Abstract
Purpose
The objective of this study is to characterize the impact of exposure to cryoprotectants followed by vitrification on primordial follicle survival and activation using a fetal bovine model.
Methods
In the first study, fetal bovine cortical pieces were exposed to cryoprotectants with or without sucrose and cultured up to 7 days in the presence or absence of insulin. In the second study, cortical pieces were exposed to cryoprotectants with or without sucrose, vitrified, and cultured up to 7 days after warming in the presence or absence of insulin. Viability and morphology of follicles, as well as proliferation and/or DNA repair in ovarian tissue were analyzed.
Results
When compared to non-exposed controls, normal follicular morphology was affected in groups exposed to cryoprotectants only immediately post-exposure and after 1 day of culture, but improved by day 3 and did not significantly differ by day 7. Similarly, normal follicular morphology was compromised in vitrified groups after warming and on day 1 compared to controls, but improved by days 3 and 7. Proliferation and/or DNA repair in ovarian tissue was not affected by vitrification in this model. Cryoprotectant exposure and vitrification of ovarian tissue did not impair the activation of primordial follicles in response to insulin, although activation was delayed relative to non-exposed controls. Interestingly, sucrose had no noticeable protective effect.
Conclusion
Vitrified fetal bovine ovarian tissue has the intrinsic capacity to mitigate the immediate damage to primordial follicles’ morphology and retains the capacity to activate. These findings provide a basis for a successful cryopreservation protocol for ovarian cortical tissue in other species including humans.
Keywords: Ovarian tissue, Vitrification, Primordial follicle activation, Gamete rescue, Cryopreservation
Introduction
Cryopreservation of gonadal tissues allows the safeguarding and restoration of female fertility in multiple contexts. This technique is used predominantly in women to obviate ovarian failure due to cancer therapy [1]. Ovarian tissue cryopreservation also offers an attractive alternative to human patients who cannot delay their treatment to allow oocytes to be harvested following hormonal stimulation [2] and to pre-pubertal girls who are unsuitable candidates for hormonal therapies [3]. In addition, freezing gonadal tissues is used to preserve the genetic potential of biomedical models, livestock species, companion animals, and endangered wildlife species [4], including gonadal rescue of after premature death [5].
Notably, primordial follicles are present in ovaries until reproductive senescence, regardless of reproductive maturity or seasonality. They remain dormant until they receive signals for primordial follicle activation, when they resume folliculogenesis and progress to the primary stage. Interestingly, primordial follicles are more resistant to cryoinjury due to their small size and cytoplasmic volume, and their lack of a zona pellucida [6]. Therefore, the goal of ovarian tissue cryopreservation is to maintain a viable pool of primordial follicles that can resume normal folliculogenesis after thawing. Importantly, an optimal freezing protocol should neither impede primordial follicle activation nor stimulate it, as this would lead to premature follicle depletion.
For this purpose, ovarian tissue has already been successfully preserved using conventional slow-freezing protocols [2], resulting in the birth of children following autotransplantation of thawed tissue [7]. However, extra- and intracellular ice crystallization can occur, leading to tissue damage by alteration of the chemical environment, mechanical constraints, and shearing of the cells [8]. This can be avoided by using vitrification, or ultra-rapid freezing, a technique that relies on exposure to very high concentrations of cryoprotectants (CPAs) followed by immersion of the sample directly into the liquid nitrogen to form a glass-like solid [8, 9]. However, vitrified tissues remain susceptible to deleterious effects of high concentrations of CPAs, which can include cell toxicity and osmotic shock [10], as well as partial cryoinjury. It is critical to (1) better characterize the damage caused by the exposure to high concentrations of CPA and by the ultra-fast cooling and warming and (2) mitigate the detrimental effects. Specifically, the addition of a non-permeable sugar, such as sucrose, to the vitrification solution can mitigate osmotic shock, protect membranes, and improve the glass-forming capabilities of permeable CPAs (e.g., ethylene glycol, dimethylsulfoxide, glycerol) [11]. Furthermore, the assessment of damage caused by vitrification should be investigated over time after warming, either in a culture system [12, 13] or following tissue grafting [14], rather than immediately after warming as the effect of CPA exposure and vitrification on cell metabolism and function may not be detectable yet and may be mitigated over time.
The objectives of this study were to investigate the potential benefits of adding sucrose to the vitrification solution to mitigate damage to early pre-antral follicles in the ovarian cortex and to examine the effects of CPA exposure and vitrification on primordial follicle activation. Besides the positive effect of the sucrose addition, we also hypothesized that cellular damage could be repaired in an appropriate culture system. Experiments were performed with ovarian cortical pieces from fetal calves in the early third trimester of pregnancy. In cattle, follicle formation begins at the end of the first trimester; primary (activated) follicles appear during the second trimester and secondary follicles during the third trimester [15]. Thus, the cortex of fetal bovine ovaries has a large population of primordial follicles and provides an ideal model of follicle activation.
Materials and methods
All reagents were purchased from Sigma-Aldrich unless otherwise specified.
Collection of bovine fetal ovarian cortical tissue
Bovine female fetuses were obtained during the early third trimester (182 to 199 days of gestation, as estimated by crown-rump length [16]) at a local abattoir (Cargill Regional Beef, Wyalusing, PA). Ovaries were collected and transported back to the laboratory within 4 h in Leibovitz L-15 medium (Invitrogen) supplemented with 1 % fetal bovine serum (FBS; Gibco), 50 IU/ml penicillin and 50 μg/ml streptomycin sulfate (Gibco) at ambient temperature, as previously described [17]. The ovarian cortex, the outer part of the ovary where primordial follicles reside, was separated from the inner medulla and dissected into 1 × 1.5 × 0.5 mm pieces.
Ovarian cortical cultures
Cortical pieces were placed on uncoated culture well inserts (two pieces per well, two wells per time point per treatment; Millicell-CM, 0.4-μm pore size; Millipore) in the wells of 24-well Costar culture plates (Corning) with 300 μl culture medium consisting of Waymouth’s medium MB 752/1 (Invitrogen) supplemented with 25 mg/l pyruvic acid, antibiotics (50 IU/ml penicillin, 50 μg/ml streptomycin sulfate), 6.25 μg/ml transferrin (BD Biosciences), 6.25 ng/ml selenous acid, 1.25 mg/ml BSA, and 5.35 μg/ml linoleic acid, in absence (control medium) or presence of 6.25 μg/ml insulin (for this medium, the last five ingredients were added in the form of ITS+ Premix, BD Biosciences). Previous experiments showed that the control medium maintains bovine fetal ovarian cortex in a healthy condition, without promoting activation of primordial follicles, whereas the addition of insulin activates a subset of primordial follicles [15].
