Abstract
Purpose
The aim of this study was to evaluate long-term embryo cryopreservation, utilization, and success rate in patients subjected to gonadotoxic treatments in the context of cancer.
Methods
This is a retrospective study on patients (n = 54) undergoing ovarian stimulation and IVF for fertility preservation between January 1997 and June 2014. Embryos were slow-frozen and stored until the women were cured and able to undergo embryo transfer.
Results
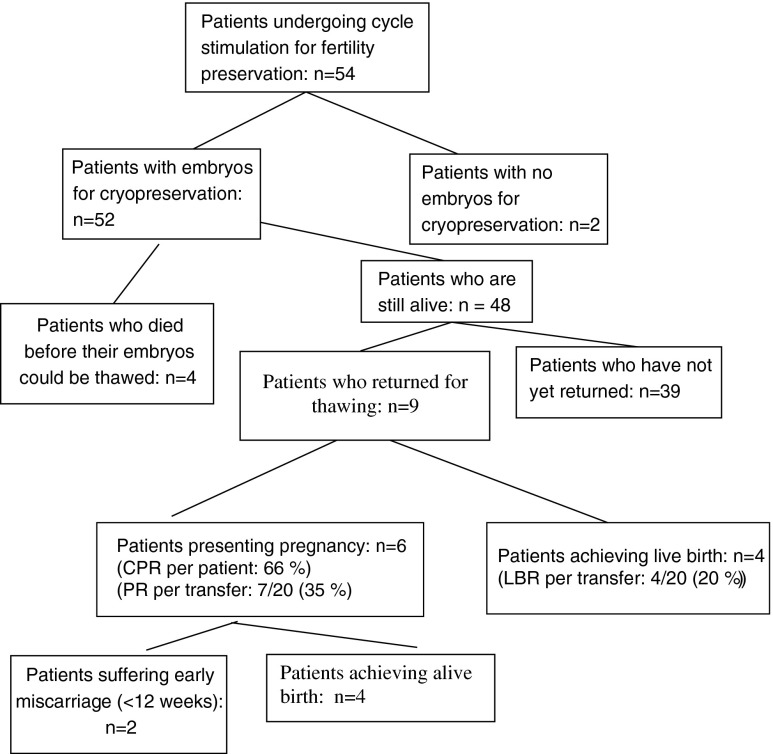
Fifty-four women underwent 66 oocyte pick-up procedures in total, and embryos were obtained from 52 of the 54 patients. Four patients died before their frozen embryos could be thawed. Of the remaining 48, 9 women returned to use their embryos, resulting in 6 pregnancies (66 % cumulative pregnancy rate), two of which ended in miscarriage. The live birth rate per patient was thus 44 % (4/9). The true come-back rate, calculated after applicable exclusions, was found to be 23 %.
Conclusion
IVF followed by embryo freezing is a widely established technique for fertility preservation, but little has been published on the outcomes in cancer patients. While we found the number of good-quality embryos to be lower than in a normal population, the cumulative live birth rate was similar to that achieved with fresh embryos in non-cancer patients. The utilization rate of this fertility preservation method can be considered high.
Keywords: IVF, Cancer patients, Embryo banking, Fertility preservation, Cryopreservation, Utilization rate, Thawing
Introduction
With recent advances in cancer treatments, the number of young cancer survivors with impaired fertility is growing. The quality of life of these patients may be severely impaired if fertility preservation options are not discussed prior to treatment [1].
Women having to undergo gonadotoxic treatments have several options to preserve their fertility and enable them to conceive when they have recovered: embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation. Currently, embryo and mature oocyte cryopreservation are the only methods endorsed by the American Society for Reproductive Medicine (ASRM) Committee [2]. While ovarian tissue cryopreservation is the only option available for young prepubertal patients and those needing to start chemotherapy right away, if there is time for ovarian stimulation and the patient has a partner, embryo cryopreservation should be proposed.
As a widely established technique, embryo cryopreservation has reliable success rates [3]. Moreover, frozen embryo transfer has been found to yield significantly higher ongoing and clinical pregnancy rates than fresh embryo transfer, which could be explained by superior embryo-endometrium synchrony [3, 4].
On the other hand, there is still some controversy concerning the impact of cancer or systemic diseases on the number and/or quality of oocytes retrieved from these patients. The ovarian response to stimulation in cancer patients may be poorer than expected [5–7], but this remains a matter of debate, as Tulandi and Holzer [8] found that malignancies do not affect oocyte numbers.
