Abstract
Purpose
The study aims to assess the protective effects of dimethyl sulfoxide (DMSO)-free solution based on trehalose on the cryopreservation of a whole sheep ovary and evaluate its use as an efficient cryoprotectant.
Method
Twenty-one ovaries collected from 6- to 8-month-old non-pregnant female sheep were randomly distributed into three groups, namely, a fresh group, a DMSO-free group, and a DMSO group. The morphology, cell apoptosis (by hematoxylin and eosin (HE) staining and terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) assay), and mRNA transcript of Bcl-2-associated X protein (BAX) and cold inducible RNA-binding protein (CIRP) (by real-time PCR) of the thawed sheep ovaries and fresh controls were tested to establish a criterion for appraising the results of the cryopreservation.
Results
(i) The histological assessment indicated that the structure of the DMSO-free ovaries remained largely intact and comparable to those of the fresh control groups; whereas, significant damage was observed in the ovaries of the DMSO group (P < 0.05). (ii) The TUNEL assay and mRNA transcript of the BAX assessment showed that the apoptosis parameter in the fresh group was the lowest among all the groups (P < 0.05), and the parameter in the DMSO-free group was significantly lower than that in the DMSO group (P < 0.05). (iii) The level of the CIRP transcripts increased the most in the DMSO-free group followed by the DMSO group and the fresh control group (P < 0.05).
Conclusions
These results indicate that a DMSO-free cryoprotectant solution, especially a trehalose cryoprotectant, is an efficient cryoprotectant and has a beneficial effect on the cryopreservation of whole sheep ovaries.
Keywords: Whole ovary, Sheep, Cryopreservation, Trehalose, Fertility preservation
Introduction
Early diagnosis and multiple treatments improve the long-term survival rates of young women with malignant ovarian cancer [1]. However, cancer therapies such as radial and chemical therapy cause severe injuries to the ovarian reserve, which may subsequently lead to ovarian failure and infertility [2]. Maintaining their fertility is of the utmost importance to many patients diagnosed with cancer during their child-bearing years, and research on the cryopreservation of ovarian tissue has been proved to have great potential for clinical applications in transplantation therapy, making it a promising area for female infertility treatment [3]. There are two options available to achieve such cryopreservation: the preservation of an ovarian cortical fragment or the preservation of the whole ovary. The first technique is most commonly used in clinical applications due to its effective cryopreservation [3, 4]; however, this method has the damaging side effect of follicle loss, caused by ischemia, most of which occurs during the transplantation process [5, 6]. Therefore, whole ovary cryopreservation along with vascular reanastomosis, which can minimize ischemia damage to the tissue and prolong graft longevity, has been proposed as a promising alternative to the cryopreservation of only an ovarian cortical fragment. In fact, the transplantation of frozen/thawed whole ovaries has been achieved in animal experiments [7, 8].
As with the cryopreservation of an ovarian cortical piece, dimethyl sulfoxide (DMSO), fetal bovine serum (FBS), and glucose are widely used for the cryopreservation of the whole ovary. Among these, DMSO is extensively applied as a cryoprotectant in most research on the cryopreservation of intact ovaries because of its ability to protect during the freezing procedure. However, it is unsuitable for clinical application and should be removed prior to transplantation because of certain detrimental effects, such as neurological toxicity [9] and gene mutation [10] associated with DMSO when it is exposed to temperature change. Trehalose, a small disaccharide molecule of glucose, is considered safe to consume [11] and possesses an exceptional ability to stabilize the biomembrane and preserve cells and tissues during the freeze-thaw procedures [12]. Subsequently, a number of studies have shown that trehalose can be used as an excellent alternative cryoprotectant in the cryopreservation of red blood and germ cells [13–15]. Another advantage of trehalose, as previously demonstrated, is its ability to inhibit the apoptosis level [16, 17]. Because of the aforementioned advantages, trehalose has become a research hot spot in the cryopreservation field in recent years. We previously applied trehalose and FBS as the main cryoprotectant, supplemented with cholesterol and lecithin, on the cryopreservation of mesenchymal stem cells and obtained a significant reduction in apoptosis (data not shown). However, to the best of our knowledge, few studies have yet investigated its ability to protect cells from apoptosis in whole organ cryopreservation, like the sheep’s ovary. Therefore, in this study, we investigate whether trehalose has a beneficial effect on the morphology of thawed intact sheep ovaries by programmed freezing, with the intention of discovering an effective cryopreservation solution for a whole sheep’s ovary.
Materials and methods
Sample preparation
Unless otherwise indicated, chemicals were purchased from Sigma-Aldrich. Ovaries, along with the ovarian pedicle, were collected from sheep (small-tailed Han sheep), aged 6 to 12 months at the local slaughterhouse. All procedures were approved by the Department of Medical Ethic Committee of Shandong University affiliated Qilu Hospital. The ovarian vessel was identified at the part where it meets the dorsal artery, and the dorsal artery was removed to keep the opening of the ovarian artery on the wall of this artery. Ovaries and vessels were transported to the laboratory in a cold (0–4 °C) 0.9 % saline solution with 1 IU/L penicillin G, 1 IU/L gentamicin, 0.25 mg/L amphotericin B, and 0.01 IU/L heparin to minimize ischemia and contamination.
Ovaries were obtained and randomly assigned to fresh control, DMSO-free, and DMSO groups. Each experimental group contained seven ovaries.
