Abstract
Background and aims
Many epidemiological studies have revealed a positive correlation between medical radiation exposure and the reproductive health in female childhood cancer survivors. However, because of variations in the samples size, such studies showed partly inconsistent conclusions. The aim of this meta-analysis was to clarify the association between radiotherapy and the risk of reproductive health impairment for female who survived from childhood cancer.
Methods
Fourteen cohort studies involving childhood radiotherapy were selected as the exposure of interest and the impaired reproductive health condition during the childbearing age as the outcome. Among meta-analysis of observational studies found in Pubmed and Embase from 1900 to 2014, we evaluated those relevant observational studies which surveyed the association of medical radiation and reproductive health in female childhood cancer survivors. Review Manager 5.2 and STATA 12.0 software were used to perform the meta-analysis. Study-specific estimations for each outcome were combined into a pooled relative risk (RR) with 95 % confidence interval (CI) by a meta-analytic approach.
Results
Based on a random-effects meta-analysis, significant association between infertility (RR = 1.28, 95 % CI = 1.16–1.42), acute ovarian failure (AOF) (RR = 9.51, 95 % CI = 5.03–17.96), low level of anti mullerian hormone (AMH) (<1 ng/mL) (RR = 14.79, 95 % CI = 3.36–66.64), stillbirth (RR = 1.19, 95 % CI = 1.02–1.39) and low birth weight (RR = 2.22, 95 % CI = 1.55–3.17) were identified. Conversely, no significant results were found in abortion and congenital malformations.
Conclusions
To the best of our knowledge, this is the first meta-analysis assessing the effect of medical radiation on female childhood cancer survivors’ reproductive capability and pregnancy outcomes. Although there were some limitations, our meta-analysis further supported that radiotherapy was a risk factor for reproductive health problems of female who survived from childhood cancer.
Keywords: Radiotherapy, Infertility, Reproductive capability, Pregnancy outcomes, Meta-analysis
Introduction
People are exposed to ionizing radiation every day from the food, building materials, soil and air from outer space [1]. In addition to the natural radiation, people might also be exposed to the radiation from medical tests and treatments. Although in developed countries, childhood cancer is the second most common cause of death [2]. In recent years, radiotherapy has become increasingly a successful tool for the treatment of cancers in children, providing high survival rate expectations for many years after treatment.
Increasing attention has been paid on the reproductive health of women who survived from childhood cancer. Radiotherapy impacts all aspects of female reproductive system, including hypothalamic, pituitary gland and endometrial receptivity. Childhood radiotherapy increases the risk of damage to the hypothalamus and pituitary gland, decreasing the pituitary gonadotropins with subsequent ovaries dysfunction and highly likely leading to infertility. In Simone Reinmuth’s epidemiological study, women treated with radiotherapy during childhood showed high rate of infertility (31 %) [3]. The human oocyte is exquisitely sensitive to radiation. In childhood cancer survivors who have received radiotherapy, the diminished ovarian reserve was detected by an AMH value <1 ng/mL [4]. AMH, which is a sensitive indicator of the longitudinal decline of ovarian reserve, is produced in the granulosa cells in late preantral and small antral follicles and plays an essential role in the regulation of egress from the primordial follicle pool adjusting the follicles reentering the meiosis. The serum AMH declines with age and is an important and sensitive predictor of menopause. In vivo studies have shown that ionizing radiation may cause low level of serum AMH. In Yasmen and colleages’ study, the serum level of AMH was found to decrease in rats treated with total body gamma radiation [5]. Conversely, as another aspect of the ability of female fertility, the proportion of abortion in cancer survivors was similar to the population from control subjects. Moreover, the risk of low birth weight and stillbirth increased among the offspring of females who received radiotherapy during childhood [6]. Untill now, no evidences have suggested the role of childhood radiotherapy on congenital malformation.
Because most of the studies are focused on the effects of radiation on the fathers or female patients with cancer in offspring, there is a lack of consistency on the effects of childhood radiotherapy on female reproductive health condition. The objective of this study was to comprehensively and systematically review the epidemiological studies and to evaluate the association between childhood medical radiation and female reproductive health conditions.