Very little has been published on the efficacy and utilization rates of long-term embryo cryopreservation in cancer patients [9–11].
In the light of this growing interest in fertility preservation and restoration, the aim of our study was to evaluate the success of the technique and review utilization and pregnancy rates after IVF and long-term embryo cryopreservation prior to gonadotoxic treatment.
Patients and methods
Patients
A retrospective study was conducted on patients undergoing IVF cycles between January 1997 and June 2014. During this period, 54 women underwent ovarian stimulation and IVF for fertility preservation, resulting in 66 oocyte pick-up procedures. Patient age at the time of oocyte retrieval ranged from 21 to 41 years (mean 30 ± 4.6 years).
Patients gave their informed consent for their medical and administrative data to be communicated to external bodies in a coded manner. Data were analyzed retrospectively from medical files and our internal computerized laboratory database, developed to ensure quality control assessment and comparison on a national level.
Ovarian stimulation
The type of stimulation administered depended on the patient cycle phase at the time of referral. Before 2012, patients in the follicular phase received a short agonist or antagonist protocol, while those in the luteal phase received a long agonist protocol. However, the majority of patients were taking oral contraceptives and they all underwent a short protocol after discontinuation of the pill. Since February 2012, all patients have undergone random start stimulation (n = 14).
Twenty-eight cycles were downregulated with GnRH agonists and 38 with GnRH antagonists (Orgalutran®, Organon, Oss, the Netherlands, or Cetrotide® Merck-Serono, Darmstadt, Germany). Gonadotropins used were recombinant (Gonal-F®, Merck-Serono, and Purégon®, MSD, Darmstadt, Germany,) or urinary purified (Menopur®, Ferring, Kiel, Germany). Trigger was performed by subcutaneous injection of human chorionic gonadotropin (hCG) (Pregnyl®, Shering-Plough) when the dominant follicle reached at least 15–16 mm in size. The patients suffering from breast cancer did not receive any specific treatment as aromatase inhibitors.
In vitro fertilization and embryo cryopreservation
Fertilization was mostly achieved by intracytoplasmic sperm injection (ICSI) to avoid the risk of non-fertilization, except in 3 cycles. All embryos were frozen on day 2 or 3 (slow-freezing protocol using a programmable freezer), depending on the day of pick-up. Slow freezing was performed according to the protocol described by Van Langendonckt et al. [12], with the use of Freeze-Kit1™ (Vitrolife, Sweden).
Thawing cycles
Estrogen replacement therapy (Progynova, Bayer Schering, Berkshire, UK), 4 to 6 mg daily, was administered to patients for a minimum of 14 days. Luteal support with progesterone (Utrogestan, Goodlife Pharma, Leylstad, the Netherlands) was initiated when endometrial thickness was at least 8 mm and, respectively, 2 or 3 days before embryo transfer depending on whether embryos had been frozen on day 2 or 3. A maximum of two thawed embryos were transferred.
Results
Patients
During the study period, 54 patients underwent 66 oocyte pick-up procedures, yielding embryos for cryopreservation in all but 2 (Fig. 1). Fifty-two patients were referred for neoplastic pathologies and two for non-neoplastic conditions (scleroderma and sarcoidosis). The most frequent oncological indications were lymphoma (n = 18) (13 Hodgkin’s lymphoma and 5 non-Hodgkin lymphoma) and breast cancer (n = 12), followed by borderline ovarian tumors (BOT, n = 8) and colorectal cancer (n = 7). Other pathologies included sarcoma (n = 2), ovarian (n = 1) or cervical (n = 1) cancer, medullar aplasia (n = 1), chronic leukemia (n = 1), and pseudomyxoma (n = 1). In the woman with ovarian cancer (stage II), one IVF attempt was approved after unilateral adnexectomy, before chemotherapy and debulking surgery were initiated.
Fig 1.
Utilization rates and pregnancy outcomes per patient undergoing stimulation for fertility preservation. CPR cumulative pregnancy rate, PR pregnancy rate, LBR live birth rate
When possible, more than one stimulation cycle was authorized, so 10 patients were able to undergo 2–3 cryopreservation cycles, 6 of them presenting with BOT.
Stimulation and cryopreservation
There was never a delay in cancer treatment due to ovarian stimulation, as oncologists only referred patients whose treatment and prognosis would not be affected by waiting 3 weeks before starting chemotherapy.
