Abstract
Purpose
The aim of this study was to determine the best combination in terms of cryopreservation techniques and vascular bed preparation before grafting in order to obtain functional ovarian tissue after transplantation.
Methods
Five cynomolgus monkeys were used. Strips from 10 ovaries were cryopreserved, 5 by vitrification (V), and 5 by slow-freezing (SF). Pieces of fresh ovarian tissue were used for controls. After 1 month, the strips were autografted to two different vascular beds, healed (HB) or freshly decorticated (FDB), constituting four study groups: SF-HB, SF-FDB, V-HB, and V-FDB. These were compared to fresh tissue. After 6 months, the ovaries were removed and several parameters analyzed: follicle quality, stage, density, proliferation, apoptosis, functionality, vascularization, and fibrosis. Mixed effect linear regression models were built to assess the impact of cryopreservation and vascular bed preparation on ovarian tissue viability and functionality. p values were adjusted for multiple testing using the Benjamini-Hochberg method, and q values < 0.20 were considered significant in order to achieve a 20 % false discovery rate.
Results
Compared to fresh tissue, no difference was observed in the percentage of morphologically normal follicles, while a significant increase was noted in the follicle proliferation rate (41 %, q = 0.19), percentage of antral follicles (12 %, q = 0.14), and number of vessels per area (3.3 times, q = 0.07) in the V-FDB group.
Conclusions
Vitrification associated with FDB vascular bed preparation is the best combination to obtain functional autografted ovarian tissue. Further studies are nevertheless required, with confirmed pregnancies and live births before introducing the procedure into clinical practice.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0542-y) contains supplementary material, which is available to authorized users.
Keywords: Ovarian tissue, Vitrification, Slow-freezing, Vascular bed, Freshly decorticated bed, Healed bed, Autotransplantation of ovarian tissue, Grafting, Cynomolgus monkey
Introduction
Transplantation of cryopreserved ovarian tissue is an emerging technique to restore fertility in patients who have undergone chemo- or radiotherapy. Indeed, 42 live births have so far been published worldwide using this approach [1–8]. Moreover, it is the only option for prepubertal patients and those in whom cancer treatment cannot be delayed, as these patients are unable to undergo oocyte or embryo cryopreservation commonly offered to the healthy postpubertal adult patients.
Results in terms of endocrine ovarian function restoration are excellent, showing up to 100 % recovery after 4–5 months if the ovarian reserve of the transplanted tissue is high enough [1]. Outcomes in terms of live births are also satisfactory, 20 healthy babies born out of a series of 80 patients [9]. Nevertheless, oocytes obtained after freezing and grafting of ovarian tissue can be of lower quality [10], which may be due to damage suffered during both the freezing procedure and post-transplantation ischemia [1, 11–13]. This oocyte damage often translates to a high empty follicle rate (30 %) during in vitro fertilization (IVF) after transplantation [10].
It is well known that both freezing and surgical techniques are of crucial importance to the survival of delicate oocytes enclosed in primordial follicles. Vitrification is already widely and successfully used to cryopreserve embryos and oocytes [14]. Although still considered experimental for ovarian tissue, it is looking increasingly promising [15–18] and offers an alternative means of cryopreserving ovarian tissue, even if almost all pregnancies to date have been achieved after slow-freezing and grafting [2]. So far, two live births have been obtained after vitrification [4, 5]. In experienced hands, vitrification of human ovarian tissue allows better preservation of the stroma and avoids ice crystal formation [16, 17]. In previous studies, a new vitrification solution was developed containing lower concentrations of penetrating cryoprotectants combined with non-penetrating cryoprotectants [19–21].
The main mechanism provoking loss of follicles after ovarian tissue transplantation is ischemia, since initiation of graft reperfusion takes place only on day 5 [12, 22]. Preparation of the vascular bed prior to grafting is also very important [2] and needs to be further explored. Recent studies in baboons have demonstrated that grafting to a freshly decorticated ovarian hilum yields good follicular survival [21]. Therefore, in order to optimize grafting procedures with the goal of increasing primordial follicle survival and hence pregnancy rates after ovarian grafting, we aim to study both the available cryopreservation techniques (vitrification versus slow-freezing) and preparation of the vascular bed before grafting in an experimental autologous cynomolgus monkey model.
Material and methods
Animals
Five naïve female adult non-human primates, namely cynomolgus macaques (Macaca fascicularis) (6 ± 0.42 years of age and 3.2 ± 0.2 kg), were obtained from R.C. Hartelust BV (the Netherlands) from their supplier, Beijing Prima Resources (Puliyuan) Ltd. (China). Bacteria (TB, shigella, salmonella) and viral infections (herpes B, SRV, SIV, STLV-1, and filovirus/Ebola-like) were all negative. Two female monkeys were housed per cage (cage size 1.7 m × 1.7 m × 1.7 m) in the animal house facility (Université Catholique de Louvain), no SPF section, where room temperature is maintained at 21 °C, with 60 % relative humidity, and a 12:12 h light/dark cycle. Animals were fed ab libitum on commercial monkey pellets (diet 107, SAFE, Augy, France) and seasonal fruit and vegetables, with free access to water. Clinical examinations were performed daily.
In the present study, cynomolgus monkeys were used as a primate model because of their evolutionary closeness to humans and similarities with our reproductive system [23, 24]. Approval for this study was obtained from the Animal Ethics Review Board of the Catholic University of Louvain (2011/UCL/MD/012). All procedures were conducted according to guidelines established by the abovementioned committee and according to Belgian (AR 29, May 2013) and European (Directive 2010/63/EU) legislation on the care and use of laboratory animals.
Surgical procedure for the ovariectomy
Surgical procedures were always performed in the presence of a veterinary surgeon (JPD). The animals were anesthetized with a mixture of 6 mg/kg zolazepam/tiletamine (Zoletil, Eurovet NV/SA, Heusden-Zolder, Belgium) and 15 mg/kg xylazine (Rompun 2 %, Bayer SA-NV, Belgium) administered intramuscularly (IM) for induction, and 1–2 % isoflurane (Isoba, Schering-Plough Ltd., UK) with N2O/O2 (70 %/30 %) for maintenance. The abdomen was shaved and disinfected with povidone iodine (Iso-Betadine, Meda Pharma, Belgium).