Methods
Publications screening
We searched published studies in Pubmed and Embase databases updated to May 2014 with the following search terms: radiation therapy AND (fertility OR infertility OR reproduction OR pregnancy) AND (female OR women) AND (case control OR case–control OR cohort). Furthermore, reference lists of main reports and review articles were also reviewed to identify additional relevant publications. Our study was conducted and reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Selection criteria
Two authors (W.G and J.L) reviewed the retrieved titles and abstracts to identify the eligible studies for our meta-analysis. Published studies were included based on the following criteria: 1, written and published in English; 2, case–control study or cohort study; 3, studies about radiation therapy and fertility in female; 4, study about childhood cancer survivors; 5, entire paper with sufficient data on the relationship between radiation therapy and fertility. We excluded studies with the following criteria: 1, written and published in a language other than English; 2, not a case–control study or cohort study; 3, studies with no sufficient data on the relationship between radiation therapy and fertility; 4, studies that are not related to childhood cancer survivors; 5, review articles without original data; 6, a commentary, letter to the editor, or monograph.
Data extraction
Two authors (W.G and J.L) performed the data evaluation independently. The following data were extracted from each study: the first author’s last name; publication year; country; number of enrolled patients; fertility factors (infertility, stillbirth, spontaneous abortion, congenital anomaly, low birth weight, AMH value, AOF and preterm birth).
Quality assessment
The quality of eligible studies was estimated by two authors (W.G and J.L), and the disagreements were discussed and resolved by the reviewer (Q.Y). The appraisal followed the guidelines of the modified Newcastle-Ottawa Quality Assessment Scale (NOS) documents.
Data synthesis and statistical analysis
Exposure to radiotherapy was analyzed as dichotomous variables, as high-dose exposure versus non- or low-dose exposure. The fertility factors were also conducted as dichotomous variables (with vs. without) or (high vs. low). These data were analyzed by random-effect method, and were measured in RR with 95 % CI. Statistical heterogeneity was estimated by means of Cochran’s Q test and I-squared test. The I-squared test represents the percentage of variation to heterogeneity, which is categorized as low (0–40 %), moderate (40–60 %), high (60–90 %), and very high (>90 %). To identify any potential publication bias, we used Begg’s test and Egger’s test. All statistical analyses were performed with Review Manager 5.2 and STATA 12.0.
Results
Systematic review
Among the 400 studies selected, only 34 matched to our strategy with inclusion criteria and content (Fig. 1). After reviewing full text, 4 of the 34 studies were excluded because of ineligible study objects; 16 studies were excluded because lacking sufficient information for effects estimations. We reviewed reference lists of these articles, and there were no additional relevant publications to be included. Finally, we identified 14 studies for analysis [4, 6–18] (Table 1).
Fig. 1.
Flow chart of the literature search and article selection
Table 1.
Characteristics of studies included in the systemastic review and meta-analysis of radiotherapy and risk of reproductive health impairment
| The first author’s last name (year) [reference] | Study design | Country | Target population | Comparison group | Study sample size (exposed/ unexposed) | Fertility factors | Radiation therapy methods | NOS score |
|---|---|---|---|---|---|---|---|---|
| Chiarelli (1999) [6] | Cohort | Canada | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 225/494 | Infertility | Abd-pelvic radiation | 7 |
| Chiarelli (2000) [7] | Cohort | Canada | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 70/165 | Congenital anomaly, spontaneous abortion, low birth weight, stillbirth | Abd-pelvic radiation | 9 |
| Green (2002) [8] | Cohort | United States | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 232/1937 | Stillbirth or neonatal death | Ovarian radiation | 7 |
| Ronckers (2002) [9] | Cohort | Netherlands | Childhood cancer survivors | Nonexposed subjects | 1178/1057 | Infertility | NRI | 9 |
| Chemaitilly (2006) [10] | Cohort | United States | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 2110/961 | Acute ovarian failure | Radiation therapy | 7 |
| Signorello (2006) [11] | Cohort | United States | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 1116/622 | Preterm birth, low birth weight | Uterus radiation | 9 |
| Winther (2008) [12] | Cohort | Denmark | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 578/1082 | Infertility, spontaneous abortion | Radiation therapy | 8 |
| Signorello (2010) [13] | Cohort | United States | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 1713/1075 | Stillbirth or neonatal death | Uterus and ovarian radiation | 7 |
| Green (2011) [14] | Cohort | United States | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 258/1960 | Infertility | Hypothalamic/pituitary radiation | 7 |
| Signorello (2012) [15] | Cohort | United States and Canada | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 1020/607 | Congenital anomaly | Ovarian radiation | 8 |
| Thomas-Teinturier (2012) [16] | Cohort | French | Childhood cancer survivors | Childhood cancer survivors with low dose radiation therapy | NA | Acute ovarian failure | Radiation therapy | 7 |
| Van Dorp (2013) [17] | Cohort | Netherlands | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 12/164 | Anti-müllerian hormone | Abdominal or total body radiation | 9 |
| Barton (2013) [18] | Cohort | United States | Childhood cancer survivors | Childhood cancer survivors without radiation therapy | 2194/962 | Infertility | Radiation therapy | 7 |
| Lunsford (2014) [4] | Cohort | United States | Childhood cancer survivors | Childhood cancer survivors without pelvic radiation | 11/42 | Anti-müllerian hormone | Pelvic radiation | 7 |
Detailed characteristics of these studies are provided in Table 1. The included studies were published between 1999 and 2014, and all of them were cohort studies [4, 6–18]. A total of 10,717 women with radiation therapy and 11,128 women without radiation therapy from 13 studies were evaluated [4, 6–15, 17, 18]. Among the 14 studies analyzed, 1 study did not have the exact number of included participants, but only had effect estimates [16]. A total of 10 studies were performed in North America [4, 6–8, 10, 11, 13–15, 18], and 4 studies in Europe [9, 12, 16, 17]. In fertility factors, 5 studies were described about infertility [6,9.12,14,18], 2 studies were based on AMH values [4, 17], 2 studies on AOF [10, 16], 3 studies on stillbirth or neonatal death [7, 8, 13], 2 studies on spontaneous abortion [7, 12], 2 studies on congenital anomaly [7, 15], 2 studies on low birth weight [7, 11] and 1 study on preterm birth [11]. In radiation therapy methods, 4 studies were performed in radiation therapy without specific description [10, 12, 16, 2], 2 studies on abd-pelvic radiation [6, 7], 2 studies on ovarian radiation [8, 15] and each of the 6 last studies were performed on pelvic radiation [4], abdominal or total body radiation [17], hypothalamic or pituitary radiation [14], nasopharyngeal radium irradiation [9], uterus radiation [11], uterus and ovarian radiation [13].
The NOS quality point of included studies is provided in Table 1. We defined the NOS quality point > 6 as higher quality and ≤ 6 as lower quality. All of included studies were higher quality.
Infertility
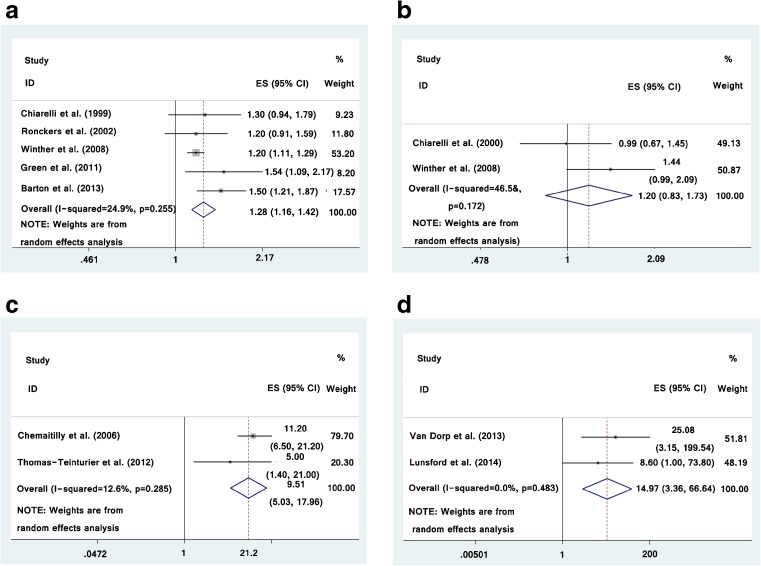
Among the 5 included studies that identified the relationship between radiotherapy and infertility [6–10], the study-specific RRs for infertility range were from 1.20 to 1.54. The pooled RR (exposed to radiotherapy vs. non-exposed) for infertility was 1.28 (95 % CI was 1.16–1.42) with low heterogeneity (I2 = 24.9 %) (Fig. 2a). There was no indication of publication bias with Begg’s test (p = 0.327) and Egger’s test (p = 0.180).
Fig. 2.
Forest plot for the meta-analysis of the association between radiotherapy during childhood and risk of infertility (a), abortion (b), AOF (c) and low AMH value (d)
Abortion
Two studies demonstrated the relationship between radiotherapy and spontaneous abortion [7, 12] and the study-specific RRs for spontaneous abortion range were from 0.99 to 1.44. The pooled RR (exposed to radiotherapy vs. non-exposed) for spontaneous abortion was 1.20 (95 % CI was 0.83–1.73) with significantly heterogeneity (I2 = 46.5 %) (Fig. 2b).
AOF
Two studies identified the relationship between radiotherapy and AOF [10, 16]. The pooled RR (exposed to radiotherapy vs. non-exposed) for AOF was 9.51 (95 % CI was 5.03–17.96) with low heterogeneity (I2 = 12.6 %) (Fig. 2c).
Low AMH values
Two studies described the influence of radiotherapy exposure in AMH concentration [4, 17]. The pooled RR (exposed to radiotherapy vs. non-exposed) for low level of AMH was 14.97 (95 % CI was 3.36–66.64) with low heterogeneity (I2 = 0.0 %) (Fig. 2d).
Stillbirth
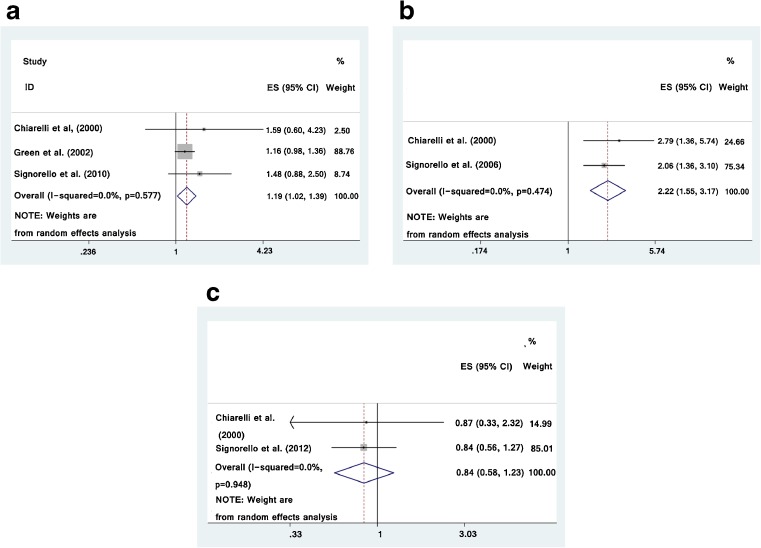
Three studies described radiotherapy associated with stillbirth or neonatal death [7, 8, 13], the study-specific RRs for stillbirth or neonatal death range were from 1.16 to 1.59. The pooled RR (exposed to radiotherapy vs. non-exposed) for stillbirth or neonatal death was 1.19 (95 % CI was 1.02–1.39) with significantly heterogeneity (I2 = 0.0 %) (Fig. 3a). There was no indication of publication bias with Begg’s test (p = 0.602) and Egger’s test (p = 0.185).
Fig. 3.
Forest plot for the meta-analysis of the association between radiotherapy during childhood and risk of stillbirth (a), low birth weight (b) and congenital malformation (c) in offspring
Low birth weight
Two included studies identified radiotherapy associated with low birth weight [7, 11], the study-specific RRs for low birth weight range were from 2.06 to 2.79. The pooled RR (exposed to radiotherapy vs. non-exposed) for low birth weight was 2.22 (95 % CI was 1.55–3.17) with low heterogeneity (I2 = 0.0 %) (Fig. 3b).
Congenital malformation
Two included studies identified radiotherapy associated with congenital malformation [7, 15], the study-specific RRs for congenital anomaly range were from 0.84 to 0.87. The pooled RR (exposed to radiotherapy vs. non-exposed) for congenital anomaly was 0.84 (95 % CI was 0.58–1.23) with low heterogeneity (I2 = 0.0 %) (Fig. 3c).
There was 1 study which described the effect of radiation therapy in preterm birth [19], the RR of preterm birth was 1.83 (95 % CI was 1.37–2.46). Because of the limitation of the studies, the meta-analysis for these factors could not be proceeded.
Discussion
Receiving radiotherapy during childhood may cause late effects of ionizing radiation and this was demonstrated by studies on childhood cancer survivors who received thoracic radiation, high rate of right ventricular dysfunction and restrictive lung disease [20]. Increasing attention has been paid to the radiation-induced damage to human health, especially to reproductive health. Radiation affecting ovarian and uterine functions has been linked to pregnancy complications including infertility, spontaneous abortion, acute ovarian failure, low birth weight and stillbirth. Also, sexual malfunction among female survivors of childhood cancers was partially related to cranial radiation treatment [21].
The most important issue for female reproductive health is the ability to successfully conceive a pregnancy and give birth to healthy children. To our knowledge, this is the first meta-analysis evaluating the effect of medical radiation on the reproductive capability and pregnancy outcomes of female childhood cancer survivors. We identified and reviewed 14 studies with a total of >20,000 female childhood cancer survivors.
Reproductive capability
Infertility is known as the impossibility of conceiving after unprotected intercourse. Approximately 8–12 % of couples with women at child-bearing age carry this condition [22]. It is reported that radiotherapy during childhood is associated with abnormal female reproductive capability which can be related with ovarian, endometrial or unexplained causes. In Xing and colleagues’ follow up study, women who suffered from acute radiation sickness showed severe ovarian failure and uterine dysfunction [23]. Our meta-analysis suggests high frequency of infertility among women who received radiotherapy during childhood (Fig. 2a). However, there is no significant association between abortion and childhood radiation. Among the potential late effects of radiotherapy, evaluation of ovarian function remains to be one of the most important aspects of the survivors’ management. Childhood radiotherapy increases the risk of hypothalamus and pituitary gland damages and results in decreased pituitary gonadotropins subsequently leading to ovaries dysfunction [24]. Ovarian radiation results in loss of hormone production and infertility because the normal hormone level is critical for the ootid development and plays an essential role in the maturation of the primary follicle [25]. Our meta-analysis also demonstrated the significant association between AOF and radiotherapy during childhood (Fig. 2c). AOF is a potential impairment factor for female reproductive health, and radiation is known as a potent ovotoxicants capable of accelerating ovarian reserve depletion. AMH, a predictor of menopause, is a sensitive indicator of the longitudinal decline of ovarian reserve. The diminished ovarian reserve was diagnosed when AMH value is less than 1 ng/mL [4]. We found that childhood radiation is associated with low level (ovarian reserve) of AMH value (Fig. 2d). The association of childhood radiotherapy and ovarian failure is only found in limited meta-analyses. Our study comprehensively assessed the significant association between radiotherapy and ovarian dysfunction among female childhood cancer survivors.
Pregnancy outcomes
Previous studies on the effects of childhood radiation in stillbirth, low birth weight and congenital malformation were inconsistent. In this study, we employed meta-analysis to comprehensively assess the association between female childhood radiotherapy and pregnancy outcomes. The association between childhood radiation and stillbirth, as well as low birth weight has been previously described [26]. From Signorello and colleagues’ analysis [13], radiation-induced toxicity to the uterus was the main cause of stillbirth. The underlying mechanism by which the radiation was most likely leading to low birth weight is the radiation induced somatic damage to the women’s abdominopelvic structures such as uterine vascular insufficiency and fibrosis [7]. From our meta-analysis, we found an increased risk of stillbirth and low birth weight in offspring of women who received radiotherapy during childhood. In the meantime, interestingly, we found the pooled RR for congenital anomaly on childhood radiotherapy was 0.84. This indicates that childhood radiotherapy may not be associated with increased risk of congenital anomaly. Findings of our study are coterminous with Nori Nakamura’s study, which demonstrated that no increased risk of malformation was observed in the offspring of the childhood cancer survivors [27].
Limitation
Several limitations of this meta-analysis should be noted. First, all published studies and papers were written in English. Some related published or unpublished studies that meet the inclusion criteria were missed, because edited in other languages. Second, the number of available references was limited. Third, there were insufficient data to allow subgroup analysis, most of the studies selected in our meta-analysis failed to provide the dose degree of radiation. Therefore, we were unable to conduct a dose–response analysis to assess the association between radiotherapy and female reproductive health. More studies about radiotherapy and reproductive health among females survived from childhood cancer should also be considered in the future.
Conclusion
The female reproductive health is one of great significance for familial and social harmony. It is predicted that girls who accepted radiotherapy treatment are at high risk of ovarian failure, which results in the increase of infertility, incidence of stillbirth and low birth weight among offspring. We found that childhood radiation is not a significant risk factor for abortion or congenital malformation of the offspring. Whether the doses of radiation, time post radiation, the individual differences or other unknown causes are also involved, still needs to be further explored. Our meta-analysis provided an important significance on counseling and management of the childbearing aged women who received radiotherapy during childhood, and called attention for the preservation of childrens who are suffering from unavoidable radiation impair during radiotherapy. Patients and physicians must be aware of the potential side effects which may affect the offspring.
Acknowledgments
The project was supported by National Natural Science Foundation of China Research grants no. 30672753 and 31270866.
The quality of eligible studies was estimated by two authors (W.G and J.L), and the disagreements were discussed and resolved by the reviewer (Q.Y).
Footnotes
Capsule Childhood radiotherapy is significantly associated with female infertility, acute ovarian failure, low AMH value, stillbirth of the mothers. Also, it is significantly associated with low birth weight of the offspring. Childhood radiotherapy is not significantly associated with abortion or congenital malformation of the offspring.
References
- 1.Groen RS, Bae JY, Lim KJ. Fear of the unknown: ionizing radiation exposure during pregnancy. Am J Obstet Gynecol. 2012;206(6):456–62. doi: 10.1016/j.ajog.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–85. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Reinmuth S, Hohmann C, Rendtorff R, Balcerek M, Holzhausen S, Muller A, et al. Impact of chemotherapy and radiotherapy in childhood on fertility in adulthood: the FeCt-survey of childhood cancer survivors in Germany. J Cancer Res Clin Oncol. 2013;139(12):2071–8. doi: 10.1007/s00432-013-1527-9. [DOI] [PubMed] [Google Scholar]
- 4.Lunsford AJ, Whelan K, McCormick K, McLaren JF. Antimullerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril. 2014;101(1):227–31. doi: 10.1016/j.fertnstert.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 5.Mahran YF, El-Demerdash E, Nada AS, et al. Insights into the protective mechanisms of tamoxifen in radiotherapy-induced ovarian follicular loss: impact on insulin-like growth factor 1. Endocrinology. 2013;154(10):3888–99. doi: 10.1210/en.2013-1214. [DOI] [PubMed] [Google Scholar]
- 6.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am J Epidemiol. 1999;150(3):245–54. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 7.Chiarelli AM, Marrett LD, Darlington GA. Pregnancy outcomes in females after treatment for childhood cancer. Epidemiology. 2000;11(2):161–6. doi: 10.1097/00001648-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB, et al. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187(4):1070–80. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 9.Ronckers CM, Land CE, Hayes RB, Verduijn PG, Stovall M, van Leeuwen FE. Late health effects of childhood nasopharyngeal radium irradiation: nonmelanoma skin cancers, benign tumors, and hormonal disorders. Pediatr Res. 2002;52(6):850–8. doi: 10.1203/00006450-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91(5):1723–8. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 11.Signorello LB, Cohen SS, Bosetti C, Stovall M, Kasper CE, Weathers RE, et al. Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98(20):1453–61. doi: 10.1093/jnci/djj394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winther JF, Boice JJ, Svendsen AL, Frederiksen K, Stovall M, Olsen JH. Spontaneous abortion in a Danish population-based cohort of childhood cancer survivors. J Clin Oncol. 2008;26(26):4340–6. doi: 10.1200/JCO.2007.15.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Signorello LB, Mulvihill JJ, Green DM, Munro HM, Stovall M, Weathers RE, et al. Stillbirth and neonatal death in relation to radiation exposure before conception: a retrospective cohort study. Lancet. 2010;376(9741):624–30. doi: 10.1016/S0140-6736(10)60752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DM, Nolan VG, Kawashima T, Stovall M, Donaldson SS, Srivastava D, et al. Decreased fertility among female childhood cancer survivors who received 22–27 Gy hypothalamic/pituitary irradiation: a report from the Childhood Cancer Survivor Study. Fertil Steril. 2011;95(6):1922–7. doi: 10.1016/j.fertnstert.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Signorello LB, Mulvihill JJ, Green DM, Munro HM, Stovall M, Weathers RE, et al. Congenital anomalies in the children of cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol. 2012;30(3):239–45. doi: 10.1200/JCO.2011.37.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas-Teinturier C, Fayech C, El Oberlin O, De Vathaire F. Acute ovarian failure in survivors of childhood cancer: major influence of surgery and radiotherapy. Horm Res Paediatr. 2012;78:201. [Google Scholar]
- 17.van Dorp W, van den Heuvel-Eibrink MM, Stolk L, Pieters R, Uitterlinden AG, Visser JA, et al. Genetic variation may modify ovarian reserve in female childhood cancer survivors. Hum Reprod. 2013;28(4):1069–76. doi: 10.1093/humrep/des472. [DOI] [PubMed] [Google Scholar]
- 18.Barton SE, Najita JS, Ginsburg ES, Leisenring WM, Stovall M, Weathers RE, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14(9):873–81. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas-Teinturier C, Fayech C, El Oberlin O, Pacquement H, Haddy N, Labbe M, et al. Age at menopause and its influencing factors in a cohort of survivors of childhood cancer: earlier but rarely premature. Hum Reprod. 2013;28(2):488–95. doi: 10.1093/humrep/des391. [DOI] [PubMed] [Google Scholar]
- 20.Edelmann MN, Krull KR, Liu W, Glass JO, Ji Q, Ogg RJ, et al. Diffusion tensor imaging and neurocognition in survivors of childhood acute lymphoblastic leukaemia. Brain. 2014;137(Pt 11):2973–83. [DOI] [PMC free article] [PubMed]
- 21.Ford JS, Kawashima T, Whitton J, Leisenring W, Laverdiere C, Stovall M, et al. Psychosexual functioning among adult female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(28):3126–36. [DOI] [PMC free article] [PubMed]
- 22.Gnaneswaran S, Deans R, Cohn RJ. Reproductive late effects in female survivors of childhood cancer. Obstet Gynecol Int. 2012;2012:564794. doi: 10.1155/2012/564794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing ZW, Jiang EH, Du JY, et al. Long-term follow-up of the genital organs and eye lenses in three cases of acute radiation sickness from a 60Co radiation accident in China. Health Phys. 2015;108(1):1–7. doi: 10.1097/HP.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 24.Styne D, Grumbach M. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: H. Kronenberg, editor, Williams textbook of endocrinology. 2008. pp. 969–1166.
- 25.Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clin Oncol (R Coll Radiol) 1989;1(2):75–9. doi: 10.1016/s0936-6555(89)80039-1. [DOI] [PubMed] [Google Scholar]
- 26.Viswanathan AN. Childhood cancer survivors: stillbirth and neonatal death. Lancet. 2010;376(9741):570–2. doi: 10.1016/S0140-6736(10)61263-9. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura N, Suyama A, Noda A, Kodama Y. Radiation effects on human heredity. Annu Rev Genet. 2013;47:33–50. doi: 10.1146/annurev-genet-111212-133501. [DOI] [PubMed] [Google Scholar]