For stimulation cycles, antagonist protocols with rec-FSH were preferentially used (n = 37), followed by GnRH agonist protocols with urinary FSH (n = 23) or rec-FSH (n = 5), and one cycle with GnRH antagonist and urinary FSH. The mean total dose of gonadotropins used was 2997 ± 1707 IU. The estradiol level at the time of hCG trigger reached a median of 788 pg/ml, with a range from 94 to 8019 pg/ml (Table 1).
Table 1.
Stimulation results
| Number of cycles | 66 |
| Gonadotropin dose (IU) (mean ± SD)a | 2997 ± 1707 |
| E2 (pg/ml) on day of hCG (median)b | 788 (range 94–8019) |
| Number of retrieved oocytes per cycle (mean)c | 9.66 (±7.55) (range 1–32) |
| Fertilization rate | 66.4 % |
| Number of stored embryos (mean ± SD) | 4.06 ± 3.68 (range 0–17) |
aComplete information for 63 cycles
bComplete information for 65 cycles
cComplete information for 64 cycles
The number of oocytes retrieved per cycle ranged from 1 to 32, with a mean of 9.66 (±7.55). The fertilization rate was 66.4 %, and the mean number of stored embryos per patient was 4.06 (±3.68), with a range from 0 to 17 (Table 1).
Thawing
Four patients died before their embryos could be thawed (Fig. 1). Of the remaining 48 patients, 9 have had their embryos thawed, while 39 have not yet returned. This yields a “come-back rate” or embryo utilization rate of 9/48 (18.75 %).
The characteristics of patients who underwent embryo thawing are presented in Table 2. Of the 9 patients who did return, 5 underwent 2 cycles of ovarian stimulation and pick-up and 4 only 1 cycle.
Table 2.
Characteristics of patients undergoing embryo thawing
| Pathology | Age at freezing | Number of stimulation cycles | Number of frozen embryos | Number of thawed embryos | Number of transferred embryos | Pregnancy |
|---|---|---|---|---|---|---|
| Breast cancer | 34 | 1 | 17 | 1 | 1 | Live birth |
| Breast cancer | 34 | 1 | 4 | 4 | 3 | Live birth |
| Breast cancer | 41 | 2 | 3 | 3 | 3 | Early miscarriage |
| BOT | 25 | 2 | 14 | 14 | 10 | Early miscarriage |
| BOT | 36 | 2 | 6 | 2 | 2 | Live birth |
| Ovarian cancer | 26 | 1 | 4 | 4 | 4 | Live birth (twins) |
| BOT | 30 | 2 | 3 | 3 | 3 | No |
| Rectal adenocarcinoma | 34 | 1 | 8 | 8 | 6 | No |
| Colon adenocarcinoma | 35 | 2 | 2 | 2 | 1 | No |
| Total | / | 14 | 61 | 41 | 33 | 6+/9 |
BOT borderline ovarian tumor
Altogether, 20 embryo transfers were performed, with a total of 33 embryos. Of these 20 embryo transfers, 4 involved use of poor-quality embryos. Seven gestational sacs were observed at ultrasound, with a pregnancy rate per transfer of 35 % (7/20) and an implantation rate of 21 % (7/33). All those who conceived had at least one intermediate-quality embryo, except one woman who had only poor-quality embryos and later suffered a miscarriage.
Among the 9 patients who came back, 6 pregnancies occurred: 5 singleton and 1 twin (cumulative pregnancy rate per patient 66 %). Two ended in early miscarriage, while 4 culminated in live births.
Two of these 9 patients still have embryos frozen.
Discussion
According to the latest ASRM Ethics Committee guidelines, the most effective strategy for fertility preservation in women is to undergo IVF and create embryos for later use [13]. Oocyte vitrification is also considered a viable option [14], as the ASRM no longer regards this technique as experimental.
Although IVF and embryo freezing are widely applied in infertile patients, very little has been published on embryo utilization rates and results in terms of efficacy of this technique in a cancer population.
Here, we present a series of 54 patients who underwent ovarian stimulation and embryo cryopreservation for fertility preservation purposes between 1997 and 2014.
Utilization rate
Of the 52 patients who had their embryos frozen, 9 have so far come back for thawing (17 %). As 4 patients died before they were able to return, this yields a come-back rate or embryo utilization rate of 18.75 % (9/48). However, this figure is slightly misleading. Indeed, patients who are treated for cancer are frequently advised not to conceive before they are in remission, so we did not expect women who have had their embryos cryopreserved in the last 2 years to return for thawing so soon (9 patients). Hence, if we exclude these patients, the come-back rate is actually 23 % (9/39).