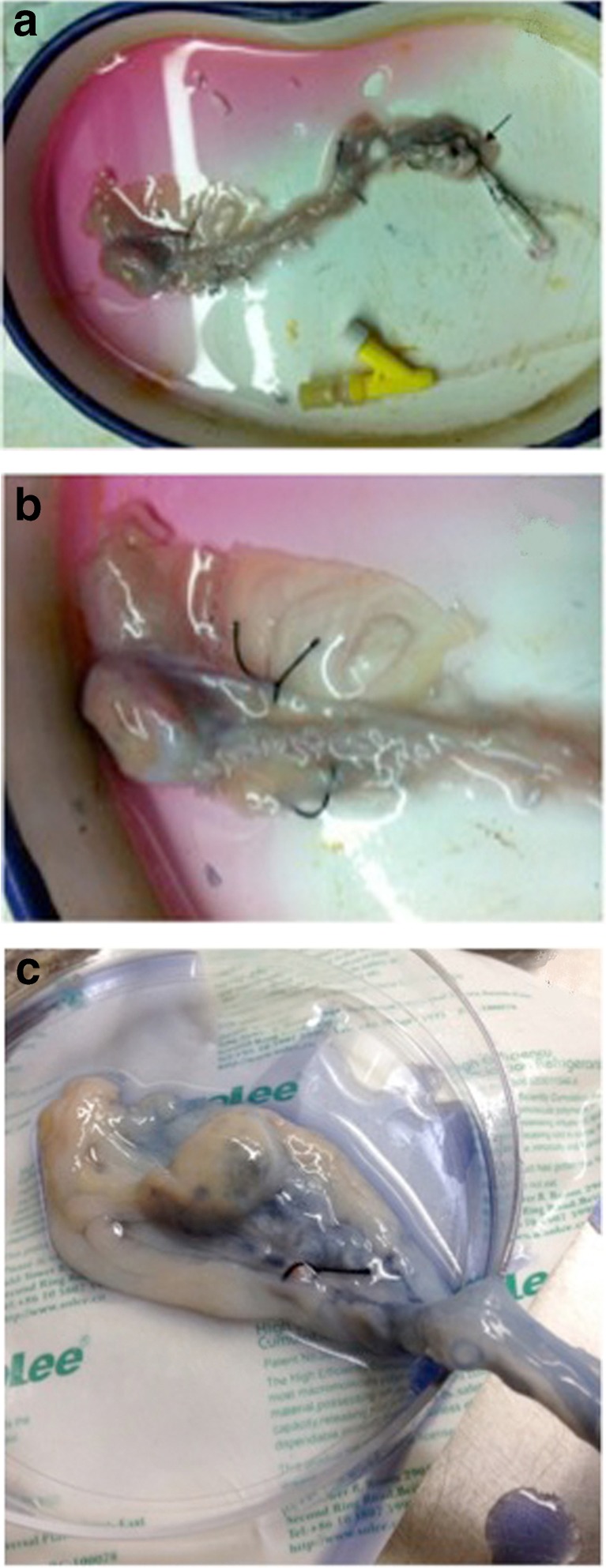
Upon arrival at the laboratory, the ovarian arteries were cannulated with a 24-G catheter (BD Insyte; America) and then secured with nylon sutures (6–0 Prolene; Ethicon, America). Artery branches (usually two per ovarian pedicle) were ligated at their origin to prevent any leakage [18] and then connected to our newly designed device (described below), which had been precooled to 4 °C (see in Fig. 1).
Fig. 1.
a, b, c Cannulation and ligation of the ovarian artery
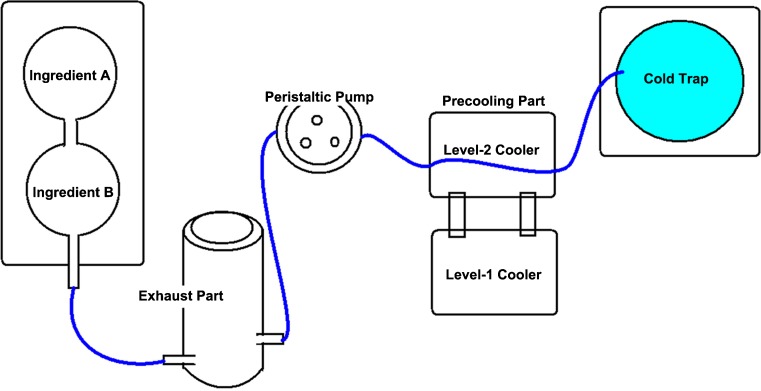
Perfusion apparatus
The concentration gradient cryoperfusion apparatus, used for the perfusion of the whole ovary, is newly designed by our research team (Fig. 2). The apparatus mainly consists of a concentration gradient-mixed instrument, an automatic filtration sterilization exhaust part, a programmed cooling control part, and a cold trap. This is a stable programmed perfusion apparatus for tissues and organs and has a variety of computer-controlled functions, described as follows: The cryoprotectant is gradient mixed by the concentration gradient-mixing instrument. The perfusion speed is controlled by the peristaltic pump of the apparatus. During perfusion, the semiconductor refrigeration module of the apparatus maintains the low temperature of the cryoprotectant while, at the same time, preventing cell and tissue damage that results from overheating the cryoprotectant. The temperature of the perfused cryoprotectant is kept between −20 and 4 °C.
Fig. 2.
The concentration gradient cryoperfusion apparatus
Perfusion of cryoprotectant solution
The samples allocated to the DMSO-free group were perfused via the ovarian artery with a cryoprotectant solution containing Roswell Park Memorial Institute (RMPI)-1640 medium (Hyclone, America), 10 % fetal bovine serum (v/v; Hyclone, America) 200 mmol/L trehalose (Sinopharm, China), 200 mg/L cholesterol (Amresco, America), 30 % (v/v) PVP 40, and lecithin-saturated solution. Samples from the DMSO group were perfused with a conventional cryoprotectant solution containing RMPI-1640 medium with 10 % DMSO and 10 % fetal bovine serum. Each group was perfused for 30 min at a flow rate of 1.3 mL/min. Then, each ovary was placed into a cryobag (Miltenyi, Germany) containing 30 mL of the same solution.
