Abstract
This paper describes the success and expansion of ovarian tissue cryopreservation and transplantation as a fertility restoration procedure, with the largest series of 60 live births worldwide reported. By repeating the procedure, ovarian activity can be restored for more than 11 years.
Keywords: Ovarian tissue transplantation, Live births, Fertility preservation, Cancer patients
Introduction
Fertility preservation (FP) in young women will be a major challenge over the next 5 years, in the context of treating cancer or benign diseases or for social reasons [1]. Unfortunately, only small fractions of patients at risk of premature ovarian failure (POF) are referred to fertility preservation specialists to discuss the different options of FP.
Mature oocyte cryopreservation
Embryo and mature oocyte cryopreservation are the only methods endorsed by ASRM.
MII oocyte vitrification represents the ideal fertility preservation option in case of benign diseases, social reasons, and even in cancer women if they are post-pubertal and if chemotherapy could be delayed.
Studies of oocyte vitrification in egg donation programs demonstrated very high oocyte survival rates (92.52 %) and ongoing pregnancy rates (as high as 43.7 %) [2]. Nevertheless, patients should be aware that around 20 vitrified oocytes are required to achieve a live birth.
Indeed, in the most experienced teams of the world, the live birth rate per vitrified oocyte is 5–7 % in egg donation program but these results cannot be extrapolated to cancer women.
Ovarian tissue cryopreservation (OTC)
For prepubertal girls and women who cannot delay the start of chemotherapy, OTC is the only FP option available [1, 3].
It has also been demonstrated in some series [1] that OTC during childhood was feasible and safe [1, 4]. Nevertheless, in that case, left oophorectomy should be performed because of the small size of the ovaries.
Otherwise, in adults, 4–5 ovarian cortical biopsies (1 cm L, 0.5 mm W, 1.5 mm T) are usually taken.
There are essentially two techniques of orthotopic reimplantation (that means “in the pelvic cavity”) [1]:
If at least one ovary is present, pieces of thawed ovarian are either fixed on the decorticated medulla [1] or pushed by a small cortical incision under the cortical capsula [5, 6].
If the ovary is absent, the ovarian pieces could be placed in a peritoneal window [7, 8].
The advantages of orthotopic ovarian tissue reimplantation are the possibility of natural conception (with demonstrated restoration of fertility) and the favorable environment for follicular development (oxygenation, pressure, presence of peritoneal fluid).
Outcomes
Restoration of ovarian activity
Restoration of ovarian activity was 100 % if primordial follicles are present. In a first series, three cases had no follicles in their grafted tissue and of course no restoration of activity was detected. It highlights the importance of an “intact” follicular density [9].
The mean duration of ovarian function after transplantation is more or less 5 years in case of high follicular density which is age dependent.
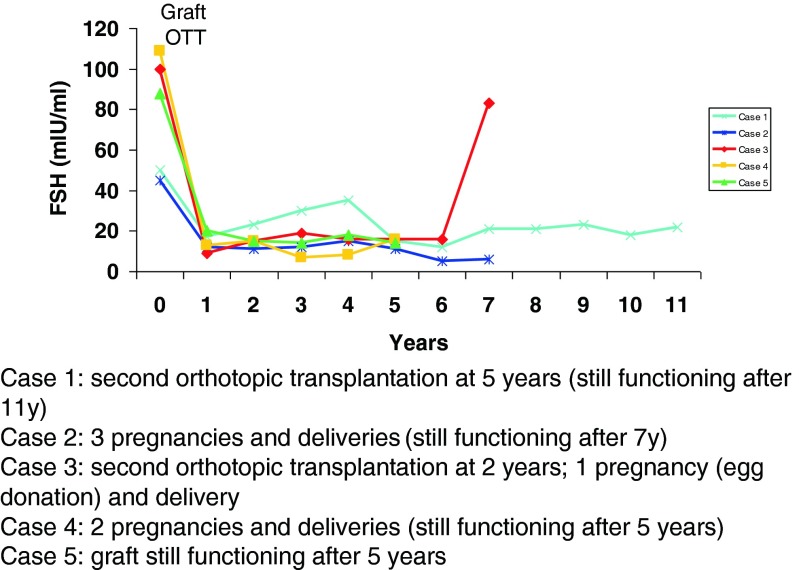
Data from a series of five women who underwent ovarian tissue cryopreservation before the age of 22 years (median 19 years) and before any kind of chemotherapy (Fig. 1) demonstrated that the duration of ovarian activity restoration was more than 5 years and that by repeating the procedure, ovarian activity can be restored for more than 11 years. These encouraging results on ovarian function restoration now lead us to speculate that in the future, ovarian cortex cryopreservation at a young age, followed by reimplantation at menopause, could be an alternative to hormonal replacement therapy.