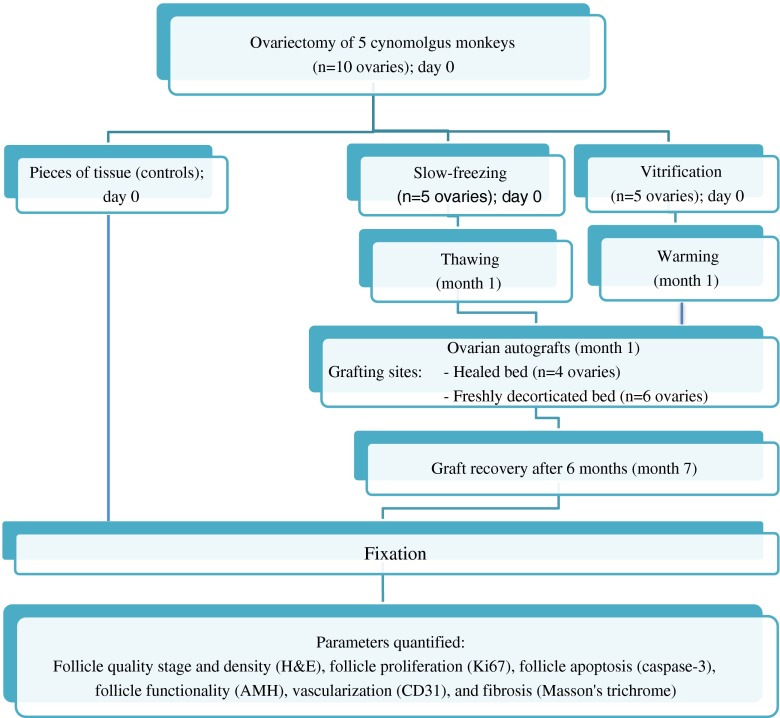
Vertical laparotomies were performed by experienced surgeons (JPD together with JD, JS or MMD) to remove both ovaries (Fig. 1). One ovary from each animal was cryopreserved by slow-freezing and the other one by vitrification. Ovariectomies were performed taking care to leave behind the ovarian hilum and a well preserved blood supply, in view of future reimplantation to this grafting site. Two different surgical techniques of ovary removal were tested. The first involved complete ovariectomy leaving just the denuded hilum behind. This group was term healed bed (HB) group (n = 2 animals, 4 beds). The second entailed removal of a large part of the cortex, leaving some ovarian tissue on the hilum which was removed on the day of autografting. This group was termed the freshly decorticated bed (FDB) (n = 3 animals, 6 beds).
Fig. 1.
Study design. Two cryopreservation techniques, slow-freezing and vitrification, and two preparatory treatments of the grafting site prior to transplantation, a healed or freshly decorticated bed, were compared using 10 ovaries. In a random manner, four ovaries were completely removed from the hilum, and six ovaries were partially removed maintaining a small part of the cortex. Randomly again, cortical strips from one ovary were slow-frozen and cortical strips from the contralateral ovary were vitrified. Some pieces of fresh tissue were immediately fixed and used as controls. One month later, the slow-frozen or vitrified ovarian strips were thawed or warmed, respectively. They were then autografted to a healed or freshly decorticated bed. Six months later, the grafts were removed and histologically analyzed for different parameters
After ovariectomy, the animals received 15 mg/kg amoxicillin (Clamoxyl LA; Pfizer, Paris, France) IM every 2 days for 1 week. Pain was controlled with 2 mg/kg subcutaneous ibuprofen daily (Ketofen; Merial, Lyon, France) and 0.01 mg/kg IM buprenorphine twice a day (Temgesic, Reckitt Benckiser SA/NV, Belgium) for 3 days.
Ovarian tissue preparation
The ovaries were immediately transported to the laboratory in minimal essential medium (MEM) + GlutamaxTM (Gibco, Carlsbad, USA) on ice, cut into halves, and the medulla was removed. The cortex was then cut into strips of approximately 5 × 1 × 1 mm and cryopreserved. Cortex from one ovary of each monkey was slow-frozen while the contralateral ovary was vitrified. One strip from the first ovary was fixed in 4 % formaldehyde in phosphate-buffered saline (PBS) (VWR Chemical, Belgium) and served as a fresh control.
Ovarian tissue vitrification and warming
For the vitrification procedure, a 30G needle (0.3 × 8 mm, BD Micro-Fine+TM, BD, Le Pont de Claix-Cedex, France) was inserted along the length of each tissue strip, according to Fathi et al. [25], in order to facilitate handling of the ovarian strips, maximize cooling rates, and optimize the vitrification process. A vitrification solution (VS) developed in our laboratory was used [19, 21]. This VS contains 10 % dimethyl sulfoxide (DMSO; Sigma-Aldrich, Belgium), 26 % ethylene glycol (Sigma-Aldrich, Belgium), 2.5 % polyvinylpyrrolidone MW 10,000 (Sigma-Aldrich, Belgium), and 1 M sucrose (Sigma-Aldrich, Belgium) in MEM + GlutamaxTM (Gibco, Carlsbad, CA, USA), supplemented with 20 mg/ml human serum albumin (HSA; Sanquin, the Netherlands).
The strips were first equilibrated in 25 % VS/MEM + HSA (7 min), then 50 % VS/MEM + HSA (4 min), and finally 100 % VS (3 min). All VS baths were performed at room temperature. The strips were then placed on aseptic absorbent gauze to remove the remaining VS and directly plunged into liquid nitrogen (LN; −196 °C). Needles containing the vitrified tissue were inserted into precooled 5 ml cryotubes (Corning Inc., Corning, New York) and stored in the LN for 4 weeks.
Before autografting, the ovarian strips were immersed in warming solution 1 (WS1: 1 M sucrose in MEM supplemented with 20 mg/ml HSA) at 37 °C for less than 15 s, just enough time to warm up. The samples were then transferred to WS2 (0.5 M sucrose in MEM supplemented with 20 mg/ml HSA), WS3 (0.25 M sucrose in MEM supplemented with 20 mg/ml HSA), and WS4 (MEM supplemented with 20 mg/ml HSA without sucrose) for 5 min each at 37 °C. The tissue was then immediately transported to the operating room to be autografted.
