Abstract
The formation of new blood vessels from pre-existing ones is a carefully orchestrated dance. A study reveals that the metabolism of sugar by glycolysis contributes to its regulation.
The breakdown of fuel by metabolism is the engine that sustains all cellular activities. But can metabolism also steer and control cellular processes? Writing in Cell, De Bock et al.1 suggest that the answer is yes, at least in the context of glucose metabolism and angiogenesis — the formation of new blood vessels*.
Glycolysis is the cellular process by which glucose is converted into pyruvate. A cell then makes a choice: it can convert pyruvate to lactate, which exits the cell, for a net yield of 2 ATP molecules (the currency of cellular energy transfer) or, in the presence of oxygen, the pyruvate can enter cellular organelles called mitochondria and become fully oxidized, producing a net yield of more than 30 ATP molecules. One would not expect any oxygenated cell to opt out of this mitochondrial bonanza, but some do, in a phenomenon first noted2 in cancer cells by Otto Warburg in 1956. Cancer cells probably make this choice because intermediate molecules formed during glycolysis support the synthesis of macromolecules needed for cellular replication3. But do any non-cancerous or even quiescent cells also display the Warburg effect? Endothelial cells, which line blood vessels throughout the body and mediate angiogenesis, do3, but until now little was known about how metabolism affects their function.
De Bock et al. began their study by confirming a previous report4 that endothelial cells are highly glycolytic but perform little pyruvate oxidation. The authors then asked the interesting question: could modulation of glycolytic activity have an effect on angiogenesis? To assess this, they altered the amount of phosphofructokinase 2 (PFK2) in endothelial cells. PFK2 is a glycolysis-regulating enzyme that was discovered only in the 1980s, long after all key enzymes of the glycolytic pathway were thought to be known5. The related enzyme PFK1, identified decades before PFK2, catalyses the crucial committing step of glycolysis: the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate. PFK2, by contrast, converts fructose-6-phosphate to fructose-2,6-bisphosphate, which is a potent allosteric activator of PFK1 (ref. 6). Activation of PFK2 thus drastically accelerates glycolytic flux through PFK1.
De Bock et al. show that reducing PFK2 levels in endothelial cells not only lowers glycolytic flux, as expected, but also impairs angiogenesis, by reducing the ability of the cells to form tip cells, migrate and form blood-vessel ‘sprouts’. Conversely, and importantly, increasing PFK2 levels has the opposite effect: angiogenesis is increased. The authors also show that PFK2 lies downstream of VEGF and Notch, two proteins that are dominant determinants of endothelial-cell characteristics during angiogenesis.
How does PFK2 achieve these effects? Perhaps most interestingly, the authors demonstrate that PFK2 localizes to structures at the margins of endothelial cells called lamellipodia and filopodia. These cellular projections, which contain meshes and filaments of the protein actin, mediate endothelial-cell movement and sprout formation during angiogenesis (Fig. 1). PFK2 activity at this site probably coincides with the cellular position of large complexes of glycolytic enzymes, known as metabolons, which facilitate the channelling of metabolic products from one enzyme to the next7. Thus, it seems that PFK2 alters angiogenic capacity by altering glycolytic flux at the site of primary cell motion.
Figure 1. Glycolysis regulates angiogenesis.
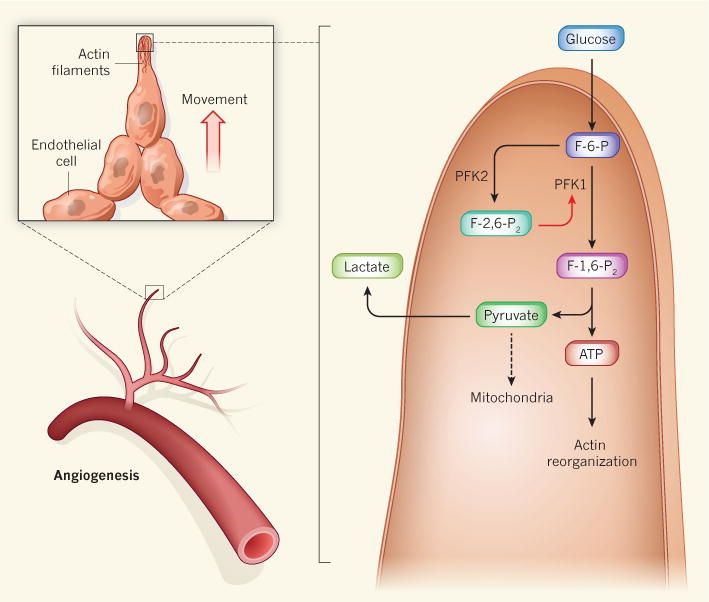
The formation of new blood vessels involves the outward movement of endothelial cells from the lining of existing blood vessels, a process that relies on the rapid reorganization of actin-protein filaments in cellular structures called filopodia and lamellipodia (not shown). The energy for this (in the form of ATP) is provided by the breakdown of glucose, but endothelial cells are unusual in that the pyruvate produced by glycolysis is converted to lactate, rather than being channelled into mitochondria for further oxidation, as occurs in most cells. De Bock et al.1 show that both angiogenesis and glycolysis are accelerated by the activity of the enzyme PFK2 in endothelial-cell lamellipodia and filopodia. PFK2 converts the glycolytic intermediate fructose-6-phosphate (F-6-P) into fructose-2,6-bisphosphate (F-2,6-P2), which, in turn, enhances the activity of the glycolytic enzyme PFK1, thereby accelerating glycolysis at these sites. Pyruvate then leaves the cell as lactate, probably because filopodia and lamellipodia are too small to accommodate mitochondria.