When we compare our data with the literature, this more realistic figure of 23 % is in accordance with the results of Robertson et al. [9], who found that 26 % of patients (10/38) returned for embryo transfer. Very recently, Cardozo et al. also published their data on IVF outcomes of cancer patients undergoing fertility preservation, covering exactly the same 17-year period (1997–2014) as in our study [11]. They obtained a relative high utilization rate, as 21/63 (33 %) patients returned for frozen embryo transfer. These return rates are much higher than for sperm banking or ovarian tissue cryobanking. For male fertility restoration, the utilization rate by cancer survivors who banked sperm prior to cancer treatment is curiously less than 10 % [15]. For cryopreserved ovarian tissue, the utilization rate was calculated to be 2.3 % in 2012 [16] but the number of patients undergoing ovarian tissue transplantation is increasing all the time as these women decide to attempt pregnancy. The latest figures for 2015 show the rate to be 3.5–8 %.
Number of oocytes and embryos
The mean number of stored embryos per patient in this study was 4.06 (±3.68), apparently similar to a control group in one of our previous studies during the same period [17]. In this control group, the mean number of good-quality frozen embryos obtained per patient on day 3 was 4.4, compared to 4.06 in the present study. In the latter, however, all embryos were frozen and only 63 % of them were of good quality, yielding an average of 2.3 good-quality embryos per patient. We can thus confirm that even if the mean number of stored embryos was a respectable 4.06 in the present study, their quality may possibly be lower than in a normal population. Nevertheless, it does not appear to impact on the cumulative live birth rate (LBR). In a systematic review and meta-analysis, Friedler et al. concluded that women with malignant disease should expect a smaller number of oocytes to be retrieved after controlled ovarian hyperstimulation for fertility preservation, compared with healthy age-matched patients [9]. However, Devesa et al. recently published that ovarian response to stimulation in women with cancer is as expected according to age [18]. This is still a matter of debate and largely depends on the type of cancer and other factors, which may constitute a bias.
Pregnancy rate
In this series of women with cancer, we report a cumulative pregnancy rate of 66 % per patient. Indeed, of the nine patients who came back for thawing, six conceived. This can be considered a success, as the cumulative LBR per patient was 44 % (4/9).
For comparative purposes, we can express this figure in LBR per transfer, which yields a rate of 20 % (4/20). In our department in 2012, the LBR per transfer with fresh embryos in non-cancer patients was 22.7 %, thus not significantly different.
Although in our study the LBR is similar with frozen embryos from cancer patients and fresh embryos from non-cancer patients, there is still some controversy in the literature regarding the quality of embryos obtained after ovarian stimulation in women with cancer.
Indeed, Sabatini et al. [10] reported in 2011 a lower LBR after transfer of frozen embryos in cancer patients compared to women who underwent fresh embryo transfer in their department (LBR 35 %). However, their study published in 2015 on a series of 57 cancer patients who had embryos cryopreserved, showed a similar LBR per transfer between cancer patients and controls [11]. Hence, even if several other studies have reported lower numbers of retrieved oocytes compared to controls [5, 7, 19, 20], this does not appear to have any impact on the pregnancy rate once the patient is cured [11, 21].
Some patients may benefit from more than one cycle and have more embryos cryopreserved, thereby enhancing their chances of becoming pregnant. Use of GnRH antagonists can also shorten the interval from oocyte retrieval to the next menses, and this in turn improves the likehood of multiple back-to-back cycles before initiating cancer treatment [22]. We believe this is a very important consideration for these patients, who want to do all they can to maximize their chances of pregnancy.
Conclusions and perspectives
This study shows that utilization rates of long-term cryopreserved embryos are relatively high (almost one in four) and that outcomes in terms of pregnancy rates are at least as good as those in the general population.
However, there are some limitations to performing oocyte or embryo cryopreservation in cancer patients. Studies by Rienzi et al. and Cobo et al. show that around 20 oocytes are required to achieve a live birth [23, 24]. This number can be obtained in egg donation programs or in case of fertility preservation for social reasons, but rarely in women with cancer. Even when a delay in treatment is possible, it is usually for no more than one cycle [24, 25]. Thus, the good results obtained in egg donation programs cannot be extrapolated to cancer patients, nor can the quality of eggs be guaranteed in these women [24, 26]. This is why, in a recent paper, we proposed a combined approach, cryopreservation of ovarian cortex followed by controlled ovarian stimulation and oocyte pick-up, if treatment can be delayed. Combining these two different methods of fertility preservation is a valuable strategy to maximize the future fertility potential of our cancer patients [18].