Freezing
Conventional slow-freezing was performed in a CryoMed Controlled Rate Freezer (Thermo scientific, America) with the following program (ControledRate Freezing Version1.0, America): (i) equilibrium at 4 °C,(ii) from 4 to 0 °C using a cooling rate of 1 °C/min and maintaining at 0 °C for 10 min, (iii) from 0 to −18 °C at 2 °C/min and maintaining at −18 °C for 15 min, (iv) from −18 to −45 °C at 2 °C/min and maintaining at −45 °C for 15 min, (v) from −45 to −90 °C at 5 °C/min and maintaining at 90 °C for 5 min, (vi) and then, plunging the ovary into liquid nitrogen for at least 1 month.
Thawing
Thawing was performed in two steps. (i) Tubes removed from the liquid nitrogen were plunged into a 37 °C water bath and gently shaken until the contents turned into an ice-water mixture. Then, they were transferred into a petri dish containing 0.9 % saline solution. (ii) RMPI-1640 medium supplemented with 10 % fetal bovine serum was perfused into the whole ovaries through the ovarian artery by the perfusion apparatus which was precooled to 4 °C at 1.5 mL/min for a total of 30 min.
Morphological analysis
The ovaries of the thawed and fresh control samples were fixed with 10 % formaldehyde, embedded in paraffin, and cut into 4-μm-thick sections, which were either stained with hematoxylin and eosin or used for the terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) assay, as described below. Slides were observed under an Olympus IX81 microscope, and pictures were acquired using a DP Controller 1.2.1.108. A minimum of five fields, randomly selected from each sample, were examined for each measure. Primordial follicles were shown as one layer of flattened granulosa cells around the oocyte. The follicles of each sample were further classified either as normal or degenerate. Normal follicles had a complete layer of flattened granulosa cells, oocytes with cytoplasm, and a non-pyknotic nucleus. Abnormal follicles were recognized as follows: pyknotic nucleus, vacuolation in cytoplasm, or granulosa cells detached from the damaged basement membrane. The number of normal and abnormal primordial follicles was counted in each group, and then the percentage of morphologically normal primordial follicles could be calculated. Similarly, the medulla of each sample was examined through the microscope, and five random fields were pictured to represent the stromal cell density, which was quantified by counting nuclei with Image J software (National Institutes of Health) as described below.
TUNEL assay
Apoptotic cells were identified with an In Situ Cell Death Detection Kit, POD (Roche Diagnostics, America) according to the manufacturer’s manual. Formalin-fixed and paraffin-embedded 4-μm-thick tissue slices were dewaxed and rehydrated through a series of decreasing concentrations of ethanol (100–70 %) to water. Endogenous peroxidase activity and non-specific sites were blocked with a solution of 3 % hydrogen peroxide. The slides were then treated with 100 mg/L Proteinase K (Tiangen, China) solution which was dissolved in 10 mM Tris-HCI (pH 8.0) for 30 min at 37 °C. The TUNEL mixture was applied to the sections that were later incubated at 37 °C for 90 min and then washed with phosphate-buffered saline (PBS) three times. The nuclei were then stained with 4′,6-diamidino-2-phenylindole (DAPI). The negative control sections were processed identically except that the labeling enzyme was omitted. The tissue sections were immediately analyzed under a fluorescent microscope. Four different optical fields (×400) were randomly selected and used to calculate the percentage of cells with apoptotic nuclei. The TUNEL-positive cells were calculated as cells per area of ovarian tissue using the image analysis software Image J, as detailed below.
RNA isolation and quantitative real-time PCR
The primers of Bcl-2-associated X protein (BAX), cold inducible RNA-binding protein (CIRP), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed and synthesized by the Shanghai ShengGong Biotechnology Company. The following primers were used: GAPDH: forward 5′-ATCGCTCTCTTTGTCGGAAG-3′, reverse 5′-GCAGAGCATTTATTCCGTCCC3-3′; BAX: forward 5′-GAGTGGCGGCTGAAATGTT-3′; reverse 5′-AAGTCCAAGGCAGTTGATGG-3′; CIRP: forward 5′-ATCGCTCTCTTTGTCGGAAG-3′, reverse 5′-TCTACTCTGCCTGCCTCAAG-3′. The total RNA was extracted from the cortical region of seven ovaries in each group using TRIzol Reagent (Life Technologies, America) following the manufacturer’s guidelines. First-strand cDNA was synthesized by a Realtime PCR Master Mix Kit (TOYOBO, Japan) in a volume of 10 μL. Complementary DNA amplifications were carried out with a Realtime PCR System (Applied Biosystems 7500, America) with a SYBR Green Realtime PCR Master Mix Kit (TOYOBO, Japan) according to the protocol provided by the manufacturer, using the appropriate conditions for BAX- and CIRP-specific primers. A melting curve analysis was used to confirm the amplification specificity. The quantification data was analyzed with 7500 System SDS Software Version 1.4 (Applied Biosystems, America), and the relative target gene expression was normalized on the basis of GAPDH for its stable expression. The results were expressed as an x-fold difference relative to the calibrator.
Image analysis
Pictures were taken with constant exposure parameters so that they could be analyzed with the image analysis software Image J 1.42 q (downloaded from http://rsbweb.nih.gov/ij/index.html). Threshold adjustments were applied to generate a black and white image, which was analyzed to quantify the fluorescent signals. In each case, the marker expression was normalized by DAPI fluorescence.