Ovarian tissue slow-freezing and thawing
Freezing of ovarian strips was performed as previously described [22], with some modifications. The strips were suspended in cryoprotectant (CPA) solution consisting of MEM-Glutamax supplemented with 4 mg/ml HSA and 10 % DMSO at 4 °C, and then transferred to 2 ml cryovials (Simport, Canada) containing 0.8 ml of the CPA solution. The cryovials were cooled in a programmable freezer (Freezer Control CL-8800i, Cryologic, Victoria, Australia) using the following program: (1) cooled from 0 to −8 °C at −2 °C/min, (2) seeded manually, (3) cooled to −40 at −0.3 °C, and (4) cooled to −140 at −30 °C/min and transferred to LN for storage.
For thawing, the cryovials were exposed to room temperature for 2 min and immersed in a water bath at 37 °C until the ice completely melted. To remove the CPA solution, the ovarian tissue was immediately transferred from the cryovials to plastic Petri dishes containing MEM-Glutamax, where it was washed three times (5 min per bath) before grafting. After thawing, fragments were transported to the operating room to be autografted.
Ovarian tissue transplantation
Four weeks after ovary removal, vitrified-warmed and frozen-thawed strips were autotransplanted to cynomolgus monkeys by vertical laparotomy performed by experienced surgeons (JD and JS), the fragments randomly assigned to either the left or right hilum, following the procedure already successfully used in primates [21] and humans [2, 26, 27].
Two different surgical procedures were evaluated for transplantation. The first involved grafting ovarian tissue to the HB and included four ovaries that had been removed by complete ovariectomy. At the time of grafting (month = 1), the grafting site was healed and fibrotic. The second entailed grafting tissue to the FDB, which was done with six ovaries. In this case, ovarian tissue had been left in place at the time of ovariectomy and was removed on the day of transplantation, provoking some bleeding.
Ovarian strips (three per grafting site) were placed directly on the grafting site (HB or FDB), stitched using non-absorbable sutures (6/0 Prolene; Ethicon, LLC, USA), and covered with GYNECARE INTERCEED® Absorbable Adhesion Barrier (Ethicon, LLC, USA). After grafting, the animals received antibiotics and painkillers, as described above.
After 6 months, the monkeys were anesthetized and euthanized using 0.3 ml/kg T61 (Hoechst GmbH, Munich, Germany) intravenously, and the grafts were recovered and fixed in 4 % formaldehyde in PBS.
Histological analysis
Histological analyses were performed on fresh, slow-frozen/thawed/grafted, and vitrified/warmed/grafted tissues. After fixation, in order to evaluate follicular morphology, the ovarian fragments were dehydrated, embedded in paraffin, and serially sectioned into 5-μm-thick sections. Every 10th slide was stained with hematoxylin-eosin (H&E) (Merck, Darmstadt, Germany) for histological evaluation; the other slides (Superfrost® Plus slides, Menzel-Glaser, Germany) were kept for immunostaining.
Follicle stage and quality
The percentage of each follicle stage was calculated. Follicles were further classified according to stage into primordial or growing (primary, secondary, and antral) follicles [28]. Follicle quality was evaluated according to a number of parameters, including integrity of the basement membrane, cellular density, presence or absence of pyknotic bodies, and integrity of the oocyte [29]. Based on these criteria, follicles were classified as morphologically normal or atretic. Morphologically normal follicles (MNFs) have a round-shaped oocyte surrounded by granulosa cells (GC) and a basal membrane. Follicles were considered atretic if one of the following characteristics was observed: pyknotic or retracted oocyte nucleus, degenerated oocyte, disorganized the granulosa cells, or those with a pyknotic nucleus.
Follicle density
Follicle density was evaluated in areas in where follicles were present but fibrosis was absent. All sections (fresh and grafted ovarian tissue fragments) were scanned using Mirax Scan (Zeiss, Jena, Germany). Surface measurements (fresh tissue and delimitation of grafts) were calculated using the Mirax Viewer program. Follicle density was estimated as the number of follicles per square millimeter by counting ovarian follicles in at least 10 sections.
Fibrosis
Fibrotic areas are characterized by poor cellularity (<25 %), as evidenced by a reduced number of cell nuclei and collagen deposits, as previously described [30]. In order to detect fibrosis, Masson’s trichrome was used to stain green tissue that had been replaced with connective collagen, rendering these areas easily recognizable. One section per graft was stained. Stained sections were scanned using Mirax Scan (Zeiss), and fibrotic areas and total section areas were measured using the Mirax Viewer program. Fibrotic areas were delimited with the freehand tool and then automatically calculated [20]. The percentage of fibrosis was determined by visualizing green area/total area × 100.
Immunohistochemistry
In order to assess follicle growth, follicle function, stromal cell apoptosis, and graft vascularization, the following markers were selected: Ki67, anti-Müllerian hormone (AMH), caspase-3, and cluster of differentiation 31 (CD31), respectively.
Paraffin sections were deparaffinized with Histosafe (Yvsolab SA, Beerse, Belgium) and rehydrated in alcohol series. After blocking endogenous peroxidase activity with 0.3 % H2O2 diluted in demineralized water (for caspase-3) or 3 % H2O2 (Merck KGaA, Germany) diluted in methanol (for Ki67, AMH, and CD31), a demasking step was performed for 75 min at 98 °C with 0.01 M citrate buffer pH 5.7 (Merck KGaA, Germany) and Triton X-100 (Merck KGaA, Germany), before the sections were subjected to an antigen retrieval step. Antibody dilutions and sources and incubation conditions are summarized in Supplementary Table I. Diaminobenzidine was used as a chromogen (SK 4100, Vector Laboratories, UK). The slides were then counterstained with H&E and mounted with DPX neutral mounting medium (Prosan, Belgium). Negative controls (absence of primary antibody) were performed in all cases.
Follicles containing at least one GC staining positive for Ki67 were classified as proliferative follicles, as determined by Gougeon et al. [31]. One section per graft was stained for Ki67.