Footnotes
Capsule
Utilization rates and pregnancy outcomes of embryos cryopreserved for fertility preservation before gonadotoxic treatments are high.
References
- 1.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15(5):587–597. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committees of American Society for Reproductive Medicine, Society for Assisted Reproductive Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Bedoschi G, Oktay K. Current approach to fertility preservation by embryo cryopreservation. Fertil Steril. 2013;99(6):1496–1502. doi: 10.1016/j.fertnstert.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Domingo J, Guillen V, Ayllon Y, Martinez M, Munoz E, Pellicer A, et al. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril. 2012;97:930–934. doi: 10.1016/j.fertnstert.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 6.Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma—evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. 2012;98:141–144. doi: 10.1016/j.fertnstert.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Friedler S, Koc O, Gidoni Y, Raziel A, Ron-El R. Ovarian response to stimulation for fertility preservation in women with malignant disease: a systematic review and meta-analysis. Fertil Steril. 2012;97:125–133. doi: 10.1016/j.fertnstert.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Tulandi T, Holzer H. Effects of malignancies on the gonadal function. Fertil Steril. 2012;98(4):813–815. doi: 10.1016/j.fertnstert.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 2011;95(2):588–591. doi: 10.1016/j.fertnstert.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Sabatini ME, Wolkovich AM, Macklin EA, Wright DL, Souter I, Toth TL. Pronuclear embryo cryopreservation experience: outcomes for reducing the risk of ovarian hyperstimulation syndrome and for fertility preservation in cancer patients. J Assist Reprod Genet. 2011;28(3):279–284. doi: 10.1007/s10815-010-9515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet. 2015;32(4):587–596. doi: 10.1007/s10815-015-0428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Langendonckt A, Demylle D, Wyns C, Nisolle M, Donnez J. Comparison of G1.2/G2.2 and Sydney IVF cleavage/blastocyst sequential media for the culture of human embryos: a prospective, randomized, comparative study. Fertil Steril. 2001;76(5):1023–1031. doi: 10.1016/S0015-0282(01)02854-0. [DOI] [PubMed] [Google Scholar]
- 13.Ethics Committee of American Society for Reproductive Medicine Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100(5):1224–1231. doi: 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Practice Committees of American Society for Reproductive Medicine; Society for Assisted Reproductive Technology Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Pacey AA, Merrick H, Arden-Close E, Morris K, Barton LC, Crook AJ, et al. Monitoring fertility (semen analysis) by cancer survivors who banked sperm prior to cancer treatment. Hum Reprod. 2012;27(11):3132–3139. doi: 10.1093/humrep/des300. [DOI] [PubMed] [Google Scholar]
- 16.Dolmans MM, Jadoul P, Gilliaux S, Amorim CA, Luyckx V, Squifflet J, et al. A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet. 2013;30(3):305–314. doi: 10.1007/s10815-013-9952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolmans MM, Marotta ML, Pirard C, Donnez J, Donnez O. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res. 2014;7:80. doi: 10.1186/s13048-014-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devesa M, Martínez F, Coroleu B, Rodríguez I, González C, Barri PN. Ovarian response to controlled ovarian hyperstimulation in women with cancer is as expected according to an age-specific nomogram. J Assist Reprod Genet. 2014;31(5):583–588. doi: 10.1007/s10815-014-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril. 2010;94:149–155. doi: 10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Pavone ME, Hirshfeld-Cytron J, Lawson AK, Smith K, Kazer R, Klock S. Fertility preservation outcomes may differ by cancer diagnosis. J Hum Reprod Sci. 2014;7:111–118. doi: 10.4103/0974-1208.138869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaan N, Ben-David G, Ben-Yosef D, Almog B, Many A, Pauzner D, et al. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol. 2010;149:175–177. doi: 10.1016/j.ejogrb.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–1369. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612. doi: 10.1093/humrep/des088. [DOI] [PubMed] [Google Scholar]
- 24.Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril. 2013;99(6):1485–1495. doi: 10.1016/j.fertnstert.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 25.Cakmak H, Rosen MP. Ovarian stimulation in cancer patients. Fertil Steril. 2013;99(6):1476–1484. doi: 10.1016/j.fertnstert.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9(12):735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]