Statistical analysis
Statistical evaluations were processed using SPSS 19.0 (SPSS Inc, Chicago, IL, USA), and pictures were formed with GraphPad Prism 5.0 (GraphPad Software Inc, San Diego, CA, USA). Cell numbers or TUNEL-positive cells were quantified with the image analysis software, Image J. The data were expressed as mean + standard error and analyzed using a one-way analysis of variance followed by a least significant difference test, and P values of <0.05 were considered to be statistically significant.
Results
Morphological assessment of cryopreservation damage
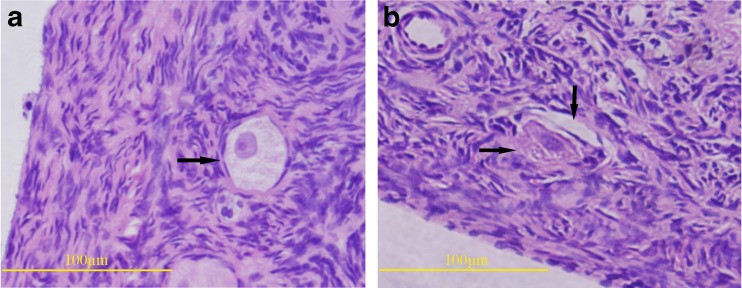
The representative images of normal and abnormal primordial follicles after cryopreservation are shown in Fig. 3, and the number of total primordial follicles and normal primordial follicle counted of each group are recorded in Table 1. The rate of intact follicles in the DMSO-free group (90.29 ± 2.30 %) was significantly higher (P < 0.01) than that in the DMSO group (80.85 ± 2.28 %), and it was no different (P = 0.07) from that in the fresh group (93.46 ± 2.30 %).
Fig. 3.
a, b Representative images of normal and abnormal follicles
Table 1.
Comparison of morphologically normal follicles in each group
| Group | Number of primordial follicles | Number of normal primordial follicles | Percentage of normal follicles (%) |
|---|---|---|---|
| Fresh group | 243 | 227 | 93.46 ± 2.30 % |
| DMSO-free group | 220 | 198 | 90.29 ± 2.30 % |
| DMSO group | 255 | 206 | 80.85 ± 2.28 %**Δ |
**P < 0.01 vs. the control group. ΔP < 0.01 vs. the DMSO-free group
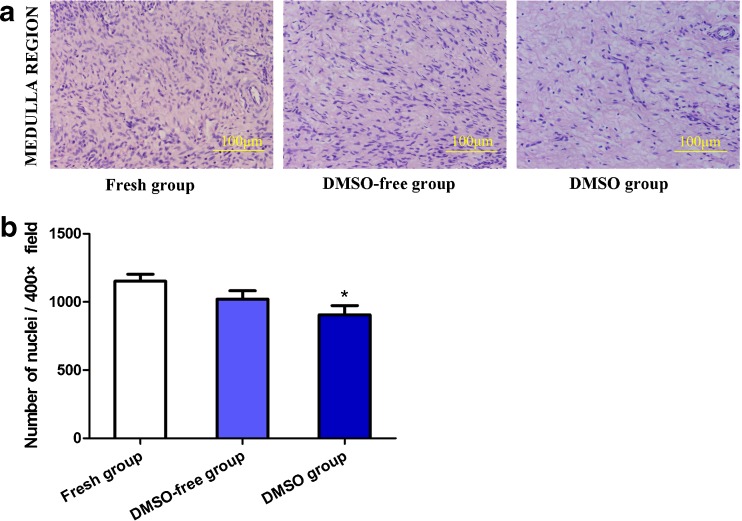
A quantitative assessment of the stromal cell density indicated that ovaries in the DMSO-free group had values (1020.83 ± 278.39 nuclei/×400 field) that were comparable to those in the fresh group (1114.68 ± 184.24 nuclei/×400 field) (P = 0.1410); whereas, a significantly lower value was visible in the DMSO group (906.14 ± 304.78 nuclei/×400 field) (P < 0.05) (Fig. 4).
Fig. 4.
a, b The stromal cell density in different groups
Cell proliferation and apoptosis
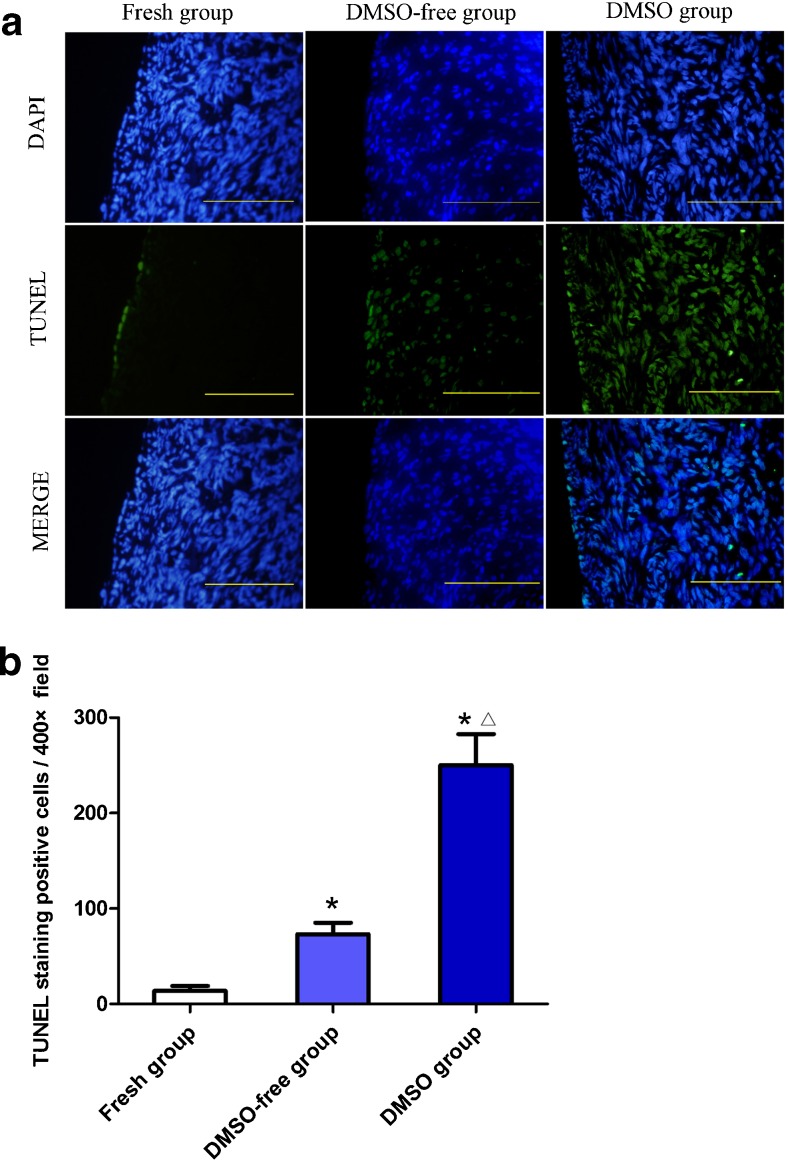
TUNEL-positive cells were detected in all the experimental groups, and the representative images are shown in Fig. 5a. Virtually, very few TUNEL-positive signals were detected in the fresh tissue. Clear signals were mainly discovered in the cortex of ovaries in the frozen/thawed group. The TUNEL assay, analyzed by Image J, showed that the number of positive cells in the fresh group (13.86 ± 13.22/×400 field) was the lowest of all the groups (P < 0.05), and the value in the DMSO-free group (73.14 ± 32.01/×400 field) was significantly lower than that in the DMSO group (250.29 ± 85.90/×400 field) (P < 0.05) (Fig. 5).
Fig. 5.
a, b TUNEL staining in different groups
mRNA level of Bcl-2-associated X protein and cold inducible RNA-binding protein
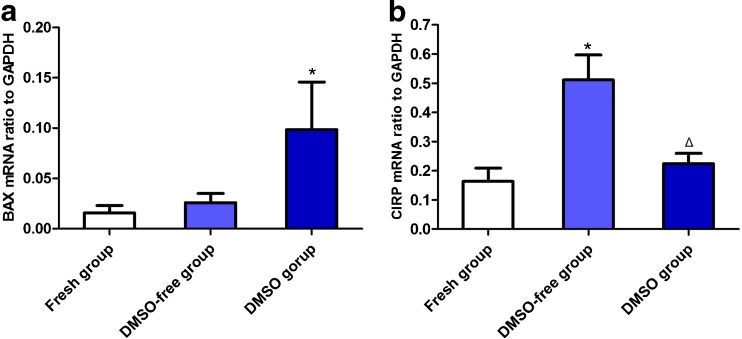
The transcription of BAX, which is another index for apoptosis, detached by the real-time PCR was slightly increased in the DMSO-free groups (0.026 ± 0.024) compared with the fresh group (0.016 ± 0.019), and the value in the DMSO group (0.099 ± 0.125) was the highest, indicating a significant difference (P < 0.05) (Fig. 6a). The effect of the cold stress response after cryopreservation was examined by means of a quantitative analysis of the CIRP transcripts at the end of the warming and cryoprotectant removal. There was a visible difference in the CIRP transcription in different groups, with significantly higher CIRP levels in the DMSO-free group (0.512 ± 0.226) than the DMSO group (0.224 ± 0.093) and the fresh group (0.164 ± 0.121) (P < 0.05) (Fig. 6b).
Fig. 6.
a, b The expression of BAX and CIRP
Discussion
This article describes the first attempt to cryopreserve a whole sheep ovary with a DMSO-free cryoprotectant solution containing trehalose and FBS, supplemented with cholesterol, lecithin, and PVP 40. We demonstrate that this solution can be used in the cryopreservation of an entire sheep’s ovary and yield satisfactory results. Additionally, the concentrated gradient-mixed programmed freezing organ perfusion apparatus, used for perfusion, satisfactorily maintained a consistent temperature and flow rate, effectively preventing cell and tissue damage resulting from wavering cryoprotectant temperatures and minimizing the variance among different groups. However, our observations are very preliminary; the results are limited due to the small number of samples. More work is required, including the functional recovery and assessment of vascular endothelial vitality, to prove or refute the significance of trehalose in the cryopreservation of the whole ovary.
We have chosen sheep ovaries as an alternative to human ovaries because their texture, which contains dense fibrous stroma and relatively high primordial follicle density, is comparable to human ovaries, although they are smaller on average [19].
It is difficult for cryoprotectant to infiltrate into the core of the ovary. In order to obtain better perfusion, we cannulated a catheter into the ovarian artery before the cryopreservation and pumped cryoprotectant through the catheter pathway according to a certain flow rate and time. JM. Zhang et al. [20] reported that a low perfusion pressure and a shorter perfusion period may not ensure the complete saturation of the tissue, causing the formation of intracellular and extracellular ice, physically damaging to the cells; whereas, a high perfusion pressure and a long perfusion period may result in rupturing the vessel and putting cells at risk of excessive chemical toxicity. This research and our preexperiments (data not shown) indicate that the perfusion conditions are crucial for cryopreservation; therefore, we applied newly designed perfusion apparatus in this study, at the speed of 1.3 mL/min for 30 min.
Quite a few attempts have been made to cryopreserve a whole sheep ovary using both slow-cooling and vitrification methodologies. The first cryopreservation of a whole sheep ovary was conducted by slow-cooling [21]. The ovaries were perfused with 10 % fetal calf serum and 1.5 M DMSO in Leibovitz L-15 medium and then cryopreserved by controlled freezing to −140 °C before being plunged into liquid nitrogen. Apoptotic signals, follicles viability, and histology were tested and yielded good results as the cryopreservation of cortical slices. The normal endocrine function of three of 11 sheep was restored, manifested by evaluating the FSH. Imhof et al. [7] also explored cryopreservation in a sheep model. In their study, RPMI-1640 solution containing 1.5 M DMSO and 10 % human albumin worked as a cryoprotectant. The freezing and thawing procedure was similar to the previous report. FSH levels reached normal physiological levels 6 months after transplantation. Two of nine sheep resumed normal ovarian function, and one achieved a spontaneous pregnancy, with the delivery of a healthy lamb. Although many attempts have been made to cryopreserve whole ovaries by slow-freezing techniques, few have been attempted using vitrification. Courbiere B et al. [22] described two vitrification solutions that may be useful for whole ovary cryopreservation. Each ovary was perfused by either VS1 or VS4 in a stepwise increase in a concentration of cryoprotectant. After perfusion, the ovaries were transferred into cryobags and immediately plunged into liquid nitrogen. More primordial follicles were preserved and fewer nuclear anomalies and general follicular anomalies with a VS4 solution containing dimethyl sulfoxide, formamide, and propylene glycol. Some studies compare vitrification versus slow-cooling by comparing parameters such as follicle viability, histological examination and apoptosis assessment, or hormone concentrations in the culture supernatants, but no consensus has yet been reached [23, 24]. Having analyzed the preexperimental data combined in previous studies, we chose slow-cooling in our study, since it enables the precise adjustment of the temperature gradients to achieve an accurate cooling rate through the entire ovary and leads to less ice formation. DMSO has been used as a cryoprotectant in most of these studies, but DMSO has been proved to cause toxic side effects to the human body [25]. Additionally, more DMSO was needed for freezing the whole ovary than just the cortex, which may cause more damage in the perfusion and washing-off steps [26]. Nevertheless, since there was obvious trend, we preferred the use of a trehalose solution to conserve the normal morphology of follicles and stromal cell density and to abate apoptosis, which may lead to necrosis in the subsequent transplantation.
N.K. Jain et al. [27] reported that huge amounts of trehalose were synthesized when cells were exposed to stress, such as cold, warm, or oxidation, and these helped to resist external stimuli. There are some advantages of trehalose in the cryopreservation field. Firstly, as a non-permeable cryoprotectant, trehalose can inhibit rapid changes in osmotic pressure which reduces osmotic shock and swelling. Secondly, trehalose helps to stabilize the structure of the cell membrane, which may prevent ice from forming because the interaction between trehalose/water is much stronger than water/water, and the water network is rearranged [28]. Furthermore, some researchers have shown that trehalose can maintain cell bioactivity, [29] improve DNA integrity [30], and result in higher embryo survival and clinical pregnancy rates at the appropriate concentration during the embryo-warming process [31].
Although all these advantages make trehalose one of the best known cryoprotectants, few studies have applied trehalose to cryopreserve a whole sheep’s ovary. In the current study, trehalose was used combined with cholesterol and lecithin, which helps protect the lipids and protein on the membrane, and PVP, which provides general protection for biological macromolecules. Cell membranes are considered to be a two-dimensional liquid, inside which lipids and protein molecules are diffused. Lipids are crucial for maintaining the structure and function of membranes when exposed to cryopreservation. As previously reported, low-density lipoprotein, such as lecithin and hen egg yolk, is widely used as a cryoprotective agent in freezing semen in order to protect the membrane’s phospholipid integrity against cold shocks [32, 33]. The possible protective mechanism of lecithin is related to the protective layer of lecithin at the surface of spermatozoa membranes against ice crystals [33]. Furthermore, these extenders could substitute some phospholipids on the membrane, thereby decreasing the phase transition temperature [34]. And the amount of cholesterol in a membrane influences its thermotropic behavior [35]. Cholesterol intercalates into the lipophilic core of the membrane, associating with the fatty acyl chains of the phospholipids, and inhibits fatty acids from interacting with other fatty acids when the membrane is cooled [36]. Therefore, cholesterol prevents lipids from undergoing the phase transition to gel state at the lipids’ phase transition temperature, and the membrane remains fluid at temperatures below those that would normally cause a membrane phase transition [37]. In this fluid state, the redistribution of membrane components that cause membrane damage does not occur. Therefore, the combination may help to stabilize the layer around the membrane, the mobility of which is restricted, and could protect it from stressful conditions. There was an obvious trend in the present study that the normal morphology of follicles and stromal cell density were preserved better in the trehalose group than the fresh group.
Another obvious advantage of trehalose is the capacity of anti-apoptosis. Apoptosis could occur in cryopreservation and subsequently transplantation, making anti-apoptotic ability a major factor for optimizing the cryoprotectant. Y.A. Lee [38] suggests that higher proliferation activity and less apoptotic cells are noticed when human spermatogonial stem cells are frozen with the appropriate concentration of trehalose. The BCL-2 family is an essential pathway to regulate cell apoptosis because BCL-2 is an inhibitory factor, while BAX is a promoting factor. Our study has shown a low expression of BAX in the trehalose group, which is consistent with some previous reports. A TUNEL assay can detect the DNA strand breaks of apoptosis cells, which are identified by the label of free 3′-OH termini, and locate the apoptosis cell under a fluorescent microscopy. In the present study, the stained cells through the TUNEL assay were in agreement with the observed transcript levels of BAX by the real-time PCR, which suggests the mechanism used by trehalose to prevent apoptosis is to stabilize the membrane and reduce the permeabilization of the mitochondrial membrane, thereby blocking the signal channel and suppressing the expression of BAX [39].
One of the best characterized responses to thermal stress is the expression of CIRP. CIRP is a highly conserved cold shock protein in the vertebrate, the expression of which is promoted by cold stress; whereas, other proteins are usually suppressed. CIRP has been proven to have the function of inhibiting apoptosis and exerting a protective effect. When exposed to stress or environmental changes, the expression of CIRP, synthesized through IRES translation mechanism, increases concurrently with the increase in the expression of TRX protein. As is well known, TRX is ubiquitous and exerts a cellular protective effect by scavenging the oxygen free radicals. Therefore, through the induction of TRX, CIRP as a stress protein may play an important role in lowering oxidative stress, inhibiting the apoptosis signaling pathway, and help the cells to rapidly adapt to environmental changes [40, 41]. There is another study that suggests that CIRP has a beneficial effect on cells, protecting them from hypothermia by activating the ERK pathway and thus promoting NF-κB activity to suppress apoptosis [42]. In the current study, the expression of CIRP in the trehalose group was higher than in the DMSO group when exposed to the same cold stress, which may indicate that the combination of the trehalose recipe provides a suitable environment for the induction of CIRP. The data of the BAX transcript was also consistent with the results; on the other hand, the results of the BAX transcript support the idea that CIRP suppresses apoptosis. However, a detailed description of this protective effect needs further investigation.
Combining the previous findings with the results in this study, we propose that the usage of a trehalose solution in whole ovary cryopreservation better preserves morphology and opposes the apoptosis and stress that may be caused by the refrigeration progress to its extended preservation. Our research is expected to provide new and direct evidence to further the understanding of the effect of trehalose on refrigerated whole ovaries and facilitate the clinical application of trehalose to the cryopreservation of the whole ovary.
Acknowledgments
The work of this article was done in the Frozen Laboratory of Qilu Hospital of Shandong University.
The authors are grateful to the Shandong Province Science and Technology Research Project (2010GSF10814) and to the National Science Council for financially supporting this study (81370711); the Frozen Laboratory of Qilu Hospital of Shandong University generously provided relevant experimental technology for this study.
Footnotes
Capsule
These results indicate that a DMSO-free and trehalose-containing cryoprotectant solution has a beneficial effect on the cryopreservation of intact sheep ovaries.
Tianqi Du and Lan Chao contributed equally to this work.
Contributor Information
Tianqi Du, Email: hellododo@126.com.
Lan Chao, Email: 570597126@qq.com.
Shuqin Zhao, Email: zzzsq110@163.com.
Linglong Chi, Email: sdqxkcll@163.com.
Dong Li, Email: qldwld@163.com.
Yanjun Shen, Email: qlszsyj@163.com.
Qing Shi, Email: qldwsq@126.com.
Xiaohui Deng, Phone: +86 18560082026, Email: dxhsdu@163.com.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 3.Macklon KT, Jensen AK, Loft A, et al. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31(11):1557–1564. doi: 10.1007/s10815-014-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst E, Bergholdt S, Jorgensen JS, et al. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25(5):1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- 5.Van Eyck AS, Bouzin C, Feron O, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93(5):1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Van Eyck AS, Jordan BF, Gallez B, et al. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92(1):374–381. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Imhof M, Bergmeister H, Lipovac M, et al. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85(Suppl 1):1208–1215. doi: 10.1016/j.fertnstert.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BK, Hernandez-Medrano J, Onions V, et al. Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum Reprod. 2014;29(8):1749–1763. doi: 10.1093/humrep/deu144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Windrum P, Morris TC. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31(4):315. doi: 10.1038/sj.bmt.1703848. [DOI] [PubMed] [Google Scholar]
- 10.Hakura A, Mochida H, Yamatsu K. Dimethyl sulfoxide (DMSO) is mutagenic for bacterial mutagenicity tester strains. Mutat Res. 1993;303(3):127–133. doi: 10.1016/0165-7992(93)90025-q. [DOI] [PubMed] [Google Scholar]
- 11.Schiraldi C, Di Lernia I, De Rosa M. Trehalose production: exploiting novel approaches. Trends Biotechnol. 2002;20(10):420–425. doi: 10.1016/s0167-7799(02)02041-3. [DOI] [PubMed] [Google Scholar]
- 12.Eroglu A, Russo MJ, Bieganski R, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol. 2000;18(2):163–167. doi: 10.1038/72608. [DOI] [PubMed] [Google Scholar]
- 13.Eroglu A, Bailey SE, Toner M, et al. Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol Reprod. 2009;80(1):70–78. doi: 10.1095/biolreprod.108.070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satpathy GR, Torok Z, Bali R, et al. Loading red blood cells with trehalose: a step towards biostabilization. Cryobiology. 2004;49(2):123–136. doi: 10.1016/j.cryobiol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Hu JH, Zan LS, Zhao XL, et al. Effects of trehalose supplementation on semen quality and oxidative stress variables in frozen-thawed bovine semen. J Anim Sci. 2010;88(5):1657–1662. doi: 10.2527/jas.2009-2335. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Zhu Z, Dong L, et al. Lack of trehalose accelerates H2O2-induced Candida albicans apoptosis through regulating Ca2+ signaling pathway and caspase activity. PLoS ONE. 2011;6(1):e15808. doi: 10.1371/journal.pone.0015808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benaroudj N, Lee DH, Goldberg AL. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem. 2001;276(26):24261–24267. doi: 10.1074/jbc.M101487200. [DOI] [PubMed] [Google Scholar]
- 18.Torre A, Momier M, Mazoyer C, et al. Validation of a new metabolic marker to assess the vascular viability of vitrified whole sheep ovaries. Hum Reprod. 2012;27(6):1811–1821. doi: 10.1093/humrep/des100. [DOI] [PubMed] [Google Scholar]
- 19.Revel A, Elami A, Bor A, et al. Whole sheep ovary cryopreservation and transplantation. Fertil Steril. 2004;82(6):1714–1715. doi: 10.1016/j.fertnstert.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JM, Zhang YC, Ruan LH, et al. Optimizing cryoprotectant perfusion conditions for intact ovary: a bovine model. J Assist Reprod Genet. 2012;29(11):1255–1260. doi: 10.1007/s10815-012-9845-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedaiwy MA, Jeremias E, Gurunluoglu R, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003;79(3):594–602. doi: 10.1016/s0015-0282(02)04842-2. [DOI] [PubMed] [Google Scholar]
- 22.Courbiere B, Massardier J, Salle B, et al. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84(Suppl 2):1065–1071. doi: 10.1016/j.fertnstert.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JM, Sheng Y, Cao YZ, et al. Cryopreservation of whole ovaries with vascular pedicles: vitrification or conventional freezing? J Assist Reprod Genet. 2011;28(5):445–452. doi: 10.1007/s10815-011-9539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Wang X, Wu Y, et al. Slow-controlled freezing versus speed-cooling for cryopreservation of whole guinea pig ovaries. Theriogenology. 2012;77(3):483–491. doi: 10.1016/j.theriogenology.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Martino M, Morabito F, Messina G, et al. Fractionated infusions of cryopreserved stem cells may prevent DMSO-induced major cardiac complications in graft recipients. Haematologica. 1996;81(1):59–61. [PubMed] [Google Scholar]
- 26.Wang HY, Lun ZR, Lu SS. Cryopreservation of umbilical cord blood-derived mesenchymal stem cells without dimethyl sulfoxide. Cryo Lett. 2011;32(1):81–88. [PubMed] [Google Scholar]
- 27.Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18(1):24–36. doi: 10.1002/pro.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Branca C, Maccarrone S, Magazu S, et al. Tetrahedral order in homologous disaccharide-water mixtures. J Chem Phys. 2005;122:17451317. doi: 10.1063/1.1887167. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Wang S, Bao J, et al. Trehalose maintains bioactivity and promotes sustained release of BMP-2 from lyophilized CDHA scaffolds for enhanced osteogenesis in vitro and in vivo. PLoS ONE. 2013;8(1):e54645. doi: 10.1371/journal.pone.0054645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TM, Kikuchi K, Nakai M, et al. Effect of trehalose on DNA integrity of freeze-dried boar sperm, fertilization, and embryo development after intracytoplasmic sperm injection. Theriogenology. 2013;80(9):1033–1044. doi: 10.1016/j.theriogenology.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo K, Takahashi T, Igarashi H, et al. Effects of different trehalose concentrations in a warming medium on embryo survival and clinical outcomes in vitrified human embryos. Gynecol Obstet Investig. 2013;76(4):214–220. doi: 10.1159/000355318. [DOI] [PubMed] [Google Scholar]
- 32.Amirat L, Tainturier D, Jeanneau L, et al. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: a comparison with Optidyl, a commercial egg yolk extender. Theriogenology. 2004;61(5):895–907. doi: 10.1016/s0093-691x(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 33.Moussa M, Marinet V, Trimeche A, et al. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen-thawed bull semen. Theriogenology. 2002;57(6):1695–1706. doi: 10.1016/s0093-691x(02)00682-9. [DOI] [PubMed] [Google Scholar]
- 34.Graham JK, Foote RH. Effect of several lipids, fatty acyl chain length, and degree of unsaturation on the motility of bull spermatozoa after cold shock and freezing. Cryobiology. 1987;24(1):42–52. doi: 10.1016/0011-2240(87)90005-8. [DOI] [PubMed] [Google Scholar]
- 35.Johnson LA, Weitze KF, Fiser P, et al. Storage of boar semen. Anim Reprod Sci. 2000;62(1–3):143–172. doi: 10.1016/s0378-4320(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 36.Dasiman R, Rahman NS, Othman S, et al. Cytoskeletal alterations in different developmental stages of in vivo cryopreserved preimplantation murine embryos. Med Sci Monit Basic Res. 2013;19:258–266. doi: 10.12659/MSMBR.884019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Meyer F, Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc Natl Acad Sci USA. 2009;106(10):3654–3658. doi: 10.1073/pnas.0809959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YA, Kim YH, Kim BJ, et al. Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells. PLoS ONE. 2013;8(1):e54889. doi: 10.1371/journal.pone.0054889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Xu L, Jiao S, et al. Trehalose inhibited the phagocytosis of refrigerated platelets in vitro via preventing apoptosis. Transfusion. 2009;49(10):2158–2166. doi: 10.1111/j.1537-2995.2009.02254.x. [DOI] [PubMed] [Google Scholar]
- 40.Al-Fageeh MB, Smales CM. Cold-inducible RNA binding protein (CIRP) expression is modulated by alternative mRNAs. RNA. 2009;15(6):1164–1176. doi: 10.1261/rna.1179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Zhang Z, Xue J, et al. Cold-inducible RNA binding protein inhibits H2O2-induced apoptosis in rat cortical neurons. Brain Res. 2012;1441:47–52. doi: 10.1016/j.brainres.2011.12.053. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai T, Itoh K, Higashitsuji H, et al. Cirp protects against tumor necrosis factor-alpha-induced apoptosis via activation of extracellular signal-regulated kinase. Biochim Biophys Acta. 2006;1763(3):290–295. doi: 10.1016/j.bbamcr.2006.02.007. [DOI] [PubMed] [Google Scholar]