | PCR-RFLP | OC | 170 | 51 | 8 | 267 | 53 | 0 | 0.106 | |
| Tao et al. | 2012 | HB | Chinese | PCR-RFLP | ESC | 345 | 81 | 0 | 355 | 77 | 0 | 0.042 | |
| Huang et al. | 2012 | HB | Chinese | PCR-RFLP | CRC | 341 | 69 | 0 | 382 | 68 | 0 | 0.083 | |
| Guo et al. | 2012 | HB | Chinese | PCR-RFLP | CRC | 120 | 41 | 9 | 122 | 33 | 5 | 0.151 | |
| Tang et al. | 2014 | HB | Chinese | PCR | Osteosarcoma | 132 | 28 | 0 | 205 | 45 | 0 | 0.118 | |
| Zhou et al. | 2015 | HB | Chinese | PCR | BC | 149 | 26 | 1 | 421 | 78 | 0 | 0.058 | |
| Zhou et al. | 2015 | HB | Chinese | PCR | BC | 126 | 27 | 3 | 421 | 78 | 0 | 0.058 | |
| RS181206 | Wei et al. | 2009 | HB | Chinese | PCR-RFLP | NPC | 241 | 61 | 0 | 253 | 57 | 0 | 0.075 |
| Zhao et al. | 2009 | HB | Chinese | PCR-RFLP | Glioma | 166 | 44 | 0 | 182 | 38 | 0 | 0.161 | |
| Pan et al. | 2012 | HB | Chinese | PCR-RFLP | NPC | 157 | 33 | 0 | 158 | 42 | 0 | 0.097 | |
| Tao et al. | 2012 | HB | Chinese | PCR-RFLP | ESC | 335 | 91 | 0 | 354 | 78 | 0 | 0.039 | |
| Huang et al. | 2012 | HB | Chinese | PCR-RFLP | CRC | 331 | 79 | 0 | 373 | 77 | 0 | 0.047 | |
| Tang et al. | 2014 | HB | Chinese | PCR | Osteosarcoma | 131 | 29 | 0 | 207 | 43 | 0 | 0.137 | |
NPC – nasopharyngeal carcinoma; HCC – hepatocellular carcinoma; PTC – papillary thyroid cancer; CRC – colorectal cancer; BC – bladder cancer; ESC – esophageal cancer; EOC – epithelial ovarian cancer; BRC – breast cancer; PCR-RFLP – polymerase chain reaction-restriction fragment length polymorphism; HWE – Hardy-Weinberg equilibrium; H-B: hospital based.
Meta-analysis
The results of the meta-analysis for the association of IL-27 polymorphisms (rs17855750, rs181206 and rs153109) with susceptibility to cancer are presented in Table 2. Obvious heterogeneity was identified in IL-27 rs17855750 polymorphism (GG vs. TT: Pheterogeneity=0.036, I2=61.2% and GG vs. GT+TT: Pheterogeneity=0.046, I2=58.7%) and rs153109 polymorphism under all 5 genetic models (G vs. A: Pheterogeneity=0.003, I2=35.5%; GG vs. AA: Pheterogeneity=0.038, I2=20.4%; GA vs. AA: Pheterogeneity=0.047, I2=18.8%; GG+GA vs. AA: Pheterogeneity=0.006, I2=32.6%; GG vs. GA+AA: Pheterogeneity=0.038, I2=4.9%) (Table 2). Therefore, we chose the random-effects model to generate wider CIs in these genetic models.
Table 2.
Results of meta-analysis for the polymorphisms in IL-27 and cancer risk.