Cortical pieces were cultured at 38.5 °C in a humidified incubator gassed with 5 % CO2 and 95 % air for 1, 3, or 7 days. Two thirds of the volume of culture medium was exchanged for fresh medium every 2 days throughout the study interval. Following culture, one piece from each well per treatment group was fixed for histological analysis, and the other piece was used to assay viability, for a total of two replicates per fetus.
Assessment of follicle morphology
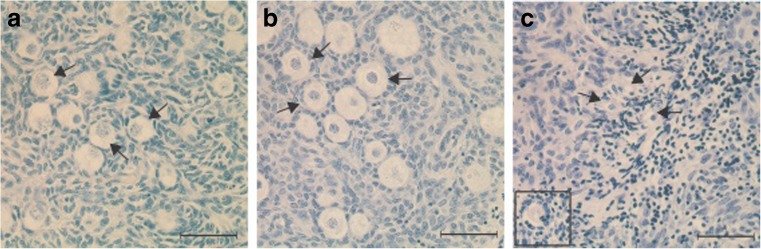
Cortical pieces were fixed in Bouin’s solution, dehydrated, embedded in paraffin, and sectioned at a thickness of 5 μm. A minimum of five non-consecutive sections were taken from the middle of each piece at 15 μm intervals to avoid double counting of follicles and were stained with hematoxylin-eosin for morphological analysis. Follicles were classified as primordial (an oocyte surrounded by one layer of flattened pregranulosa cells; Fig. 1a) or activated (an oocyte surrounded by one or more layers of cuboidal granulosa cells; Fig. 1b), as previously described [18]. Follicles were further classified as morphologically “normal” (nuclei of oocyte and surrounding granulosa cells were structurally intact; Fig. 1a, b) or “abnormal” (the oocyte and/or granulosa cells contained a pyknotic, fragmented or shrunken nucleus, or cytoplasmic vacuoles; Fig. 1c). Only follicles containing oocytes with a visible nucleus were included to avoid double counting. The number of normal or activated follicles was divided by the total number of follicles evaluated for each section to determine the proportions of normal and activated follicles per treatment.
Fig. 1.
Sections of bovine fetal ovarian cortex (182–199 days of gestation) stained with hematoxylin and eosin. a Normal primordial follicles (arrows). b Normal primary follicles (arrows). c Abnormal follicles (arrows and insert). Scale bar = 50 μm
Assessment of follicle viability
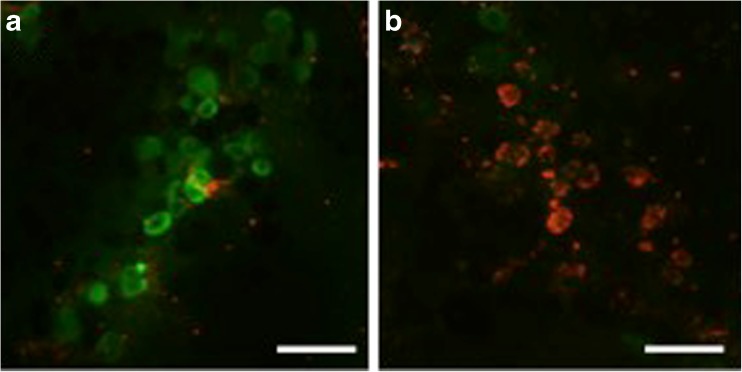
Follicle viability was determined using a cytoviability assay (LiveDead Viability/Cytotoxicity kit, Life Technologies). Cortical pieces were incubated in PBS with 2 μl/ml calcein AM and 5 μl/ml ethidium homodimer for 20–30 min at 38.5 °C, gently teased using 22-G needles, and visualized under fluorescence at 518 and 593 nm. Follicles were considered viable when the oocyte and surrounding granulosa cells fluoresced green and were considered dead when the oocyte and/or at least 50 % of the surrounding granulosa cells fluoresced red. The number of viable follicles was divided by the total number of follicles evaluated for each cortical piece to determine the proportion of live follicles per treatment.
Proliferation/DNA repair assay
Another five non-consecutive sections per cortical piece were used for the detection of proliferating cell nuclear antigen (PCNA), which enables DNA polymerase ε to bind to DNA during DNA synthesis and can therefore be used as a marker for proliferation and DNA repair [19]. A PCNA immunohistochemistry kit (PCNA Staining Kit, Life Technologies) was used following the manufacturer’s instructions. Briefly, the sections were dewaxed and rehydrated; endogenous peroxidase activity was blocked with 3 % hydrogen peroxide and non-specific staining was blocked with serum. The sections were incubated with the primary antibody for 2 h at room temperature in a humid container and then with a horseradish peroxidase-conjugated secondary antibody, which was visualized with 3,3′-diaminobenzidine. Sections were counterstained with hematoxylin. For the negative controls, the primary antibody was omitted. The sections were observed by light microscopy. Follicles were classified as follows: negative, no nuclear staining in the granulosa cells; positive, nuclear staining in at least one granulosa cell. Stromal cells were considered PCNA positive when nuclear staining was present. The number of PCNA-positive follicles was divided by the total number evaluated for each section to determine the proportions per treatment. The proportions of PCNA-positive stromal cells were estimated in each histological section by dividing the number of PCNA-positive cells by the total number of stromal cells.
Experimental design
Study 1 examined the effects of a stepwise increase in CPA concentration, as well as the presence or absence of sucrose in the vitrification solution, on the health and function of early pre-antral follicles. The composition of each solution is described in Table 1. For each fetus (n = 3), the dissected cortical pieces were randomly assigned to one of four treatment groups, with 28 cortical pieces per group: (1) no exposure to cryoprotectants (control); (2) exposure to equilibration solution alone (ES) for 10 min on ice, followed by an ES wash gradient; (3) exposure to ES for 10 min on ice, followed by exposure to vitrification solution (VS) for 2 min on ice, then followed by a VS wash gradient; (4) exposure to ES for 10 min on ice, followed by exposure to vitrification solution + 0.5 M sucrose (VS+Suc) for 2 min on ice, then followed by a VS+Suc wash gradient (Fig. 2a).
Table 1.
Composition of equilibration solution (ES), vitrification solution (VS), and vitrification solution with sucrose (VS+Suc) and their corresponding wash gradients
| Solution | |||
|---|---|---|---|
| ES | VS | VS+Suc | |
| Composition | 7.5 % EG | 15 % EG | 15 % EG |
| 7.5 % DMSO | 15 % DMSO | 15 % DMSO | |
| 20 % FBS | 20 % FBS | 20 % FBS | |
| 0.5 M sucrose | |||
| Wash gradient | 50 % ES 5 min | 50 % VS 5 min | 50 % VS+Suc 5 min |
| 25 % ES 5 min | 25 % VS 5 min | 25 % VS+Suc 5 min | |
| 0 % ES 5 min × 3 | 0 % VS 5 min × 3 | 0 % VS+Suc 5 min × 3 | |
All solutions are shown as v/v% in PBS
EG ethylene glycol (Gibco), DMSO dimethyl sulfoxide (Gibco), FBS fetal bovine serum
Fig. 2.
Experimental design for a Study 1—cryoprotectant toxicity and b Study 2—cryoprotectant efficacy. ES equilibration solution, VS vitrification solution, VT vitrification
Following exposure to cryoprotectants, two cortical pieces from each treatment group were fixed immediately for histological analysis, and two pieces were assayed for viability (day 0). The remaining 24 pieces per treatment group were cultured for 1, 3, or 7 days; half of the pieces in each treatment group were cultured in medium supplemented with insulin to stimulate primordial follicle activation and the other half in medium without insulin as a control.
In study 2, vitrification by needle immersion of fetal bovine ovarian tissue in VS with or without sucrose was performed to compare the efficacy of the permeable and non-permeable CPAs to prevent cryoinjury and osmotic shock. The vitrification protocol was adapted from Wang et al. [14]. For each fetus (n = 4), the dissected cortical pieces were threaded on to 30-G needles (up to five pieces per needle) and were randomly assigned to one of three treatment groups, with 28 cortical pieces per group: (1) no exposure to cryoprotectant or vitrification (control), (2) exposure to ES and VS as described above (VT), (3) exposure to ES and VS+Suc as described above (VT+Suc). Following the exposure to VS, excess solution was absorbed with a Kimwipe, and the needle was plunged into liquid nitrogen. Samples were transferred to a pre-cooled cryovial containing liquid nitrogen and were stored in liquid nitrogen for 24 h (Fig. 2b).
Warming was performed by removing the needles from the cryovials in liquid nitrogen and transferring them quickly to the appropriate wash gradient at 37 °C. The cortical pieces were cultured and analyzed as described above.
Statistical analysis
Data are presented as means ± standard error mean (SEM). The experimental designs were replicated with cortical pieces from 3 (study 1) or 4 (study 2) fetuses, obtained on different days. Treatments were applied in duplicate within each fetus. The proportions of follicles in each treatment group were assessed by an ANOVA using a mixed random effect model (with treatment and fetus as two fixed effects, and cortical pieces as a random effect), followed by a Tukey’s all-comparison test. Differences were considered significant at P < 0.05 (JMP ver. 10.0, SAS Institute Inc.).Line art was created in GraphPad Prism 6 (GraphPad Software Inc.); halftone art was created in Inkscape (version 0.91; www.inkscape.org).
Results
Influence of exposure to cryoprotectants with or without sucrose on the morphology, viability, and activation of primordial follicles
Immediately following exposure to CPAs and washing on day 0, the percentage of morphologically normal follicles was significantly lower in all treatment groups compared to non-exposed tissues (P < 0.05; Table 2). The decrease was still significant after 1 and 3 days of culture (P < 0.05; Table 2); however, the proportions of morphologically normal follicles increased within each treatment group on day 3 compared to day 1 (P < 0.05; Table 2). On day 7, the percentages of morphologically normal follicles were not significantly different between ES+VS, ES+VS+Suc and the control group (P ≥ 0.05; Table 2). The percentage of normal follicles was slightly lower in ES compared to controls (P < 0.05; Table 2), but not significantly different from ES+VS or ES+VS+Suc (P ≥ 0.05). Normal follicle morphology did not vary among time points for the non-exposed controls (P ≥ 0.05; Table 2).
Table 2.
Effects of exposure to equilibration (ES) and vitrification (VS) solutions, with or without sucrose (suc), on follicle morphology and viability in fetal bovine ovarian tissue after 0, 1, 3, and 7 days of culture
| Morphology treatment | Normal follicles (%) | |||
| Day 0 | Day 1 | Day 3 | Day 7 | |
| Control | 94.7 ± 0.7A,a | 94.2 ± 0.9A,a | 91.6 ± 1.4A,a | 90.8 ± 2.1A,a |
| ES | 47.2 ± 9.1A,b | 48.6 ± 8.6A,b | 67.4 ± 3.5B,b | 78.2 ± 4.1B,b |
| ES+VS | 40.0 ± 5.6A,b | 47.8 ± 7.5A,b | 75.9 ± 2.7B,b | 82.2 ± 3.1B,a,b |
| ES+VS+Suc | 51.7 ± 5.2A,B,b | 51.9 ± 8.3A,b | 71.4 ± 3.7B,C,b | 85.6 ± 3.0C,a,b |
| Viability treatment | Live follicles (%) | |||
| Day 0 | Day 1 | Day 3 | Day 7 | |
| Control | 96.0 ± 0.7A,a | 92.9 ± 1.5A,a | 85.8 ± 1.2B,a | 81.5 ± 2.2B,a |
| ES | 97.1 ± 2.9A,a | 93.2 ± 0.7A,B,a | 87.8 ± 1.2B,C,a | 85.5 ± 1.4C,a |
| ES+VS | 95.1 ± 0.7A,a | 92.8 ± 1.3A,a | 89.1 ± 1.9A,B,a | 80.0 ± 3.3B,a |
| ES+VS+Suc | 94.1 ± 0.9A,a | 92.5 ± 1.7A,a | 88.6 ± 1.4A,a | 87.0 ± 1.9A,a |
Data are means ± SEM (n = 6 cultures, 2 from each of 3 fetal ovaries). A total of 16,393 follicles were observed for morphology (range, 576 to 1611 per treatment group per day); 10,029 follicles were observed for viability (range, 174 to 1113 per treatment group per day)
A,B,CMeans with no common letters within treatment groups across time points significantly differ (P < 0.05)
a,bMeans with no common letters among treatment groups within a time point significantly differ (P < 0.05)
Interestingly, the percentage of viable follicles as determined by calcein AM and ethidium homodimer staining (Fig. 3) did not vary between treatment groups at any time point (P ≥ 0.05; Table 2). While viability started to decrease by Day 3 in the non-exposed and ES treatment groups compared to earlier time points (P < 0.05; Table 2), it did not decline until Day 7 in the ES+VS treatment group (P < 0.05; Table 2) and did not vary among time points in the ES+VS+Suc treatment group (P ≥ 0.05; Table 2).
Fig. 3.
Sections of bovine fetal ovarian cortex (182–199 days of gestation) assessed for viability. a Viable follicles appear green (Calcein AM). b Dead follicles stain red (ethidium homodimer). Scale bar = 50 μm
In the absence of insulin in the culture medium, the proportion of activated follicles did not vary among treatment groups at any time during culture (P ≥ 0.05; Fig. 4), although the ES+VS group had a lower percentage of activated follicles than controls on day 0 (P < 0.05; Fig. 4). In the presence of insulin, the proportion of activated follicles was higher starting on day 3 and through day 7 in all exposed treatment groups compared to day 0 and day 1 (P < 0.05; Fig. 4). The percentages of activated follicles did not differ among groups cultured with insulin regardless of presence or absence of sucrose in the vitrification solution (P ≥ 0.05; Fig. 4), except on day 1 when the non-exposed control group had a higher percentage (P < 0.05). Only the non-exposed control group cultured with insulin had an increased percentage of activated follicles at day 1 compared to day 0 (P < 0.05). By day 3, all treatment groups cultured with insulin had significantly higher percentages of activated follicles compared to those cultured without insulin regardless of exposure (P < 0.05; Fig. 4).
Fig. 4.
Effects of exposure to equilibration (ES) and vitrification (VS) solutions, with or without sucrose (suc), on primordial follicle activation in fetal bovine ovarian tissue after 0, 1, 3, and 7 days of culture in the presence or absence of insulin. Data are means ± SEM (n = 6 cultures, 2 from each of 3 fetal ovaries). Means without common letters among treatment groups within a time point significantly differ (P < 0.05)
The mean total number of follicles per section did not vary between treatment groups for all biometrics (P ≥ 0.05; data not shown).
Influence of vitrification with or without sucrose on the morphology, viability, proliferation/DNA repair, and activation of primordial follicles
Immediately following warming on day 0, the percentage of morphologically normal follicles was significantly lower (P < 0.05; Table 3) in both treatment groups compared to fresh tissue. After 1 day of culture, only the VT group was significantly different from the control (P < 0.05; Table 3). After 3 and 7 days of culture, there were no significant differences in the proportions of morphologically normal follicles between the treatment groups and control, regardless of presence or absence of sucrose in the vitrification solution (P ≥ 0.05; Table 3). There were no significant differences in the percentages of morphologically normal follicles among time points within each treatment group (P ≥ 0.05; Table 3).
Table 3.
Effects of vitrification (VT), with or without sucrose (suc), on follicle morphology and viability in fetal bovine ovarian tissue after 0, 1, 3, and 7 days of culture
| Morphology treatment | Normal follicles (%) | |||
| Day 0 | Day 1 | Day 3 | Day 7 | |
| Control | 95.3 ± 1.1A,a | 93.0 ± 2.1A,a | 91.7 ± 2.8A,a | 88.4 ± 3.9A,a |
| VT | 79.0 ± 3.4A,b | 76.8 ± 8.1A,b | 84.3 ± 4.8A,a | 84.9 ± 4.7A,a |
| VT+Suc | 74.2 ± 5.5A,b | 81.8 ± 6.4A,a,b | 90.9 ± 2.3A,a | 85.2 ± 6.5A,a |
| Viability treatment | Live follicles (%) | |||
| Day 0 | Day 1 | Day 3 | Day 7 | |
| Control | 93.9 ± 1.3A,a | 94.0 ± 0.9A,a | 89.6 ± 1.8A,a | 64.8 ± 4.9B,a |
| VT | 90.4 ± 1.9A,a | 89.5 ± 1.2A,b | 80.9 ± 4.1A,a,b | 56.5 ± 6.5B,a |
| VT+Suc | 92.0 ± 1.3A,a | 87.1 ± 1.5A,B,b | 78.4 ± 2.8B,b | 56.1 ± 4.9C,a |
Data are means ± SEM (n = 8 cultures, 2 from each of 4 fetal ovaries). A total of 20,804 follicles were observed for morphology (range, 954 to 1611 follicles per treatment group per day); 10,389 follicles were observed for viability (range, 459 to 1351 follicles per treatment group per day)
A,B,CMeans with no common letters within treatment groups across time points significantly differ (P < 0.05)
a,bMeans with no common letters among treatment groups within a time point significantly differ (P < 0.05)
A slightly lower proportion of viable follicles was observed on day 1 for both vitrification groups and on day 3 for the VT+Suc group compared to non-vitrified controls (P < 0.05; Table 3). On day 7, there was no significant difference in viability among treatment groups (P ≥ 0.05; Table 3). For both the non-vitrified controls and the VT group, the percentage of viable follicles was significantly lower at day 7 compared to all other days, whereas it was lower by day 3 for VT+suc (P < 0.05; Table 3).
The effect of vitrification on primordial follicle activation was similar to that of cryoprotectant exposure alone. In the absence of insulin, the proportion of activated follicles did not vary either among treatment groups or among time points within a treatment group (P ≥ 0.05; Fig. 5). Starting on day 3, all treatment groups cultured with insulin had significantly higher percentages of activated follicles compared to those cultured without insulin, regardless of vitrification or presence of sucrose (P < 0.05; Fig. 5). Percent of activated follicles was greater in the non-vitrified control group compared to the vitrified treatment groups on day 1 (P < 0.05; Fig. 5), but was not significantly different on days 3 and 7 (P ≥ 0.05; Fig. 5). The non-vitrified control group cultured with insulin was the only one to have an increased percentage of activated follicles at day 1 compared to day 0 (P < 0.05).
Fig. 5.
Effects of vitrification (VT), with or without sucrose, on primordial follicle activation in fetal bovine ovarian tissue after 0, 1, 3, and 7 days of culture in the presence or absence of insulin. Data are means ± SEM (n = 8 cultures, 2 from each of 4 fetal ovaries). Means without common letters among treatment groups within a time point significantly differ (P < 0.05)
PCNA staining was localized to the granulosa cell nuclei, the oocyte nuclei, and the stromal nuclei in all treatment groups (Fig. 6). Similar proportions of PCNA-positive stromal cells (ranging from 15 to 20 % of the total stromal cell population) were found among treatment groups within each time points (Fig. 6). The proportion of PCNA-positive follicles did not vary among treatment groups after warming (day 0) or on day 1 regardless of presence or absence of insulin in the culture medium (P ≥ 0.05; Fig. 7). On day 3, the vitrified treatment groups had a significantly higher percentage of PCNA-positive follicles when cultured with insulin compared to cortical pieces cultured without insulin (P < 0.05; Fig. 7). While the proportion of PCNA-positive follicles in the non-vitrified control group cultured with insulin was not significantly different from the VT+Suc group cultured with insulin or all three treatment groups cultured without insulin (P ≥ 0.05; Fig. 7), it was significantly lower than the VT group cultured with insulin (P < 0.05; Fig. 7). On day 7, all treatment groups cultured with insulin had a significantly higher percentage of PCNA-positive follicles compared to treatment groups cultured without insulin (P < 0.05; Fig. 7); the VT and VT+Suc treatment groups were not significantly different from the non-vitrified control (P ≥ 0.05; Fig. 7).
Fig. 6.
PCNA staining of cortical pieces from non-vitrified controls (a–c) and VT+Suc group (d–f), before culture (day 0 (a, d)) and after 7 days of culture in the absence (b, e) and presence of insulin (c, f). Black arrows show PCNA-positive granulosa cells, and black arrow heads show PCNA-positive stromal cells. Scale bar = 50 μm
Fig. 7.
Effects of vitrification (VT), with or without sucrose, on percentage of follicles with PCNA-positive granulosa cells in fetal bovine ovarian tissue after 0, 1, 3, and 7 days of culture in the presence or absence of insulin. Data are means ± SEM (n = 8 cultures, 2 from each of 4 fetal ovaries). Means without common letters among treatment groups within a time point significantly differ (P < 0.05)
The mean total number of follicles per section did not vary among treatment groups for all biometrics (P ≥ 0.05; data not shown).
Discussion
In this work, fetal bovine ovarian tissue was successfully cryopreserved using needle immersed vitrification, and while exposure to CPAs caused morphological damage to the follicles, the damage was mitigated by maintaining the tissue in an appropriate culture system for 7 days. The capacity of primordial follicles to activate was not altered either by exposure to CPAs or by vitrification. However, we found no evidence to suggest that the addition of sucrose to the vitrification solution improved the vitrification of early follicle stages in fetal bovine ovarian tissue with respect to CPA toxicity or cryoinjury.
Conventional freezing of ovarian tissue can result in extra- and intracellular ice formation, which causes shearing damage to the cell structures and organelles. Vitrification bypasses the danger of ice crystal formation by forming a glassy solid, due to the extreme viscosity of the sample [9]. This high rate of viscosity can be achieved by ultra-fast cooling and increasing the concentration of cryoprotectants. However, high concentrations of CPA have cytotoxic effects on tissue [20], although adding low concentrations of a combination of cryoprotectants has been effective at reducing the total toxicity of cryoprotectant exposure [21]. In this study, exposure to CPAs did not affect the morphology of early pre-antral follicles in a dose-dependent manner, as there were no differences in the percentage of morphologically normal follicles at any time point among the exposed treatment groups. Exposure to the equilibration solution, which had half the CPA concentration of the vitrification solution, caused as much morphological damage immediately after washing and 1 day of culture as exposure to the vitrification solution, with and without sucrose. For all three exposed treatment groups, normal follicular morphology began to recover by day 3 of culture and was similar to the unexposed controls by day 7.
As each research group uses ovarian tissue from different species, and varying types and concentrations of cryoprotectants, it is difficult to compare results among studies. Furthermore, the few which have investigated the effects of cryoprotectant exposure alone on follicular health, without subsequent vitrification, have looked at the follicular health and morphology either immediately after exposure to CPAs [22] or after a short incubation period (15 min [23]; 2 h [24, 25]). Because cryopreservation acts by suspending cellular metabolism, the tissue must be cultured long enough for the effects of CPA exposure on cell metabolism and function to be evident. By measuring the biometrics of ovarian tissue exposed to CPAs or vitrification after a few days of culture, rather than immediately after washing, our study provides novel evidence on the tissue’s capability to withstand and mitigate damage induced by the CPAs and ultra-rapid cooling. This highlights the importance of allowing adequate recovery of the ovarian tissue in a culture system when optimizing vitrification protocols.
Successful cryopreservation of fetal bovine ovarian tissue was achieved using needle immersed vitrification. This novel technique, first developed by Wang et al. in 2008 [14] and successfully used for cryopreservation of ovarian tissue from many species (mouse [26]; human [27–29]; baboon [30]; Japanese quail [31]), increases the cooling rate within the tissue [30]. In the current study, the morphology of early pre-antral follicles in vitrified ovarian tissue was not significantly different from the non-vitrified controls after 3 days of culture, regardless of the presence or absence of sucrose in the vitrification solution. The viability of early pre-antral follicles did not decrease until after 3 days of culture in ovarian tissue exposed to CPAs and was never significantly different from non-exposed controls. In contrast, viability was lower in vitrified ovarian tissue cultured for 1 day compared to non-vitrified controls, which indicates that ultra-rapid cooling, rather than exposure to CPAs, affects the viability of follicles in fetal bovine ovarian tissue. This suggests that early pre-antral follicles in fetal bovine ovarian tissue are susceptible to morphological damage from exposure to CPA and vitrification, but are able to mitigate this damage when cultured in an appropriate environment for at least 3 days. Follicular morphology of ovarian tissue immediately after warming was better compared to the CPA exposure trial, which may due to the fact that using an insulin needle as a carrier improved ease of handling and allowed for quicker transfer of the ovarian pieces from one solution to the next and decreased trauma compared to forceps. Though adult bovine ovarian tissue has been used to study CPA exposure and vitrification, this is the first study to use fetal bovine ovarian tissue. Successful vitrification of adult bovine ovarian tissue was first performed by Kagawa et al. [32] using the Cryotop method, followed by autotransplantation. The duration of exposure to CPAs was then found to have an effect on adult bovine tissue, with shorter exposure times resulting in decreased toxicity, but longer exposure times improving vitrification of the tissue [33].
The present study was the first to objectively investigate the effect of CPA exposure and vitrification on primordial follicle activation. Primordial follicles comprise a finite pool and represent the reproductive potential of a female. If ovarian tissue cryopreservation and grafting are to be a viable solution for gamete rescue, it is necessary for the follicle reserve to remain dormant and activate only at progressive intervals. If vitrification causes spontaneous and ubiquitous activation of primordial follicles, this will deplete the follicle reserve at a faster rate than desirable. It is therefore necessary to determine whether vitrification has an effect on the endogenous pathways of primordial follicle activation. Due to the wide variety of protocols for ovarian tissue vitrification, conflicting results have been reported on the efficacy of vitrification for the cryopreservation of both dormant and growing follicles [34]. It is also important to recognize that many studies report the effects of vitrification shortly, if not immediately, after thawing the cryopreserved tissue. While there is overwhelming evidence that grafting ovarian tissue following warming leads to activation of the primordial follicle reserve due to ischemia-reperfusion [30, 35, 36], there is contradictory evidence as to whether cryoprotectant exposure and/or vitrification of ovarian tissue induces activation of primordial follicles. One study found that the type of cryoprotectant used affects the results, with propanediol increasing the activation rate following grafting more than with DMSO [37]. Oktem et al. [38] have found decreased concentrations of anti-Mϋllerian hormone, known to maintain primordial follicles quiescent, in vitrified ovaries compared to fresh controls following a 3-day culture, as well as a lower primordial follicle count. Others have found no differences in the proportions of primordial follicles in vitrified and control ovarian tissue cultured for 5 days in two different media [12]. While some studies have suggested that the method of cryopreservation affects the primordial follicle reserve, results are inconsistent. Huang et al. [39] found no differences in primordial follicle proportions between slow-freezing and solid surface vitrification protocols, while Amorim et al. [40] found higher proportions of primordial follicles in vitrified groups compared to slow-freezing groups after xenografting.
Primordial follicles first appear in the fetal bovine ovary around 90 days of gestation, but only acquire the capacity to activate around 140 days of gestation, when they have achieved meiotic arrest [18], as demonstrated by a decrease in the proportion of primordial follicles and an increase in the proportion of primary follicles in ovarian tissue cultured in the presence of insulin [15]. In this study, primordial follicles within fetal bovine tissue exposed to EG and DMSO as permeable CPAs and sucrose as a non-permeable CPA retained the capacity to activate in the presence of insulin; correspondingly, primordial follicles cultured without insulin remained dormant. Similar results were found for vitrified tissue. Primordial follicles within ovarian tissue cultured in the presence of insulin were able to activate to a similar extent as fresh tissue, though activation may have been delayed as it was first noticeable at 3 days of culture for exposed and vitrified ovarian tissue compared to 1 day for non-exposed tissue. This demonstrates that vitrification neither impedes nor causes spontaneous activation of primordial follicles, and vitrified ovarian tissue can be used as an adequate source of primordial follicles that can resume normal folliculogenesis. This also provides evidence that the loss of primordial follicles and increase in growing follicles found in warmed and xenografted tissue is more likely caused by ischemia-reperfusion rather than by vitrification of the tissue.
Vitrification of fetal bovine ovarian tissue did not affect proliferation and DNA repair as detected by PCNA immunohistochemistry. Similar proportions of follicles with PCNA-positive granulosa cells were found in the fresh controls and vitrified groups throughout the study, which would suggest that exposure to CPAs and vitrification does not impede the proliferation of granulosa cells and stromal cells. In addition to being a marker for proliferation and DNA repair [19], PCNA can be used as an indicator of primordial follicle activation. While PCNA is absent from quiescent primordial follicles, it is expressed in the proliferating granulosa cells of activated primary follicles in rats [41], cows [17], and baboons [42]. It can also be found in the oocytes of primary follicles [17, 43], as PCNA is a binding platform for replicative DNA polymerase involved in chromatin dynamics during transcription and cell cycle regulation [44]. The increase in the percentage of follicles with PCNA-positive granulosa cells in both the control and vitrified ovarian tissue at day 7 in tissue cultured in the presence of insulin compared to those cultured without insulin is consistent with the activation of primordial follicles in response to insulin. The increased proportion of PCNA-positive follicles in the vitrified groups at day 3 compared to non-vitrified controls could be due to DNA damage repair occurring during culture in an appropriate environment; this would be consistent with our findings that follicular morphology of vitrified tissue improves after 3 days of culture. As a marker for proliferation, DNA repair and primordial follicle activation, PCNA could be considered a global indicator of tissue health; it may therefore be used for a preliminary assessment of successful vitrification of ovarian tissue. More specific assay such as Ki67 and RAD51 should be used for adequate quantification of cell proliferation and DNA repair, respectively.
Throughout this study, we found no significant differences in the evaluated biometrics related to increased concentration of CPA or the addition of sucrose to the vitrification solution. Sucrose is traditionally added to the vitrification solution as a non-permeable sugar which reduces osmotic shock and improves the glass-forming properties of permeable CPAs [11]. Moniruzzaman et al. [22] found that the addition of sucrose to the vitrification solution increased abnormalities of the oocyte nucleus and cytoplasm in a dose-dependent manner in neonatal pigs, and Amorim et al. [25] reported that using trehalose instead of sucrose improved follicle morphology in women. Bao et al. [45] found that adult bovine ovarian tissue vitrified using a sucrose-containing vitrification solution and the Cryotop method had a higher percentage of morphologically abnormal primordial follicles, compared to tissue vitrified using sucrose-free solutions, suggesting that the presence of sucrose increased osmotic injury. Additionally, higher concentrations of sucrose (0.5 M) led to follicular degeneration while lower concentrations (0.25 M) improved follicular morphology in caprine ovarian tissue [46]. The interactions between sucrose and permeable CPAs may also play a role, as the addition of sucrose to EG and DMSO in the vitrification solution resulted in better follicular morphology in prepubertal rats, compared to other vitrification solutions without sucrose or with different CPAs [47]. The results from the present study are consistent with reports for adult bovine ovaries [33] that the presence or absence of sucrose in the vitrification solution does not affect follicular morphology. It therefore appears that the role sucrose plays in the vitrification of ovarian tissue is dependent on concentration, permeable CPAs, tissue species, and age.
Our success in vitrifying fetal bovine ovarian tissue may be attributed to different factors. First, the fibrous content is lower in fetal ovaries than in adult ovaries [48], which could improve the tissue’s permeability to CPAs and allow non-permeable sucrose to effectively reduce osmotic shock. Second, fetal bovine ovaries between 140 and 210 days of gestation consist of many primordial follicles and some growing follicles. Evidence from previous studies on ovarian cortex vitrification suggests that primordial follicles are more resistant to vitrification damage than growing follicles, as they are smaller and have lower metabolic rates and no zona pellucida [39, 49]. This would contribute towards increasing the permeability to CPAs compared to larger follicles and possibly diminish the toxic effect on the oocyte. It is also possible that fetal bovine ovaries are capable of mitigating the formation of free radicals, which can occur from exposure to CPAs [50].
In conclusion, we found that fetal bovine ovarian tissue had the intrinsic capacity to mitigate the immediate impact of cryopreservation on the morphology, viability, and functionality of primordial follicles. Furthermore, these characteristics were not affected by the presence or absence of sucrose in the vitrification solution, and neither exposure to CPAs nor vitrification of ovarian tissue affected the capacity of primordial follicles to activate. This procedure can therefore be used as a baseline for developing gamete rescue protocols for endangered species and women undergoing fertility preservation.
Acknowledgments
The authors thank Cargill Regional Beef for the donation of bovine ovaries. The cooperation of John Couture at Cargill is gratefully acknowledged. The authors also thank Dr. Mark Roberson for the use of his storage facilities and Mary Lou Norman for her assistance with histological preparations.
Conflict of interest
The authors declare they have no competing interests.
Funding
This research was supported by the United States Department of Agriculture Multistate Project (NE-1227).
Footnotes
Capsule
Cryoprotectant exposure and vitrification of fetal bovine ovarian tissue do not cause long-term damage to follicle structure or affect the capacity of primordial follicles to activate.
Contributor Information
Lara Mouttham, Email: moutthaml@si.edu.
Pierre Comizzoli, Email: comizzolip@si.edu.
References
- 1.Chung K, Donnez J, Ginsburg E, Meirow D. Emergency IVF versus ovarian tissue cryopreservation: decision making in fertility preservation for female cancer patients. Fertil Steril. 2013;99(6):1534–42. doi: 10.1016/j.fertnstert.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans M-M. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol. 2010;53(4):787–96. doi: 10.1097/GRF.0b013e3181f97a55. [DOI] [PubMed] [Google Scholar]
- 3.West ER, Zelinski MB, Kondapalli LA, Gracia C, Chang J, Coutifaris C, et al. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53(2):289–95. doi: 10.1002/pbc.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comizzoli P, Songsasen N, Wildt DE. Protecting and extending fertility for females of wild and endangered mammals. Cancer Treat Res. 2010;156:87–100. doi: 10.1007/978-1-4419-6518-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos RR, Amorim C, Cecconi S, Fassbender M, Imhof M, Lornage J, et al. Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim Reprod Sci. 2010;122(3-4):151–63. doi: 10.1016/j.anireprosci.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Depalo R, Loverro G, Selvaggi L. In vitro maturation of primordial follicles after cryopreservation of human ovarian tissue: problems remain. Med Pediatr Oncol. 2002;38(3):153–7. doi: 10.1002/mpo.10053. [DOI] [PubMed] [Google Scholar]
- 7.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–50. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17(3):243–56. doi: 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- 9.Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21(4):407–26. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 10.Fahy GM. The relevance of cryoprotectant “toxicity” to cryobiology. Cryobiology. 1986;23(1):1–13. doi: 10.1016/0011-2240(86)90013-1. [DOI] [PubMed] [Google Scholar]
- 11.Courbière B, Baudot A, Mazoyer C, Salle B, Lornage J. Vitrification: a future technique for ovarian cryopreservation? Physical basis of cryobiology, advantages and limits. Gynecol Obstet Fertil. 2009;37(10):803–13. doi: 10.1016/j.gyobfe.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Castro SV, Carvalho AA, Silva CMG, Santos FW, Campello CC, Figueiredo JR, et al. Fresh and vitrified bovine preantral follicles have different nutritional requirements during in vitro culture. Cell Tissue Bank. 2014 Mar 8. [DOI] [PubMed]
- 13.Wang X, Catt S, Pangestu M, Temple-Smith P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction. 2011;141(2):183–91. doi: 10.1530/REP-10-0383. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Xiao Z, Li L, Fan W, Li S-W. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23(10):2256–65. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- 15.Fortune JE, Yang MY, Muruvi W. In vitro and in vivo regulation of follicular formation and activation in cattle. Reprod Fertil Dev. 2011;23(1):15–22. doi: 10.1071/RD10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans HE, Sack WO. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Zentralbl Veterinarmed C. 1973;2(1):11–45. doi: 10.1111/j.1439-0264.1973.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 17.Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod. 1996;55(5):942–8. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- 18.Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod. 2008;78(6):1153–61. doi: 10.1095/biolreprod.107.066688. [DOI] [PubMed] [Google Scholar]
- 19.Moldovan G-L, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129(4):665–79. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007;67(1):81–9. doi: 10.1016/j.theriogenology.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod BioMed Online. 2006;12(6):779–96. doi: 10.1016/s1472-6483(10)61091-7. [DOI] [PubMed] [Google Scholar]
- 22.Moniruzzaman M, Bao RM, Taketsuru H, Miyano T. Development of vitrified porcine primordial follicles in xenografts. Theriogenology. 2009;72(2):280–8. doi: 10.1016/j.theriogenology.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Fathi R, Valojerdi MR, Eimani H, Hasani F, Yazdi PE, Ajdari Z, et al. Sheep ovarian tissue vitrification by two different dehydration protocols and needle immersing methods. Cryo Letters. 2011;32(1):51–6. [PubMed] [Google Scholar]
- 24.Salehnia M, Sheikhi M, Pourbeiranvand S, Lundqvist M. Apoptosis of human ovarian tissue is not increased by either vitrification or rapid cooling. Reprod BioMed Online. 2012;25(5):492–9. doi: 10.1016/j.rbmo.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Amorim CA, David A, Van Langendonckt A, Dolmans M-M, Donnez J. Vitrification of human ovarian tissue: effect of different solutions and procedures. Fertil Steril. 2011;95(3):1094–7. doi: 10.1016/j.fertnstert.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 26.Fatehi R, Ebrahimi B, Shahhosseini M, Farrokhi A, Fathi R. Effect of ovarian tissue vitrification method on mice preantral follicular development and gene expression. Theriogenology. 2013 Oct 1. [DOI] [PubMed]
- 27.Xiao Z, Wang Y, Li L, Luo S, Li S-W. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010;94(6):2323–8. doi: 10.1016/j.fertnstert.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Xiao Z, Wang Y, Li L-L, Li S-W. In vitro culture thawed human ovarian tissue: NIV versus slow freezing method. Cryo Letters. 2013;34(5):520–6. [PubMed] [Google Scholar]
- 29.Klocke S, Bündgen N, Köster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. Springer Berlin Heidelberg; 2014 Aug 13;:1–8. [DOI] [PubMed]
- 30.Amorim CA, Jacobs S, Devireddy RV, Van Langendonckt A, Vanacker J, Jaeger J, et al. Successful vitrification and autografting of baboon (Papio anubis) ovarian tissue. Hum Reprod. 2013;28(8):2146–56. doi: 10.1093/humrep/det103. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Cheng KM, Silversides FG. Novel needle-in-straw vitrification can effectively preserve the follicle morphology, viability, and vascularization of ovarian tissue in Japanese quail (Coturnix japonica) Anim Reprod Sci. 2012;134(3-4):197–202. doi: 10.1016/j.anireprosci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod BioMed Online. 2009;18(4):568–77. doi: 10.1016/s1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 33.Celestino JJH, Santos RRD, Melo MAP, Rodrigues APR, Figueiredo JR. Vitrification of bovine ovarian tissue by the solid-surface vitrification method. Biopreserv Biobank. 2010;8(4):219–21. doi: 10.1089/bio.2010.0019. [DOI] [PubMed] [Google Scholar]
- 34.Amorim CA, Curaba M, Van Langendonckt A, Dolmans M-M, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod BioMed Online. 2011;23(2):160–86. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Amorim CA, David A, Dolmans M-M, Camboni A, Donnez J, Van Langendonckt A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J Assist Reprod Genet. 2011;28(12):1157–65. doi: 10.1007/s10815-011-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David A, Van Langendonckt A, Gilliaux S, Dolmans M-M, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum Reprod. 2012;27(4):1088–95. doi: 10.1093/humrep/des013. [DOI] [PubMed] [Google Scholar]
- 37.von Schönfeldt V, Chandolia R, Kiesel L, Nieschlag E, Schlatt S, Sonntag B. Advanced follicle development in xenografted prepubertal ovarian tissue: the common marmoset as a nonhuman primate model for ovarian tissue transplantation. Fertil Steril. 2011;95(4):1428–34. doi: 10.1016/j.fertnstert.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Oktem O, Alper E, Balaban B, Palaoglu E, Peker K, Karakaya C, et al. Vitrified human ovaries have fewer primordial follicles and produce less anti-Müllerian hormone than slow-frozen ovaries. Fertil Steril. 2011;95(8):2661–1. doi: 10.1016/j.fertnstert.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):193–8. doi: 10.1016/j.ejogrb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Amorim CA, Dolmans M-M, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertility and Sterility. 2012 Nov;98(5):1291–8.e1–2. [DOI] [PubMed]
- 41.Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995;53(2):295–301. doi: 10.1095/biolreprod53.2.295. [DOI] [PubMed] [Google Scholar]
- 42.Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. Oxford University Press; 1997 Sep 1;12(9):1993–2001. [DOI] [PubMed]
- 43.Lan C, Xiao W, Xiao-Hui D, Chun-Yan H, Hong-Ling Y. Tissue culture before transplantation of frozen-thawed human fetal ovarian tissue into immunodeficient mice. Fertil Steril. 2010;93(3):913–9. doi: 10.1016/j.fertnstert.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Ulrich HD, Takahashi T. Readers of PCNA modifications. Chromosoma. 2013;122(4):259–74. doi: 10.1007/s00412-013-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao R-M, Yamasaka E, Moniruzzaman M, Hamawaki A, Yoshikawa M, Miyano T. Development of vitrified bovine secondary and primordial follicles in xenografts. Theriogenology. 2010;74(5):817–27. doi: 10.1016/j.theriogenology.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho AA, Faustino LR, Silva CMG, Castro SV, Luz HKM, Rossetto R, et al. Influence of vitrification techniques and solutions on the morphology and survival of preantral follicles after in vitro culture of caprine ovarian tissue. Theriogenology. 2011;76(5):933–41. doi: 10.1016/j.theriogenology.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Fathi R, Valojerdi MR, Salehnia M. Effects of different cryoprotectant combinations on primordial follicle survivability and apoptosis incidence after vitrification of whole rat ovary. Cryo Letters. 2013;34(3):228–38. [PubMed] [Google Scholar]
- 48.Figueiredo JR, Hulshof SC, van den Hurk R, Ectors FJ, Fontes RS, Nusgens B, et al. Development of a combined new mechanical and enzymatic method for the isolation of intact preantral follicles from fetal, calf and adult bovine ovaries. Theriogenology. 1993;40(4):789–99. doi: 10.1016/0093-691x(93)90214-p. [DOI] [PubMed] [Google Scholar]
- 49.Bos-Mikich A, Marques L, Rodrigues JL, Lothhammer N, Frantz N. The use of a metal container for vitrification of mouse ovaries, as a clinical grade model for human ovarian tissue cryopreservation, after different times and temperatures of transport. J Assist Reprod Genet. 2012;29(11):1267–71. doi: 10.1007/s10815-012-9867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang AW, Zhang H, Ikemoto I, Anderson DJ, Loughlin KR. Reactive oxygen species generation by seminal cells during cryopreservation. Urology. 1997;49(6):921–5. doi: 10.1016/s0090-4295(97)00070-8. [DOI] [PubMed] [Google Scholar]