For quantitative analysis of AMH expression, a minimum of 10 follicles from each follicle stage (primordial, primary, secondary, and antral) were evaluated for each animal and group. If fewer than 10 follicles were present, all follicles were taken into account. Follicles were considered AMH-positive when at least one GC was immunostained. Two sections per graft were stained for AMH.
For caspase-3 and CD31 immunostaining, the area around each ovarian tissue graft was defined and all caspase-3-positive stromal cells and all vessels present in the grafts were counted. A follicle was considered apoptotic when at least one GC was positive. Vascularization was evaluated using CD31 staining in ovarian tissue. The number of vessels was normalized by taking into account the total area (μm2). The area occupied by antral follicles and invagination of the tissue was subtracted from the total area of the graft. Six sections per graft were stained for CD31 and two sections per graft for caspase-3.
For all immune histological analyses, the percentage of staining was calculated as the number of follicles positive for the selected staining/number of follicles counted × 100.
Statistical analysis
Statistical analyses were performed on 11 distinct primary experimental outcomes, including (1) follicle morphology (percentage of MNFs); follicle stages (percentage of follicles at (2) primordial, (3) primary, (4) secondary and (5) antral stage); (6) follicle functionality (percentage of AMH-positive follicles); (7) proliferation rate (percentage of Ki67-positive follicles; (8) apoptosis rate (percentage of caspase-3-positive follicles); (9) fibrosis (percentage of the ovary area covered with fibrosis); (10) follicle density (number of follicles/area); and (11) number of CD31-positive vessels/area.
For each outcome, the impact of the cryopreservation method (slow-freezing or vitrification) and the surgical technique (grafting to an FDB or HB site) was assessed using a mixed effect linear regression model [32]. In this model, the “monkey effect” was assumed to follow normal distribution and was therefore defined as a random effect, while surgery/cryopreservation was taken as a fixed effect. Given our study design, the surgery/cryopreservation variable had five distinct categories, including fresh tissue used as a reference, slow-frozen freshly decorticated bed (SF-FDB), slow-frozen healed bed (SF-HB), vitrified freshly decorticated bed (V-FDB), and vitrified healed bed (V-HB).
Because the first nine outcomes were expressed as a percentage, these outcomes were transformed using the arcsine square root transformation in order to meet the assumptions of the statistical models (i.e., residuals with normal distribution and homogeneity of variance). For the last two outcomes (follicle density and number of CD31-positive vessels), log transformation was applied.
Since multiple outcomes (n = 11) were analyzed in this study, the Benjamini-Hochberg correction method was used to correct p values (convert into q values) and maintain a false discovery rate of 0.20. Differences were considered significant when q values were <0.20. All statistical analyses were performed with R statistical software (version 3.1.2, R Statistical Computing, Vienna).
Results
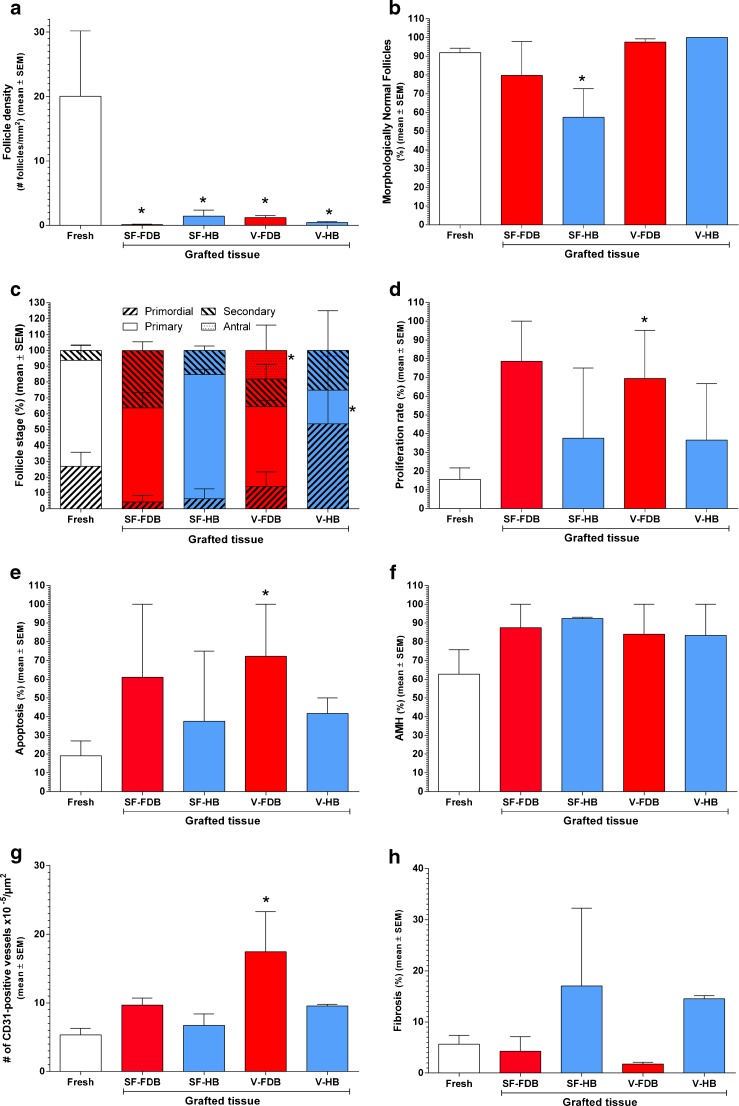
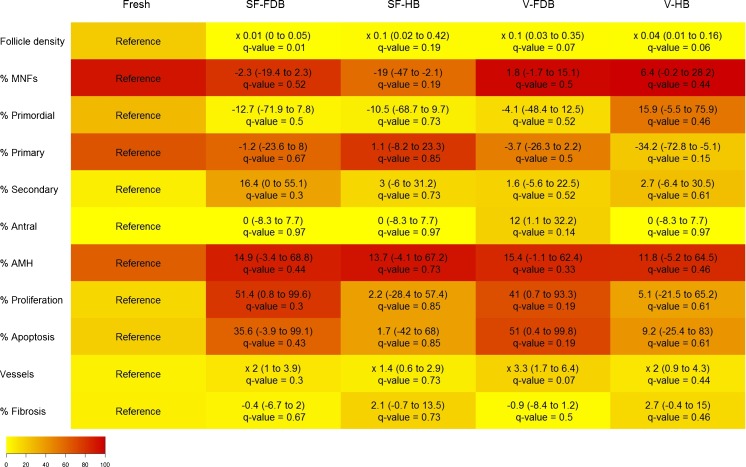
The impact of surgery and cryopreservation techniques on all outcomes is reported in Figs. 2 and 3. Real values (absolute numbers) are depicted in Fig. 2 by means of graphs for each outcome. In Fig. 3 (heatmap), the effect of the different treatments (SF-HB, SF-FDB, V-HB, and V-FDB) is expressed as an increased or decreased value compared to the reference group (fresh tissue).