| Variables (rs17855750) | Case/Control | G vs. T | GG vs. TT | GT vs. TT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | ||
| Total | 1.177 (1.034–1.341)* | 0.156 | 31.5 | 3.529 (0.803–15.515) | 0.036 | 61.2 | 1.120 (0.972–1.290) | 0.747 | 0.0 | |
| Bladder cancer | 1.196 (0.877–1.630) | 0.322 | 0.0 | 14.600 (1.653–128.994)* | 0.647 | 0.0 | 1.042 (0.742–1.464) | |||
| Colorectal cancer | 1.211 (0.925–1.586) | 0.511 | 0.0 | 1.830 (0.596–5.619) | – | – | 1.177 (0.872–1.588) | 0.746 | 0.0 | |
| Genotyping method | 0.554 | 0.0 | ||||||||
| PCR-RFLP | 1.194 (1.028–1.387)* | 0.074 | 47.9 | 2.035 (0.356–11.628) | 0.037 | 69.6 | 1.155 (0.981–1.361) | 0.550 | 0.0 | |
| PCR | 1.125 (0.865–1.464) | 0.472 | 0.0 | 14.600 (1.653–128.994)* | 0.647 | 0.0 | 1.019 (0.766–1.354) | 0.815 | 0.0 | |
| Case/ control | GG+GT vs. TT | GG vs. GT+TT | ||||||||
| OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | |||||
| Total | 1.156 (1.005–1.329)* | 0.410 | 3.3 | 3.413 (0.818–14.250) | 0.046 | 58.7 | ||||
| Bladder cancer | 1.121 (0.804–1.563) | 0.421 | 0.0 | 14.480 (1.640–127.836)* | 0.658 | 0.0 | ||||
| Colorectal cancer | 1.205 (0.899–1.615) | 0.602 | 0.0 | 1.733 (0.568–5.286) | – | – | ||||
| Genotyping method | ||||||||||
| PCR-RFLP | 1.185 (1.008–1.392)* | 0.232 | 25.7% | 1.974 (0.380–10.249) | 0.051 | 66.4 | ||||
| PCR | 1.073 (0.811–1.420) | 0.645 | 0.0% | 14.480 (1.640–127.8360)* | 0.658 | 0.0 | ||||
| Variables (rs181206) | Case/ control | C vs. T | CC vs. TT | CT vs. TT | ||||||
| OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | ||
| Total | 1.110 (0.947–1.302) | 0.823 | 0.0% | – | – | – | 1.124 (0.950–1.330) | 0.784 | 0.0 | |
| NPC | 0.983 (0.730–1.322) | 0.314 | 1.2% | – | – | – | 0.981 (0.716–1.342) | 0.287 | 11.8 | |
| Genotyping method | ||||||||||
| PCR-RFLP | 1.116 (0.943–1.321) | 0.709 | 0.0% | – | – | – | 1.131 (0.946–1.351) | 0.662 | 0.0 | |
| Case/ control | CC+CT vs. TT | CC vs. CT+TT | ||||||||
| OR (95% CI) | Pa | I2 | OR (95% CI) | Pa | I2 | |||||
| Total | 1.124 (0.950–1.330) | 0.784 | 0.0 | – | – | – | ||||
| NPC | 0.981 (0.716–1.342) | 0.287 | 11.8 | – | – | – | ||||
| Genotyping method | ||||||||||
| PCR–RFLP | 1.131 (0.946–1.351) | 0.662 | 0.0 | – | – | – | ||||
| Variables (rs153109) | Case/ control | G vs. A | GG vs. AA | GG vs. AA | ||||||
| OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | ||
| Total | 3526/4732 | 1.080 (0.972–1.201) | 0.003 | 35.5 | 1.137 (0.929–1.391) | 0.038 | 20.4 | 1.095 (0.961–1.249) | 0.047 | 18.8 |
| Cancer type | ||||||||||
| NPC | 492/510 | 0.895 (0.746–1.075) | 0.661 | 0.0 | 0.814 (0.540–1.226) | 0.706 | 0.0 | 0.870 (0.667–1.134) | 0.875 | 0.0 |
| Colorectal cancer | 580/610 | 1.325 (0.911–1.927) | 0.044 | 57.0 | 1.671 (0.859–3.253) | 0.098 | 40.3 | 1.386 (0.910–2.110) | 0.123 | 33.5 |
| Bladder cancer | 332/998 | 1.190 (0.991–1.428) | 0.948 | 0.0 | 1.230 (0.831–1.819) | 0.932 | 0.0 | 1.413 (1.080–1.848)* | 0.725 | 0.0 |
| Genotyping method | ||||||||||
| PCR–RFLP | 3034/3484 | 1.057 (0.928–1.205) | 0.001 | 44.9 | 0.943 (0.890–1.000)* | 0.094 | 27.9 | 1.035 (0.896–1.195) | 0.077 | 17.8 |
| PCR | 492/1248 | 1.178 (1.009–1.375)* | 0.979 | 0.0 | 1.144 (0.899–1.456) | 0.792 | 0.0 | 1.357 (1.081–1.703)* | 0.805 | 0.0 |
| Case/ control | GG+GA vs. AA | GG vs. GA+AA | ||||||||
| OR (95% CI) | Pa | I2 (%) | OR (95% CI) | Pa | I2 (%) | |||||
| Total | 3526/4732 | 1.112 (0.963–1.283) | 0.006 | 32.6 | 1.064 (0.930–1.218) | 0.038 | 4.9 | |||
| Cancer type | ||||||||||
| NPC | 664/827 | 0.857 (0.666–1.104) | 0.786 | 0.0 | 0.878 (0.598–1.289) | 0.708 | 0.0 | |||
| Colorectal cancer | 160/250 | 1.465 (0.897–2.393) | 0.786 | 51.4 | 1.328 (0.934–1.889) | 0.237 | 8.0 | |||
| Bladder cancer | 492/510 | 1.368 (1.061–1.764) | 0.794 | 0.0 | 1.029 (0.715–1.481) | 0.812 | 0.0 | |||
| Genotyping method | 210/220 | |||||||||
| PCR–RFLP | 426/432 | 1.061 (0.898–1.253) | 0.006 | 37.2 | 1.065 (0.917–1.237) | 0.175 | 8.6 | |||
| PCR | 229/320 | 1.331 (1.072–1.652)* | 0.890 | 0.0 | 1.061 (0.775–1.453) | 0.923 | 0.0 | |||
I2: 0–25, means no heterogeneity; 25–50, means modest heterogeneity; >50, means high heterogeneity; Pa: P value of Q test for heterogeneity test;
means statistically significant (P<0.05).
The present work identified that rs17855750 polymorphism of IL-27 was significantly associated with cancer susceptibility (G vs. T: OR=1.177, 95%CI=1.304–1.341, Pheterogeneity=0.156, Figure 2A; GG+GT vs. TT: OR=1.156, 95%CI=1.005–1.329, Pheterogeneity=0.410, Figure 2B), particularly for bladder cancer (GG vs. TT: OR=14.600, 95%CI=1.653–128.994, Pheterogeneity=0.647; GG vs. GT+TT: OR=14.480, 95%CI=1.640–127.836, Pheterogeneity=0.658). Nevertheless, no association was identified between IL-27 rs153109 and rs181206 polymorphisms and cancer susceptibility. When a stratification analysis was performed by cancer type, we identified an increased susceptibility of bladder cancer in rs153109 polymorphism (GA vs. AA: OR=1.413, 95%CI=1.080–1.848, Pheterogeneity=0.725). Moreover, in the stratification analysis by genotyping method, we identified an increased susceptibility for PCR-RFLP group in rs17855750 polymorphism (G vs. T: OR=1.194, 95%CI=1.028–1.387, Pheterogeneity=0.074; GG+GT vs. TT: OR=1.185, 95%CI=1.008–1.392, Pheterogeneity=0.232), whereas a decreased susceptibility was identified in rs153109 polymorphism (GG vs. AA: OR=0.943, 95%CI=0.890–1.000, Pheterogeneity=0.094) (Table 2).
Figure 2.
(A) OR estimates with the corresponding 95% CI for the association of IL-27 rs17855750 polymorphism with overall cancer risk (G vs. T). The sizes of the squares represent the weighting of included studies; OR: odds ratio; CI: confidence interval. (B) OR estimates with the corresponding 95% CI for the association of IL-27 rs17855750 polymorphism with overall cancer risk (GG+GT vs. TT). The sizes of the squares represent the weighting of included studies; OR: odds ratio; CI: confidence interval.
Sensitivity analyses and publication bias
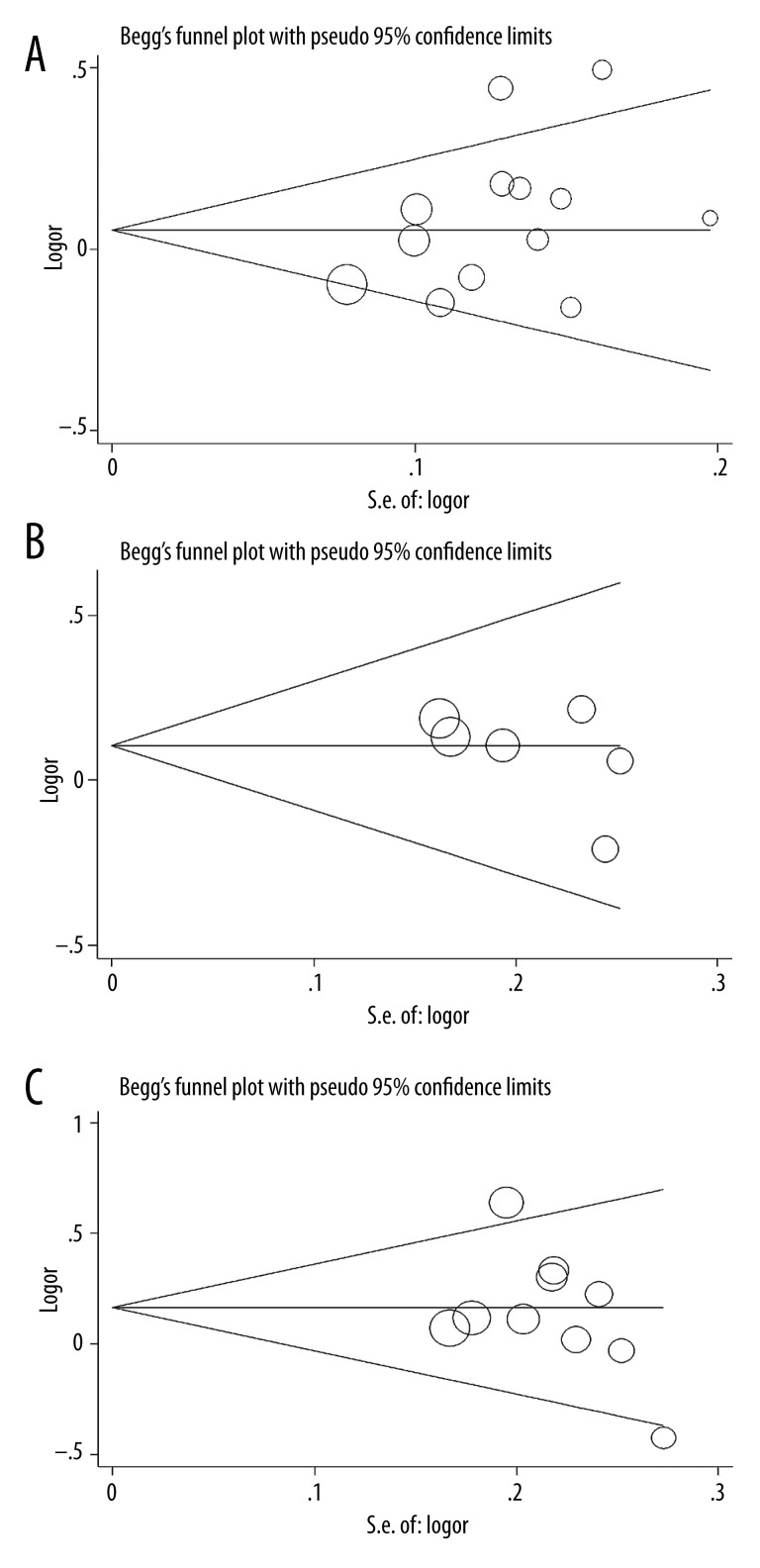
We performed sensitivity analysis by omitting each study sequentially, suggesting that the results for the overall population were statistically robust and reliable (Figure 3A–3C). Egger’s test and Begg’s funnel plot were performed to examine the publication bias risk in our research. No publication bias was identified (rs153109: G vs. A: P=0.300 for Begg’s test, P=0.112 for Egger’s test, Figure 4A; rs181206: C vs. T: P=0.133 for Begg’s test, P=0.252 for Egger’s test; Figure 4B; rs17855750: G vs. T: P=0.283 for Begg’s test, P=0.322 for Egger’s test; Figure 4C).
Figure 3.
(A) Sensitivity analysis of overall OR coefficients for IL-27 rs153109 polymorphism (G vs. A). Results were calculated by omitting each study in turn. The 2 ends of the dotted lines represent the 95%CI. (B) Sensitivity analysis of overall OR coefficients for IL-27 rs181206 polymorphism (C vs. T). Results were calculated by omitting each study in turn. The 2 ends of the dotted lines represent the 95%CI. (C) Sensitivity analysis of overall OR coefficients for IL-27 rs17855750 polymorphism (G vs. T). Results were calculated by omitting each study in turn. The 2 ends of the dotted lines represent the 95%CI.
Figure 4.
(A) Publication bias in studies of the association between the IL-27 rs153109 polymorphism and cancer susceptibility assessed by Begg’s funnel plot and Egger’s test (G vs. A). Log (OR): the natural logarithm of the odds ratio. (B) Publication bias in studies of the association between the IL-27 rs181206 polymorphism and cancer risk assessed by Begg’s funnel plot and Egger’s test (C vs. T). Log (OR): the natural logarithm of the odds ratio. (C) Publication bias in studies of the association between the IL-27 rs17855750 polymorphism and cancer susceptibility assessed by Begg’s funnel plot and Egger’s test (G vs. T). Log (OR): the natural logarithm of the odds ratio.
Discussion
Interleukin-27 is a newly discovered member of the IL-12 family, which is regarded as a mediator of naive T cell proliferation, and an inducer of IFN-g secretion, specifically in synergy with IL-12 [28]. Recently, Chiyo et al. [29] investigated the antitumor ability of IL-27 against a murine tumour model and observed that IL-27 could induce tumour-specific antitumor activity. The relationship between IL-27 polymorphisms and cancer susceptibility had been investigated in recently published case-control studies, but conflicting results were reported. Meta-analysis is regarded as a crucial method to accurately define the influence of specific genetic polymorphisms on cancer susceptibility. After searching the databases, 2 meta-analyses were found focussing on the relevance of the IL-27 polymorphisms and cancer susceptibility [14,15]. In 2014, Hu et al. [14] conducted a meta-analysis and concluded that IL-27 rs153109 polymorphism was associated with cancer susceptibility in Chinese, whereas the rs17855750 and rs181206 polymorphisms were not. The results were consistent with another meta-analysis conducted by Xu et al. [15], which reported that IL-27 rs153109 polymorphism might enhance cancer susceptibility. Additionally, their results also demonstrated that IL-27 rs153109 polymorphism increased colorectal cancer susceptibility. However, several limitations in both analyses were obvious. First, the number of eligible published studies used was limited to a total of 6, with 1684 patients and 1837 controls. Second, there were 8 case-control studies, including 2044 cancer cases and 2197 controls focussing only on a single IL-27 polymorphism. Therefore, we performed the current meta-analysis to comprehensively elucidate the effects of IL-27 polymorphisms (rs153109, rs17855750 and rs181206) in a total of 12 publications, including 27 case-control studies comprising 7570 patients and 9839 controls. Interestingly, the results were inconsistent with previous studies. Except for IL-27 rs17855750 polymorphism, there was no evident relationship between the IL-27 rs153109 and rs17855750 polymorphisms and cancer susceptibility. In addition, when we performed a stratification analysis by cancer type, an increased susceptibility to bladder cancer in rs153109 polymorphism was identified.
Nevertheless, there exist several drawbacks in our meta-analysis. Stratifications may be introduced by the combination of genetic studies on various cancers in the meta-analysis. The results of IL-27 polymorphisms on cancer susceptibility might be affected by several complicated factors, such as age, sex, ethnicity, source of controls, and matching criteria when we lack the original data. Additionally, only papers published in a limited number of databases were searched, and some studies might have been missed; therefore, the eligible case-control samples included into the current meta-analysis were insufficient. Thirdly, all the studies were conducted in Chinese, and no research in whites or Africans was identified.
Conclusions
This meta-analysis illustrated that IL-27 rs17855750 polymorphism enhanced cancer susceptibility in a Chinese population, and an increased susceptibility of bladder cancer was identified in rs153109 polymorphism when a stratification analysis was performed by cancer type. Further well-planned studies on these variants are warranted to discover the mechanisms of IL-27 polymorphisms in the tumorigenesis of these cancers.
Footnotes
Competing interests
The authors have declared that no competing interests exist.