-
2.
Live birth
Fig. 1.
Data from a series of five women who underwent ovarian tissue cryopreservation before the age of 22 years (median 19 years) and before any kind of chemotherapy. Case 1—second orthotopic transplantation at 5 years (still functioning after 11 years), case 2—three pregnancies and deliveries (still functioning after 7 years), case 3—second orthotopic transplantation at 2 years; 1 pregnancy (egg donation) and delivery, case 4—two pregnancies and deliveries (still functioning after 5 years), and case 5—graft still functioning after 5 years
So far, 60 live births were reported either in peer reviewed journals or in abstracts of congress (Table 1). All of them but two were obtained when the slow freezing technique was applied.
Table 1.
Series of 60 live births after transplantation of frozen-thawed ovarian cortex
| Cryopreservation procedure | Number of transplanted women desiring pregnancy | Number of live births (..) = ongoing pregnancies | |
|---|---|---|---|
| Donnez and Dolmans et al. | SF | 19 | 8 (+1) |
| Meirow et al. | SF | NA | 6 |
| Demeestere et al. | SF | NA | 3 |
| Andersen’s et al. | SF | 25 | 8 |
| Silber et al. | SF | 6 | 4 |
| Piver et al. and Roux et al. | SF | NA | 3 (+1) |
| Pellicer et al. | SF | 33 | 6a (+3) |
| Revel et al. | SF | NA | 2 |
| Dittrich et al. | SF | 20 | 6 |
| Revelli et al. | SF | NA | 1 |
| Callejo et al. | SF | NA | 1 |
| Stern, Gook, and Rozen | SF | 14 | 3a |
| Kawamura and Suzuki et al. | VF | NA | 2 |
| Burmeister and Kovacs, et al. | SF | 2 | 1 |
| Rodriguez-Wallberg and Hovatta et al. | SF | NA | 1 |
| Tanbo et al. | SF | 2 | 2 |
| Agarwal et al.b | SF | NA | 1 |
| Makolkin et al., and Kalugina et al.b | SF | NA | 2 |
As the number of transplantations (the denominator) is not known, results for three centers (Denmark, Spain, Belgium) were collected in a large series [9], and 2 years later in the largest series taking into account a fourth center (Germany) [10]. The number of women who conceived is 25 %. Importantly, at least, two women delivered each three babies proving the long-term efficacy of the technique in terms of fertility [10, 11].
Combined technique
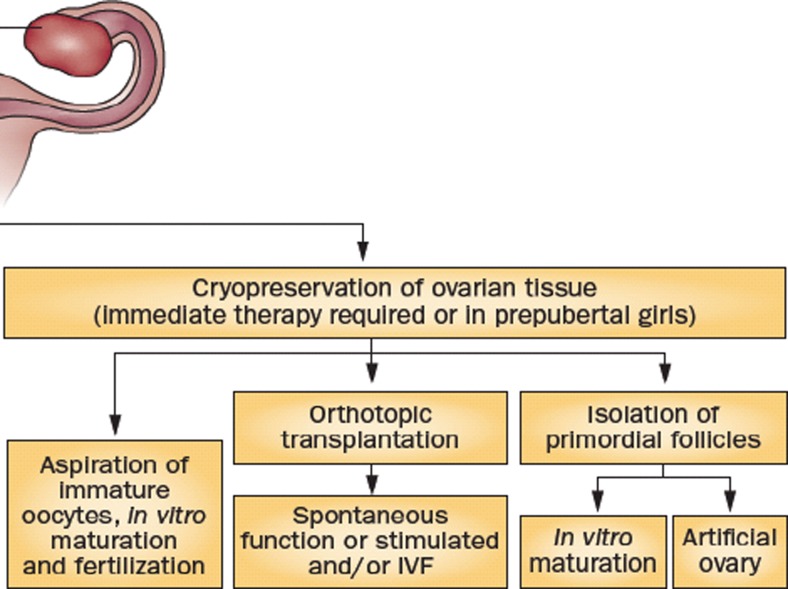
OTC might be combined with the removal of small antral follicles (by puncture) including those present in the dissection medium (Fig. 2). The first pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient was recently published by Prasath et al. [12], followed by a second live birth reported by Segers et al. at the Brussels meeting [13].
OTC could be performed between days 0 and 3 of controlled ovarian stimulation (COS) and then, by continuing COS, ovum pick-up with a view of vitrifying oocytes could increase the efficacy of the fertility preservation. It was indeed recently demonstrated that the number of oocytes obtained after COS, following immediately OTC, was similar to a control group of the same age [14].
Fig. 2.
OTC might be combined with the removal of small antral follicles (by puncture) including those present in the dissection medium. Adapted from Donnez and Dolmans (Nature End Rev)
Conclusion
Only a small fraction of patients at risk of POF is referred to specialists to discuss fertility preservation options; of this group, only a few women actually undergo fertility preservation owing to social, economic, or technical hurdles. In addition, women are increasingly postponing childbearing to later in life for social reasons, and the incidence of most cancers increases with age.
Health-care providers, particularly the oncologists and hematologists, should address the possibility of infertility in patients requiring gonadotoxic drugs and/or irradiation [15].
They should advise them as to available methods of FP, referring them to appropriate reproduction specialists. All patients should receive correct information based on evidence in terms of fertility, and it is time not to consider anymore ovarian tissue cryopreservation and reimplantation as an experimental procedure.
Footnotes
Capsule
Ovarian tissue transplantation restores ovarian secretion and allow natural conception.
References
- 1.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–49. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 2.Cobo A, Garcia-Velasco JA, Domingo J, Remohí J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril. 2013;99(6):1485–95. doi: 10.1016/j.fertnstert.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM. Ovarian tissue freezing: current status. Curr Opin Obstet Gynecol. 2015;27(3):222–30. doi: 10.1097/GCO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 4.Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16(6):617–30. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 5.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 6.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–72. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 7.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 8.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–5. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet. 2015;385(9967):506–7. doi: 10.1016/S0140-6736(15)60198-2. [DOI] [PubMed] [Google Scholar]
- 11.Andersen CY. Success and challenges in fertility preservation after ovarian tissue grafting. Lancet. 2015;385(9981):1947–8. doi: 10.1016/S0140-6736(15)60960-6. [DOI] [PubMed] [Google Scholar]
- 12.Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29(2):276–8. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 13.Segers I, Mateizel I, Guzman L, Romero S, Van Moer E, Smitz J, et al. Congress on “Freezing oocytes embryos and ovarian tissue”. Brussels; 2015.
- 14.Dolmans MM, Marotta ML, Pirard C, Donnez J, Donnez O. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res. 2014;7:80. doi: 10.1186/s13048-014-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolmans MM, Jadoul P, Gilliaux S, Amorim CA, Luyckx V, Squifflet J, et al. A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet. 2013;30(3):305–14. doi: 10.1007/s10815-013-9952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozen G, Agresta F, Gook D, Braat D, Stern CJ. Success and challenges in fertility preservation after ovarian tissue grafting. Lancet. 2015;385(9981):1947. doi: 10.1016/S0140-6736(15)60959-X. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Wallberg KA, Karlström PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94(3):324–8. doi: 10.1111/aogs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanbo T, Greggains G, Storeng R, Busund B, Langebrekke A, Fedorcsak P. Autotransplantation of cryopreserved ovarian tissue after treatment for malignant disease - the first Norwegian results. Acta Obstet Gynecol Scand. 2015. [Epub ahead of print]. [DOI] [PubMed]