Fig. 2.
Impact of the cryopreservation technique and vascular bed on different ovary and follicle characteristics after grafting. Follicle density (a), morphologically normal follicles (MNFs; b), follicle stage (c), proliferation rate (d), apoptosis rate (e), follicle functionality (expressed as a percentage of AMH-positive follicles; f), vascularization (expressed as a percentage of CD31-positive vessels ×105/μm2; g), and fibrosis (expressed as a percentage of area stained with Masson’s trichrome; h) are shown in the figure. Fresh ovarian tissue (fixed immediately, open bar), slow-frozen (SF) and grafted ovarian tissue, and vitrified (V) and grafted ovarian tissue were used. SF and V ovarian tissue were grafted to a freshly decorticated bed (FDB, red bars) or healed bed (HB, blue bars). Mean ± SEM are depicted on the graph. Differences were considered significant when q values were <0.2. Asterisk vs fresh tissue: q < 0.2
Fig. 3.
Impact of the cryopreservation technique and vascular bed on different ovary and follicle characteristics after grafting (heatmap). Increased (positive values) or decreased (negatives values) effects of different treatments applied to ovarian tissue compared to the fresh tissue used as a reference are shown on the graph. Parameters studied were follicle density, morphologically normal follicles (MNFs), follicle stage, proliferation rate, apoptosis rate, follicle functionality (expressed as a percentage of AMH-positive follicles), vascularization (expressed as a percentage of CD31-positive vessels ×105/μm2), and fibrosis (expressed as a percentage of area stained with Masson’s trichrome). Fresh ovarian tissue (fixed immediately, fresh), slow-frozen (SF) and grafted ovarian tissue, and vitrified (V) and grafted ovarian tissue were investigated by transplanting to a freshly decorticated bed (FDB) or healed bed (HB). The cell color intensity (more reddish or more yellowish) reflects the average value of each parameter in each group. 95 % CI and q values are indicated on the graph. Differences were considered significant when q values were <0.20
Graft recovery rate and macroscopic aspect
After 6 months of transplantation, all graft specimens (100 %) were easily recovered from the five cynomolgus monkeys and some vessels were found connecting the grafts to surrounding tissue. Grafts from two animals contained a visible corpus luteum on their surface, while another showed a visible follicle. Seven grafts appeared whitish and somewhat shrunken.
Follicle density
Compared to fresh tissue, all the treatments provoked a significant decrease in follicle density: 0.01× for SF-FDB (q = 0.01), 0.1× for SF-HB (q = 0.19), 0.1× for V-FDB (q = 0.07), and 0.04× for V-HB (q = 0.06) (Fig. 3).
Follicle quality
Compared to fresh tissue, a significant decrease of 19 % was observed in the proportion of MNFs in the SF-HB group (q = 0.19). Non-significant differences were also seen in the SF-FDB (−2.3 %, q = 0.52), V-FDB (1.8 %, q = 0.5), and V-HB (6.4 %, q = 0.44) groups compared to their respective fresh ovarian tissue.
Follicle stage
Primordial follicles
Compared to fresh tissue, no significant impact was observed on the percentage of primordial follicles in SF-FDB (−12.7 %, q = 0.5), SF-HB (−10.5 %, q = 0.73), V-FDB (−4.1 %, q = 0.52), or V-HB (15.9 %, q = 0.46) grafted ovarian tissue.
Primary follicles
Compared to fresh tissue, SF-FDB, SF-HB, and V-FDB treatments had no significant effect on the percentage of primary follicles (−1.2 %, q = 0.67; 1.1 %, q = 0.85; and −3.7 %, q = 0.5, respectively). However, V-HB showed a significant decrease of 34.2 % in the percentage of primary follicles (q = 0.15).
Secondary follicles
Compared to fresh tissue, a non-significant increase was observed in the percentage of secondary follicles in all four groups after grafting: SF-FDB (16.4 %, q = 0.3), SF-HB (3 %, q = 0.73), V-FDB (1.6 %, q = 0.52), and V-HB (2.7 %, q = 0.61).
Antral follicles
Compared to fresh tissue, a significant increase of 12 % was found in the percentage of antral follicles with V-FDB treatment (q = 0.14). No significant differences were observed in the SF-FDB, SF-HB, and V-HB grafted tissue groups (0 %, q = 0.97 in all three).
Follicle proliferation
Compared to fresh tissue, a significant increase was observed in the follicle proliferation rate in V-FDB grafted ovarian tissue (41 %, q = 0.19), but not in the other groups: SF-FDB (51.4 %, q = 0.3), SF-HB (2.2 %, q = 0.85), or V-HB (5.1 %, q = 0.61).
Follicle apoptosis
Compared to fresh tissue, an increased apoptosis rate was encountered in all four groups, but was only significant in V-FDB grafted ovarian tissue (51 %, q = 0.19) and not in the other groups: SF-FDB (35.6 %, q = 0.439), SF-HB (1.7 %, q = 0.85), and V-HB (9.2 %, q = 0.61).
Follicle functionality
Positive staining for AMH was observed at all follicle stages, namely primordial, primary, secondary, and antral. Weak and nonspecific AMH staining was found in oocytes, while the theca layer remained negative.