Source of support: The work by Z.C. and S.W. was supported by JCYJ (20130401114928183; JCYJ20140416180323426)
Reference
- 1.Kimman M, Norman R, Jan S, et al. The burden of cancer in member countries of the Association of Southeast Asian Nations (ASEAN) Asian Pac J Cancer Prev. 2012;13(2):411–20. doi: 10.7314/apjcp.2012.13.2.411. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4(11):850–60. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 6.Jankowski M, Kopinski P, Goc A. Interleukin-27: biological properties and clinical application. Arch Immunol Ther Exp (Warsz) 2010;58(6):417–25. doi: 10.1007/s00005-010-0098-6. [DOI] [PubMed] [Google Scholar]
- 7.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13(16):4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 8.Zhou B, Zhang P, Tang T, et al. Polymorphisms and plasma levels of IL-27: impact on genetic susceptibility and clinical outcome of bladder cancer. BMC Cancer. 2015;15(1):433. doi: 10.1186/s12885-015-1459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Gao X, Wang Y, et al. Interleukin 27-964A > G genetic polymorphism and serum IL-27p28 levels in Chinese patients with papillary thyroid cancer. Tumour Biol. 2015 doi: 10.1007/s13277-015-3570-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Wang Y, Wang M, Ji Z. IL-27–964A>G polymorphism and the risk of breast cancer: a case-control study. Tumour Biol. 2014;35(12):12099–102. doi: 10.1007/s13277-014-2512-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Zhou B, Wu Y, et al. Prognostic value of IL-27 polymorphisms and the susceptibility to epithelial ovarian cancer in a Chinese population. Immunogenetics. 2014;66(2):85–92. doi: 10.1007/s00251-013-0753-2. [DOI] [PubMed] [Google Scholar]
- 12.Peng Q, Qin X, He Y, et al. Association of IL27 gene polymorphisms and HBV-related hepatocellular carcinoma risk in a Chinese population. Infect Genet Evol. 2013;16:1–4. doi: 10.1016/j.meegid.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Huang ZQ, Wang JL, Pan GG, Wei YS. Association of single nucleotide polymorphisms in IL-12 and IL-27 genes with colorectal cancer risk. Clin Biochem. 2012;45(1–2):54–59. doi: 10.1016/j.clinbiochem.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Hu T, Zhao H, Wang K, et al. Association of IL-27 polymorphisms and cancer risk in Chinese population. J Recept Signal Transduct Res. 2015;35(2):180–83. doi: 10.3109/10799893.2014.942465. [DOI] [PubMed] [Google Scholar]
- 15.Xu XP, Hua LY, Chao HL, et al. Genetic association between IL-27 rs153109 polymorphism and cancer risk in Chinese population: a meta-analysis. J Recept Signal Transduct Res. 2014:1–6. doi: 10.3109/10799893.2014.986743. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–26. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controll Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang YJ, Wang JL, Nong LG, et al. Associations of IL-27 polymorphisms and serum IL-27p28 levels with osteosarcoma risk. Medicine. 2014;93(10):e56. doi: 10.1097/MD.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao YP, Wang WL, Li SY, et al. Associations between polymorphisms in IL-12A, IL-12B, IL-12Rbeta1, IL-27 gene and serum levels of IL-12p40, IL-27p28 with esophageal cancer. J Cancer Res Clin Oncol. 2012;138(11):1891–900. doi: 10.1007/s00432-012-1269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei YS, Lan Y, Luo B, et al. Association of variants in the interleukin-27 and interleukin-12 gene with nasopharyngeal carcinoma. Mol Carcinog. 2009;48(8):751–57. doi: 10.1002/mc.20522. [DOI] [PubMed] [Google Scholar]
- 25.Zhao B, Meng LQ, Huang HN, et al. A novel functional polymorphism, 16974 A/C, in the interleukin-12-3’ untranslated region is associated with risk of glioma. DNA Cell Biol. 2009;28(7):335–41. doi: 10.1089/dna.2008.0845. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Qin A, Li R, et al. Association of single nucleotide polymorphisms in IL-12 and IL-27 genes with colorectal cancer risk. ChongQing Medicine. 2012;41(10):948–50. [Google Scholar]
- 27.Pan G, Lu D, Liang L, Huang C. Association of the genotype and serum level of IL-27 with nasopharyngeal carcinoma. ShanDong Medicine. 2012;52(41):18–20. [Google Scholar]
- 28.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19(5):641–44. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 29.Chiyo M, Shimozato O, Yu L, et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115(3):437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]