The study is important for several reasons. The findings imply that glucose metabolism can ‘steer’ the angiogenic process, in addition to simply being its ‘engine’. This unveils glucose metabolism as a potential target for pro-angiogenic therapies (such as in patients with inadequate blood supply to the heart or limbs) or anti-angiogenic therapies (for example, to tackle tumours). Metabolic enzymes make good drug targets, so this is an exciting possibility. The study also provides an additional explanation for why endothelial cells perform glycolysis rather than oxidative breakdown of glucose: rapid local generation of ATP can occur in glycolytic metabolons located in the lamellipodia and filopodia, which are too small to accommodate mitochondria and are often found at angiogenic fronts where oxygen is scant.
Like all seminal work, this study generates several questions. Does modulation of glycolytic flux in ways other than through PFK2 also affect angiogenic sprouting? Could non-enzymatic properties of PFK2 contribute to the observed phenomena? Such behaviour has been seen for pyruvate kinase, another key enzyme in glycolysis that was recently found8 to be present in the cell nucleus and associated with transcription factors that drive gene expression. Does PFK2 modulate the activities of Rac, Akt and eNOS — key enzymes that regulate endothelial-cell motility — and, if so, how? How do Notch and VEGF signal to PFK2? Does glycolysis regulate migration of other cell types, such as smooth-muscle cells or macrophages, or even cancer cells? And is the pro-angiogenic activity of PFK2 altered when glucose homeostasis is perturbed, such as in diabetes?
These questions aside, De Bock and colleagues’ study deepens our understanding of why some cells choose to forego the lucrative use of mitochondria to break down their glucose, even when, as is the case for endothelial cells, the cells are not highly replicative. The authors’ findings also introduce a new concept in endothelial biology: that metabolic decisions can regulate the endothelial phenotype, as well as vice versa. It turns out that, much like children, endothelial cells that gorge on sugar become hyperactive.
NATURE.
50 Years Ago
Outline of Human Genetics. By Prof. L. S. Penrose — Throughout, Prof. Penrose deals with just those points which are of general interest and particularly topics about which people ignorant of genetics are always asking, for example, Is natural selection still operating in spite of civilization and medical advances? … In “Commentary” he explains in more detail how common chromosomal abnormalities, such as those causing mongolism and intersexes, are produced; mentions theories dealing with the possibility of inherited cancer; touches on pharmacogenetics; and outlines the vast amount of genetic variability which is being shown up by the complicated polymorphisms of the blood proteins. Finally, he makes the very good point that while geneticists are continually worrying about the quality of the human race we shall have doubled our numbers in the next 50 years and that birth control is far more important than the fruitless task of planning the superman.
From Nature 24 August 1963.
100 Years Ago
An exhibit illustrating the damage caused to biscuits sent out in soldered tins for the use of the troops in South Africa—especially during the Boer war—Gibraltar, Malta, Ceylon, &c., has just been placed in the central hall of the British Museum (Natural History), where it will be kept open about a month. The larvae of certain minute moths and beetles were the active agents; and it appears that since these cannot, in all probability, withstand the high temperature to which the biscuits are subjected in baking, the eggs must be laid by the moths during the period when the biscuits are being cooled before tinning.
From Nature 21 August 1913.
Footnotes
This article and the paper under discussion1 were published online on 14 August 2013.
References
- 1.De Bock K, et al. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobrina A, Rossi F. Biochim Biophys Acta. 1983;762:295–301. doi: 10.1016/0167-4889(83)90084-8. [DOI] [PubMed] [Google Scholar]
- 5.Van Schaftingen E, Hers HG. Biochem Biophys Res Commun. 1981;101:1078–1084. doi: 10.1016/0006-291x(81)91859-3. [DOI] [PubMed] [Google Scholar]
- 6.Van Schaftingen E, Hue L, Hers HG. Biochem J. 1980;192:897–901. doi: 10.1042/bj1920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srere PA. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- 8.Luo W, et al. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]