Compared to fresh tissue, all the treatments produced a non-significant increase in the percentage of AMH-positive follicles in SF-FDB (14.9 %, q = 0.44), SF-HB (13.7 %, q = 0.73), V-FDB (15.4 %, q = 0.33), and V-HB (11.8 %, q = 0.46) grafted ovarian tissue.
Vascularization
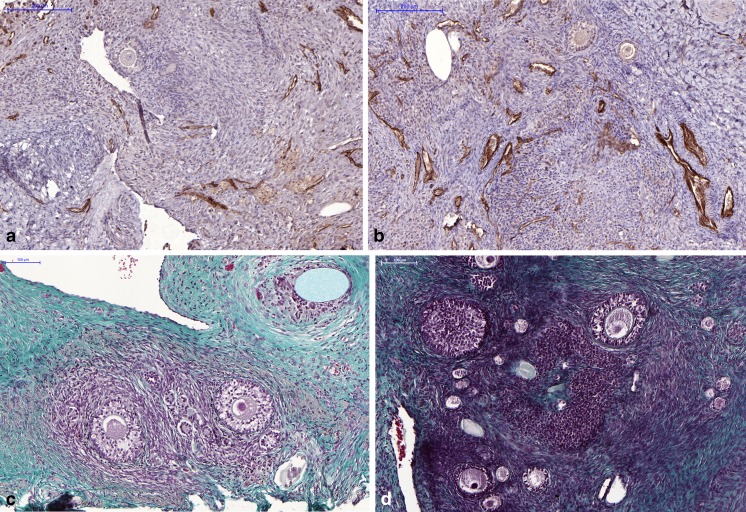
Compared to fresh tissue, V-FDB grafted ovarian tissue showed a significant increase in the number of CD31-positive vessels per area (3.3×, q = 0.07), but the increase was not significant in SF-FDB (2×, q = 0.3), SF-HB (1.4×, q = 0.73), or V-HB (2×, q = 0.44) grafted ovarian tissue (Fig. 4a, b).
Fig. 4.
Vascularization and fibrosis of ovarian tissue. a, b CD31 immunostaining. CD31 immunostaining in fresh ovarian tissue (a) and vitrified tissue grafted to a freshly decorticated vascular bed (b), showing more vessels in the latter group. The scale bar represents 200 μm (a, b). c, d Masson’s trichrome staining. Histological appearance of vitrified ovarian tissue after Masson’s trichrome staining to detect fibrotic areas, showing a higher percentage of fibrosis on a healed bed (c) than a freshly decorticated bed (d). The scale bar represents 100 μm (c, d)
Fibrosis
Compared to fresh tissue, SF-FDB, and V-FDB treatments had no effect on the percentage of fibrosis (−0.4 %, q = 0.67; −0.9 %, q = 0.5, respectively), while SF-HB and V-HB produced a non-significant increase (2.1 %, q = 0.73; 2.7 %, q = 0.46, respectively) (Fig. 4c, d).
Discussion
At present, the standard procedure for cryopreservation of ovarian tissue involves the slow-freezing technique, and numerous live births have been achieved using this method [33]. However, two live births were recently reported after vitrification, warming, and in vitro culture (2 days) of ovarian strips cut into small cubes and using Akt pathway stimulators [4, 5], so more research is needed to compare the influence of these two cryopreservation techniques after grafting. Moreover, it is uncertain whether it is possible to improve the ovarian tissue grafting site prior to transplantation. For this reason, a non-human primate animal model was used to investigate the impact of the two cryopreservation techniques, as well as the effect of grafting site or vascular bed preparation, on ovarian viability and functionality after long-term grafting. The vascular bed has previously been suggested to play a role [2], but, to the best of our knowledge, this is the first time a study has been designed to compare the effect of both the cryopreservation technique and vascular bed preparation on the survival and growth of ovarian tissue after grafting.
Statistical analysis in this study applied a mixed effect linear regression model that provides estimated values, allowing an increase or decrease in effect with each treatment to be detected and compared to a reference, in this case, fresh ovarian tissue fixed immediately after ovariectomy. We were therefore able to learn if the treatment applied to the ovarian tissue, namely slow-freezing or vitrification, and the surgical preparation of the grafting site or vascular bed before transplantation, namely a healed or freshly decorticated bed, had any impact on the analyzed parameters, always compared to fresh tissue (Fig. 3).
Our study showed that follicles can survive and grow after cryopreservation of ovarian tissue by either slow-freezing or vitrification, followed by long-term autografting. Moreover, we demonstrated that the best strategy involves grafting vitrified ovarian tissue to a freshly decorticated bed as a grafting site. Follicle survival and growth are evidenced by a number of factors. First, follicle survival is demonstrated by follicle density. Compared to fresh tissue, follicle density was significantly lower after all the treatments (Figs. 2a and 3), but this is to be expected, as it is well known that the follicle reserve decreases after ovarian tissue transplantation [34–36]. This is mainly due to the fact that grafted tissue is subjected to ischemia-reperfusion [12, 22] and needs to be revascularized as soon as possible in order to be kept alive. Vascularization was shown to begin 5 days after grafting. In this study, vascularization was evaluated by staining the CD31 antigen, and we demonstrated that vitrifying the tissue and grafting it to a freshly decorticated bed (V-FDB) resulted in a significant increase in the number of vessels (3.3 times more) compared to fresh tissue (Figs. 2g, 3, and 4a, b). This increase in vascularization could have been due to the slight trauma induced during decortication of the remaining cortex in the FDB group. Increasing vascularization in grafted tissue is important, since it has been found to enhance primordial follicle survival, not only in mice [37] but also in human ovarian tissue [38].
Second, follicle survival can be appraised by the percentage of MNFs obtained after grafting (Fig. 2b and 3). Assessment of SF-FDB, V-FDB, and V-HB grafted ovarian tissue revealed non-significant differences in MNFs compared to fresh tissue, indicating that ovarian tissues treated by these procedures do not differ greatly from fresh tissue. However, significantly fewer MNFs were obtained after SF-HB.
Third, follicle survival can also be evaluated by follicle growth after grafting. Follicle growth is demonstrated by the presence of follicles at all stages of development (primordial, primary, secondary, and antral) (Fig. 2c). Ischemic injury occurring directly after transplantation without vascular anastomosis is well known to cause dramatic follicle depletion, known as the “burn-out effect.” A large number of primordial follicles are lost after xenografting cryopreserved human ovarian tissue to mice [34]. In the present study, a significant decrease was observed in the percentage of primary follicles in V-HB grafted tissue compared to fresh tissue, and a significant increase in the percentage of antral follicles in V-FDB grafted tissue (Fig. 3). Our data on follicle survival are consistent with the study of Suzuki et al. [39], in which oocytes were retrieved after autotransplantation and 5 months of grafting of vitrified ovarian cortex in cynomolgus monkeys. Although we did not evaluate the estrous cycle of each monkey, we were careful to use animals of the same age.
Another important sign of follicle growth and functionality after grafting is follicle proliferation. In this study, a huge increase in the proliferation rate was observed after grafting to the FDB (Figs. 2d and 3). Decorticating the hilum at the moment of grafting can be useful to induce a small degree of local trauma and generate local inflammation, facilitating fibrin deposition and improving ovarian tissue attachment to the vascular bed. The present study confirms the importance of vascular bed preparation before grafting [2]. Indeed, we showed that FDB treatment induces more extensive vascularization in grafted tissue than does HB treatment, reaching statistical significance in the V-FDB group (Figs. 2g and 3). This is consistent with the significant increase in the proliferation rate and percentage of antral follicles observed in V-FDB grafted tissue.
In a study performed in baboons [21], it was demonstrated that the proliferation rate of fresh tissue was 16 %, compared to 21 % in vitrified-warmed tissue after grafting to an FDB site. In the present study, the proliferation rate was found to be around 15 % in fresh ovarian tissue (Fig. 2d), consistent with the baboon study. However, the proliferation rate in ovarian tissue vitrified and grafted to the FDB was around 70 %, hence much higher than in the baboon study. Considering that the surgical and vitrification techniques were the same, we can account for this discrepancy by interspecies variability.
The follicle apoptosis rate was higher in all grafted tissue compared to fresh control tissue, but only significant in the V-FDB group (Figs. 2e and 3). This high rate can be explained by our strict definition of apoptosis, with follicles considered apoptotic when at least one GC was positive. This significant increase in the apoptosis rate in the V-FDB group may have been due to the fact that this group had a significantly higher proportion of antral follicles (Figs. 2c and 3) containing more GCs than other stages. The probability of finding apoptotic GCs in antral follicles is indeed higher. However, because of the small number of antral follicles found in all the other groups (n = 0 or 1), this would not have changed the final conclusion of the study. Since follicle density was very low and quite similar in all the grafted groups (Figs. 2a and 3), increased follicle apoptosis in the V-FDB group was counteracted by the high follicle proliferation rate also seen in this group.
Follicle functionality is indicated by the production of AMH in follicles. In this study, the same increase was observed in the percentage of positive follicles after all the treatments (Figs. 2f and 3), demonstrating that follicles are functional after long-term autografting. These data are consistent with previous results showing ungrafted fresh tissue to contain around 60 % of follicles positive for AMH, increasing to around 80 % after slow-freezing of human ovarian tissue grafted to mice [40].
Fibrosis is a sign of cell death and its subsequent replacement by fibrous connective tissue. We therefore evaluated fibrosis in grafted ovarian tissue. No difference was found in the percentage of fibrosis after grafting any of the tissues, compared to fresh tissue (Figs. 2h and 3). However, the percentage of fibrosis was very low (less than 5 %) after grafting to an FDB site, but over 15 % after grafting to an HB site, showing that decorticating the remaining ovary on the day of grafting reduces the percentage of fibrosis in transplanted ovarian tissue (Fig. 4c, d).
In conclusion, we demonstrated in the present study that both slow-freezing/thawing and vitrifying/warming combined with long-term autografting of ovarian tissue can be easily performed in cynomolgus monkeys allowing follicular development. Indeed, follicles were detected at all stages of development (primordial, primary, secondary, and antral). Moreover, we noted a tendency towards more MNFs after vitrification than after slow-freezing. Concerning the vascular bed, our hypothesis was that an FDB site would be more “receptive” to grafting than HB. It was previously suggested that prior preparation of the grafting site could have an impact on graft survival [2], but two different procedures have never before been tested in the same study. Although some of the comparisons were non-significant, possibly due to small sample size, we demonstrated an increase in the proliferation rate in ovarian tissue grafted to the FDB (non-significant increase for SF-FDB tissue and significant increase for V-FDB tissue) compared to fresh tissue and the HB site. A lower rate of fibrosis was also noted on the FDB compared to the HB showing that decortications just before transplantation can be beneficial to the survival of ovarian tissue. Finally, we found that the best combination of treatments, cryopreservation, and graft site preparation was vitrification and grafting to the FDB. Indeed, this combination of a significantly higher proliferation rate, very low fibrosis, and 3.3-fold more vessels in V-FDB grafted tissue than in fresh tissue results in a percentage of MNFs comparable to fresh tissue, as well as significant production of antral follicles. Further studies should now be conducted with confirmed pregnancies and live births, before this technique can be introduced into the clinical practice.
Electronic supplementary material
(DOCX 12 kb)
Acknowledgments
The authors thank Maria Dolores Gonzalez and Olivier Van Kerk for their technical support and Mira Hryniuk for revising the English language of the manuscript. This work was supported by grants from the Fonds National de la Recherche Scientifique de Belgique (5/4/150/5, W.0056.15 and T.0077.14), Fonds Spéciaux de Recherche, Fondation Saint Luc, Foundation Against Cancer, and donations from Mr. Pietro Ferrero, Baron Frère, and Viscount Philippe de Spoelberch.
Compliance with ethical standards
ᅟ
Ethical approval
Approval for this study was obtained from the Animal Ethics Review Board of the Université Catholique de Louvain (2011/UCL/MD/012). All procedures were conducted according to guidelines established by the abovementioned committee and according to Belgian (AR 29, May 2013) and European (Directive 2010/63/EU) legislation on the care and use of laboratory animals. This manuscript does not involve any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interests.
Research involving human participants
Not applicable
Research involving animals
Yes
Informed consent
Not applicable
Footnotes
Capsule Vitrification associated with freshly decorticated vascular bed preparation appears to be the best combination to obtain functional autografted ovarian tissue.
M. M. Dolmans and M. M. Binda contributed equally to this work.
References
- 1.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez SM, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–49. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 3.Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384:1311–9. doi: 10.1016/S0140-6736(14)61261-7. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–9. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 6.Burmeister L, Kovacs GT, Osianlis T. First Australian pregnancy after ovarian tissue cryopreservation and subsequent autotransplantation. Med J Aust. 2013;198:158–9. doi: 10.5694/mja12.11768. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Wallberg KA, Karlstrom PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94:324–8. doi: 10.1111/aogs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanbo T, Greggains G, Storeng R, Busund B, Langebrekke A, Fedorcsak P. Autotransplantation of cryopreserved ovarian tissue after treatment for malignant disease—the first Norwegian results. Acta Obstet Gynecol Scand. 2015 doi: 10.1111/aogs.12700. [DOI] [PubMed] [Google Scholar]
- 9.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet. 2015;385:506–7. doi: 10.1016/S0140-6736(15)60198-2. [DOI] [PubMed] [Google Scholar]
- 10.Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van LA, et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009;24:2778–87. doi: 10.1093/humrep/dep289. [DOI] [PubMed] [Google Scholar]
- 11.Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet. 2012;29:489–93. doi: 10.1007/s10815-012-9757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Eyck AS, Bouzin C, Feron O, Romeu L, Van LA, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–85. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Camboni A, Martinez-Madrid B, Dolmans MM, Amorim CA, Nottola SA, Donnez J, et al. Preservation of fertility in young cancer patients: contribution of transmission electron microscopy. Reprod Biomed Online. 2008;17:136–50. doi: 10.1016/S1472-6483(10)60303-3. [DOI] [PubMed] [Google Scholar]
- 14.Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27:1606–12. doi: 10.1093/humrep/des088. [DOI] [PubMed] [Google Scholar]
- 15.Gandolfi F, Paffoni A, Papasso BE, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85(Suppl 1):1150–6. doi: 10.1016/j.fertnstert.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 17.Amorim CA, Curaba M, Van LA, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23:160–86. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Herraiz S, Novella-Maestre E, Rodriguez B, Diaz C, Sanchez-Serrano M, Mirabet V, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;101:775–84. doi: 10.1016/j.fertnstert.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Amorim CA, David A, Van LA, Dolmans MM, Donnez J. Vitrification of human ovarian tissue: effect of different solutions and procedures. Fertil Steril. 2011;95:1094–7. doi: 10.1016/j.fertnstert.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98:1291–8. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 21.Amorim CA, Jacobs S, Devireddy RV, Van LA, Vanacker J, Jaeger J, et al. Successful vitrification and autografting of baboon (Papio anubis) ovarian tissue. Hum Reprod. 2013;28:2146–56. doi: 10.1093/humrep/det103. [DOI] [PubMed] [Google Scholar]
- 22.Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van LA, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–81. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, et al. Why primate models matter. Am J Primatol. 2014;76:801–27. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinbauer GF, Niehoff M, Niehaus M, Srivastav S, Fuchs A, Van EE, et al. Physiology and endocrinology of the ovarian cycle in macaques. Toxicol Pathol. 2008;36:7S–23S. doi: 10.1177/0192623308327412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fathi R, Valojerdi MR, Eimani H, Hasani F, Yazdi PE, Ajdari Z, et al. Sheep ovarian tissue vitrification by two different dehydration protocols and needle immersing methods. Cryo Lett. 2011;32:51–6. [PubMed] [Google Scholar]
- 26.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 27.Donnez J, Martinez-Madrid B, Jadoul P, Van LA, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–35. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 28.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–7. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 29.Amorim CA, Lucci CM, Rodrigues AP, Carvalho FC, Figueiredo JR, Rondina D, et al. Quantitative and qualitative analysis of the effectiveness of a mechanical method for the isolation of preantral follicles from ovine ovaries. Theriogenology. 2000;53:1251–62. doi: 10.1016/S0093-691X(00)00269-7. [DOI] [PubMed] [Google Scholar]
- 30.Dath C, Van Eyck AS, Dolmans MM, Romeu L, Delle VL, Donnez J, et al. Xenotransplantation of human ovarian tissue to nude mice: comparison between four grafting sites. Hum Reprod. 2010;25:1734–43. doi: 10.1093/humrep/deq131. [DOI] [PubMed] [Google Scholar]
- 31.Gougeon A, Busso D. Morphologic and functional determinants of primordial and primary follicles in the monkey ovary. Mol Cell Endocrinol. 2000;163:33–42. doi: 10.1016/S0303-7207(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 32.Brown H, Prescott R. Applied mixed models in medicine. West Sussex: Willey; 2006. pp. 1–455. [Google Scholar]
- 33.Donnez J, Dolmans MM. Ovarian tissue freezing: current status. Curr Opin Obstet Gynecol. 2015;27:222–30. doi: 10.1097/GCO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 34.Dolmans MM, Martinez-Madrid B, Gadisseux E, Guiot Y, Yuan WY, Torre A, et al. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134:253–62. doi: 10.1530/REP-07-0131. [DOI] [PubMed] [Google Scholar]
- 35.Amorim CA, David A, Dolmans MM, Camboni A, Donnez J, Van LA. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J Assist Reprod Genet. 2011;28:1157–65. doi: 10.1007/s10815-011-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 37.Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng A. 2011;17:3095–104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia X, Yin T, Yan J, Yan L, Jin C, Lu C et al. Mesenchymal stem cells enhance angiogenesis and follicle survival in human cryopreserved ovarian cortex transplantation. Cell Transplant 2014. [DOI] [PubMed]
- 39.Suzuki N, Hashimoto S, Igarashi S, Takae S, Yamanaka M, Yamochi T, et al. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod. 2012;27:2420–9. doi: 10.1093/humrep/des178. [DOI] [PubMed] [Google Scholar]
- 40.David A, Van LA, Gilliaux S, Dolmans MM, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum Reprod. 2012;27:1088–95. doi: 10.1093/humrep/des013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb)