Abstract
Objective
Risk of intracerebral hemorrhage is the primary factor limiting use of tissue plasminogen activator (tPA) for stroke. Clinical studies have established an association between admission hyperglycemia and the risk of hemorrhage with tPA, independent of prior diabetes. Here we used an animal model of tPA-induced reperfusion hemorrhage to determine if this clinical association reflects a true causal relationship.
Methods
Rats underwent 90-minutes of focal ischemia, and tPA infusion was begun 10 minutes prior to vessel reperfusion. Glucose was administered during ischemia to generate blood levels ranging from 5.9 ± 1.8 mM (normoglycemia) to 21 ± 2.3 mM. In some studies, apocynin was administered to block NADPH oxidase production of superoxide. Brains were harvested 1 hour or 3 days after reperfusion to evaluate the effects of hyperglycemia and apocynin on oxidative stress, blood-brain barrier breakdown, infarct volume, and hemorrhage volume.
Results
Rats that were hyperglycemic during tPA infusion had diffusely increased blood-brain barrier permeability in the post-ischemic territory, and a 3 – 5 fold increase in intracerebral hemorrhage volumes. The hyperglycemic rats also showed increased superoxide formation in the brain parenchyma and vasculature during reperfusion. The effects of hyperglycemia on superoxide production, blood-brain barrier disruption, infarct size, and hemorrhage were all attenuated by apocynin.
Interpretation
These findings demonstrate a causal relationship between hyperglycemia and hemorrhage in an animal model of tPA stroke treatment, and suggest that the effect of hyperglycemia is mediated through an increase in NADPH oxidase - mediated superoxide production.
Keywords: ischemia, hemorrhage, glucose, thrombolytic, NADPH oxidase
INTRODUCTION
Tissue plasminogen activator (tPA) is an effective treatment for acute ischemic stroke 1, but its use is limited by the risk of hemorrhage into the reperfused brain tissue. Several studies have identified a strong association between blood glucose levels and tPA-induced brain hemorrhage, independent of pre-existing diabetes 2–8 (reviewed by Lansberg et al. 9). Three of these studies stratified risk according to severity of hyperglycemia, and all three found the risk of hemorrhage to increase by a factor of 1.6 to 2.2 with each 5.5 mM (100 mg/dl) increase in admission blood glucose 2, 3, 6. However, it remains to be established whether this clinical association reflects a true causal relationship, and if so, what is the mechanism of this effect.
A causal relationship is biologically plausible. The presence of hyperglycemia during brain ischemia promotes acidosis and production of advanced glycation endproducts, both of which damage the vasculature 10–16. Hyperglycemia can also accelerate the production of superoxide by NADPH oxidase 11, 17–19, which contributes to blood-brain barrier disruption 20, 21. On the other hand, hyperglycemia is a non-specific response to stress, and the clinical association of hyperglycemia and intracerebral hemorrhage could alternatively reflect increased severity of injury or an accentuated stress response in the hyperglycemic patients.
Hyperglycemia can be corrected with insulin in the acute stroke setting, but this intervention carries a risk of exacerbating ischemic injury if glucose levels fall excessively 22–24. It is therefore important to determine whether hyperglycemia is a causal factor promoting intracerebral hemorrhage in the setting of tPA stroke treatment, or merely an epiphenomenon. In this study we used an animal (rat) model of post-ischemic, tPA-induced brain hemorrhage to address this question. We additionally used an inhibitor of NADPH oxidase, apocynin, to test the hypothesis that hyperglycemia promotes tPA-induced hemorrhage by increasing superoxide production.
METHODS
Animals and surgical procedures
Studies were approved by the San Francisco Veterans Affairs Medical Center animal studies committee. Adult male Sprague-Dawley rats (Charles River Laboratories, Gilroy, CA), 250 – 350 g, were fasted overnight to ensure uniform basal blood glucose levels. Anesthesia was induced with 2% isoflurane in 70% N2O / balance O2. Rectal (core) temperature was maintained at 37 ± 0.5°C with a homeothermic blanket, and blood pressure was continuously monitored with the use of an indwelling femoral arterial catheter. This catheter was also used for periodic measurements of blood gases and blood glucose. Focal brain ischemia-reperfusion was produced by occluding both common carotid arteries and the left proximal middle cerebral artery (MCA) with micro-aneurysm clips for 90 minutes, as previously described 25. Experimental groups, mortality, and peri-operative physiological parameters are provided in Supplementary Table 1. Outcome measures for all studies were evaluated by persons blinded to the treatment conditions.
Hyperglycemia and drug administration
Rats were rendered hyperglycemic with intraperitoneal (i.p.) injections of 50% glucose at the indicated time points (Fig. 1A). The initial injection volume was 2.0, 3.5, or 5.0 ml / kg for the target blood glucose concentrations of 10 mM, 15 mM, and 20 mM, respectively (Fig. 1). Subsequent injection volumes were all 1.5 μl / g. Normoglycemic (5.0 – 6.0 mM glucose) rats received 3.5 μl / g saline vehicle at the initial time point, and 1.5 μl / g at the subsequent time points. Preliminary studies showed that blood glucose in all treatment groups returned to normoglycemia within 3 hours of the last injection (not shown). Tissue plasminogen activator (tPA; Genentech) was infused intravenously (i.v.) beginning 10 minutes before reperfusion, at a dose of 10 mg / kg, as a 10% bolus and balance over 30 minutes. Controls received equivalent volumes of tPA vehicle, which contains 1 mM L-arginine. When used, apocynin (2.5 mg / kg; Sigma) or its vehicle (1% DMSO) was given i.v. 20 minutes before reperfusion 26, and dihydroethidium (Invitrogen) was administered 3 mg / kg i.p. in 1 ml of 1% DMSO.
Figure 1. Experimental design.
(A) Hyperglycemia was induced by glucose injections at the designated time points. tPA infusion was begun 10 minutes before end of MCA occlusion. Controls received tPA vehicle. (B) Arterial blood glucose concentrations before, during, and after MCA occlusion. Filled symbols, tPA treatment; open symbols, vehicle treatment.
Hemorrhage and infarct volumes
Rats were euthanized 3 days after ischemia–reperfusion for these outcome measures. Brains were removed, cut into 8 2-mm coronal slices, and photographed. The photographs were used to quantify brain hemorrhage using a recently validated image analysis method 27, which estimates the volume of blood in each brain slab on the basis of the intensity and area of hemoglobin staining. Brain hemorrhage was also quantified by manual scoring of each slice as either 1 (hemorrhage visible) or 0 (no hemorrhage visible), and summing these scores for each brain. The two methods gave very similar results in all experiments. Infarct volume was determined in each brain by the 2,3,5-triphenyltetrazolium (TTC) method 28, using the same coronal slices. Rats in some experimental groups died in the 3-day interval between surgery and euthanasia (Supplementary Table 1), and brains from those rats could not be used for the histological studies.
Blood-brain barrier permeability
The same slices used to measure hemorrhage and infarct volume were also used to evaluate extravasation of IgG into brain parenchyma 29. Two slices from each brain, 4 and 6 mm from the frontal pole, were cryosectioned into 25 μm sections and fixed with 75% acetone / 25% ethanol. The mounted sections were incubated with biotinylated anti rat IgG (H+L) (1:200; Vector Lab) at 4 °C overnight, followed by incubations with avidin-biotin complex and diaminobenzidine. The mean optical density of the ischemic hemisphere from each section was measured with an image analysis system, using constant illumination and exposure parameters. Non-ischemic controls were included with each batch of stained and photographed sections, and the mean value of these control sections was subtracted from that of each ischemic section to additionally control for potential variations in staining or image capture.
Superoxide detection
Rats were injected i.p. with 3 mg / kg dihydroethidium (Invitrogen) 15 minutes before ischemia, euthanized 1 hour after reperfusion, and perfused with 0.9% saline followed by 4% formaldehyde. Forty-micrometer cryostat sections were prepared, and ethidium fluorescence was photographed by confocal microscopy 30. Ethidium fluorescence was measured in each cell captured in a 250 μm2 field centered in the post-ischemic cortex, with 2 sections analyzed per brain. Fluorescence intensity of each cell (> 50 cells / section) was normalized to the mean fluorescence intensity of cells in the homologous contralateral (non-ischemic) cortex. Prior studies have shown that ethidium fluorescence induced by ischemia-reperfusion is reduced in brains over-expressing Cu/Zn superoxide dismutase 31 or lacking NADPH oxidase activity 11, thus establishing specificity for superoxide in this setting. The brain sections from hyperglycemic rats injected with dihydroethidium were also immunostained for cell-specific markers. These sections were incubated overnight with mouse anti-NeuN (Millipore), mouse anti-CD11b (Serotec) or goat anti-PECAM1(Santa Cruz Biotechnology), followed by incubation with secondary antibodies conjugated for blue fluorescence. Sections were photographed by confocal microscopy.
Statistical Analysis
Experiments were designed to produce n = 6 for the 3-day survival studies, and n = 4 – 6 for the 1-hour survival studies (Supplementary Table 1). Brain hemorrhage and IgG staining were evaluated with two-way ANOVA and post-test for linear trend. All other data were analyzed with Student’s t-test or with one-way ANOVA followed by Dunnett’s test. Graphed data are presented as means ± s.e.m.
RESULTS
Hyperglycemia increases tPA-induced brain hemorrhage after ischemia
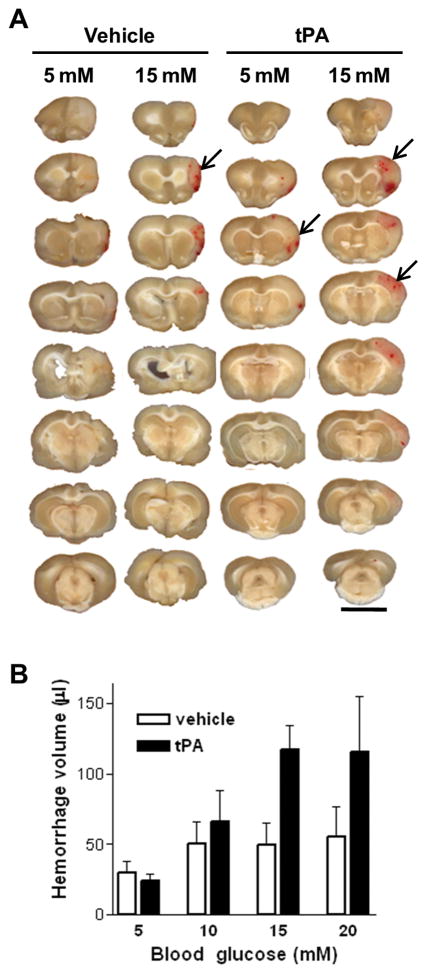
Hyperglycemia was induced by intraperitoneal glucose injections during ischemia, and tPA infusion began shortly before reperfusion (Fig. 1). Neither glucose nor tPA injections affected the mean arterial blood pressure, PaO2, PaCO2, or pH, but mortality was increased (33%) in the tPA-treated, 20 mM glucose group (Supplementary Table 1). Hyperglycemia caused an increase in hemorrhage volume in the tPA-treated rats, and this increase was progressively greater with progressively greater elevations in blood glucose concentrations (Fig. 2).
Figure 2. Hyperglycemia increases tPA-induced brain hemorrhage.
(A) Each column shows coronal sections of a representative brain from the designated treatment group. Arrows denote some of the hemorrhage areas. Scale bar = 1 cm. (B) Hemorrhage is quantified as the volume of blood per brain. Results from two separate experiments are combined. n = 6–7; P < 0.02 for effects of both glucose and tPA on hemorrhage volume and P < 0.01 for linear trend between glucose and hemorrhage volume in the tPA-treated groups.
Hyperglycemia increases superoxide production during reperfusion
Oxidative stress contributes to blood-brain barrier damage during ischemia-reperfusion 21. Here we compared superoxide production after 1 hour of reperfusion in normoglycemic and hyperglycemic brains. Hyperglycemia was found to increase superoxide production in the post-ischemic cortex, but not in the contralateral, non-ischemic cortex (Fig. 3). Brain sections from the hyperglycemic animals were also immunostained for cell-type specific markers to identify the cell types containing the superoxide signal. These markers showed increased superoxide in most of the neurons and endothelial cells of the ischemic cortex, and in a smaller fraction of the microglia (Supplemental Fig. 1). This method does not, however, definitively identify the cell type(s) in which the superoxide originated.
Figure 3. Hyperglycemia-induced superoxide production in re-perfused cortex is blocked by apocynin.
(A) Brains were harvested from normoglycemic (5 mM glucose) and hyperglycemic (15 mM glucose) rats 1 hour after reperfusion with tPA. Photomicrographs show superoxide production identified by ethidium fluorescence (red) in representative sections. Scale bar = 40 μm. (B) Histogram shows normalized ethidium fluorescence of cells in cortex of a representative brain from each treatment group, with arrows indicating the median values. Graph shows mean (± s.e.m.) of the median values from the 4 rats in each group; *P < 0.05. (C,D). In a separate experiment, hyperglycemic rats treated were treated with apocynin or vehicle during tPA reperfusion, and brain sections were evaluated as in A,B. n = 5–7; *P < 0.05.
The NADPH oxidase inhibitor apocynin blocks the effects of hyperglycemia
We performed an additional set of experiments in which hyperglycemic rats were treated with apocynin, which blocks superoxide formation by NADPH oxidase 26, 32. Apocynin was found to block the effects of hyperglycemia on both post-ischemic superoxide production (Fig. 3B) and brain hemorrhage (Fig. 4). Apocynin did not affect blood glucose levels, mean arterial blood pressure, PaO2, PaCO2, or pH (Supplementary Table 1). Hyperglycemia also increased infarct size (as previously reported 33, 34), and apocynin likewise attenuated this increase (Fig. 5A).
Figure 4. Apocynin reduces hemorrhage in tPA-treated brains.

Hyperglycemic rats were pre-treated with apocynin (or vehicle) prior to reperfusion with tPA, and brains were harvested 3 days later. (A) Each column of coronal sections shows a brain from the designated treatment group. Scale bar = 1 cm. (B) Hemorrhage is quantified as volume of blood per brain. n = 6; **P < 0.01.
Figure 5. Effects of hyperglycemia on infarct size and blood-brain barrier.
Brains were harvested 3 days after ischemia-reperfusion. (A) Infarct size in tPA-treated rats was increased by hyperglycemia and reduced by apocynin. n = 6–7; * P < 0.05 vs. 5 mM, #P < 0.05 vs. 15 mM. (B) Blood-brain barrier disruption identified by immunostaining for IgG (dark brown) in representative brain sections. (C) Graph shows quantified IgG staining. P < 0.02 for an effect of glucose on IgG staining in both tPA and vehicle-treated brains, with P < 0.01 for linear trend between glucose and IgG staining. (D) Representative images from a separate experiment in which hyperglycemic rats were pre-treated with apocynin or vehicle prior to tPA reperfusion. (E) Graph shows quantified IgG staining. n = 6–7; *P < 0.05. Panels A and C show results combined from 2 separate experiments.
Hyperglycemia increases blood-brain barrier disruption during reperfusion with tPA
We additionally measured IgG extravasation into brain parenchyma to more directly assess blood-brain barrier disruption. Rats undergoing sham ischemia showed no detectable immunoreactivity for IgG in the brain sections irrespective of blood glucose levels, but rats undergoing ischemia-reperfusion showed diffuse extravasation of IgG into the post-ischemic brain parenchyma (Fig. 5). The intensity of IgG staining was significantly greater in brains that had been hyperglycemic during reperfusion, and progressively increased with increasing elevations in blood glucose levels. The effect of apocynin was evaluated in rats maintained at 15 mM glucose during ischemia-reperfusion. Apocynin significantly attenuated the hyperglycemia-induced increase in IgG staining (Fig. 5).
DISCUSSION
Hyperglycemia was found to increase blood-brain barrier disruption, oxidative stress, infarct size, and tPA-induced brain hemorrhage after ischemia-reperfusion in this animal model. These effects increased in magnitude with increasing degrees of hyperglycemia, and were mitigated by the NADPH oxidase inhibitor, apocynin.
The mitigation of these effects by apocynin suggests a mechanism by which hyperglycemia may promote oxidative stress and tPA-induced hemorrhage during reperfusion. Reperfusion brain hemorrhage results in part from protease degradation of the extracellular matrix around and within cerebral blood vessels 35. The activation of these proteases is promoted by tPA 36, and also by superoxide produced by NADPH oxidase 20, 21. NADPH oxidase generates superoxide through a process that requires glucose as a substrate for NADPH production, such that glucose availability can be the rate-limiting factor in superoxide production by this process 11, 17–19. High glucose levels may additionally “prime” NADPH oxidase so that it is more quickly and completely activated by other signals 38.
Apocynin blocks assembly of the active NADPH oxidase (NOX2) complex, and does not block superoxide production by mitochondria 32, 37. The observation that apocynin blocks the effects of hyperglycemia on infarct size, as well as on hemorrhage volume, suggests that both of these effects of hyperglycemia may result from underlying oxidative damage to the cerebral vasculature, as previously proposed 16. This idea is further supported by the observations made here and previously 39–41 that hyperglycemia exacerbates ischemic damage to the blood-brain barrier.
Clinical studies suggest that more than 40% of stroke patients have blood glucose levels above 8.5 mM (153 mg/dl) at admission, and a substantial fraction of these patients have levels in the 10 – 15 mM (180 – 270 mg/dl) range 2, 3, 42, 43. The present studies were designed to model the effects of this acute, stress-induced hyperglycemia on tPA-induced brain hemorrhage. Results of these studies show a roughly 2-fold increase in tPA-induced hemorrhage volume with each 5 mM increase in blood glucose over the range evaluated. The animal model cannot be expected to directly map human clinical outcomes, but this result is similar in magnitude to the 1.6 – 2.2 fold increase in symptomatic hemorrhage reported over comparable blood glucose elevations in several clinical series 2–9.
The animal model has several limitations. Post-reperfusion mortality rate was significant in the 20 mM glucose plus tPA group treatment (33%; Supplementary Table 1), and since brains from those rats could not be evaluated histologically, the extent of hemorrhage may have been underestimated in this extreme hyperglycemia group. Unlike clinical stroke, there is no true clot in this model and thus no platelet or thrombus breakdown products released into the post-ischemic tissue, which could in principle modulate reperfusion and risk of hemorrhage 44. In addition, the tPA dose used in the rats was much higher than is used in humans (because of the reduced thrombolytic efficacy of human tPA in rat plasma 45), and this higher tPA dose could have additional effects, unrelated to thrombolysis 36. The present study also makes no distinction between asymptomatic and symptomatic hemorrhage (other than mortality), and the results could thus overestimate the effect of hyperglycemia on functional outcomes after tPA administration if extrapolated to clinical settings. On the other hand, clinical studies suggest that rates of symptomatic and asymptomatic hemorrhage parallel one another 2, and that long–term effects of initially “asymptomatic” brain hemorrhage may not be insignificant 46, 47.
Independent of these considerations, the results obtained with this animal model demonstrate that hyperglycemia can increase tPA-induced brain hemorrhage in post-ischemic brain. Together with the established clinical association between hyperglycemia and hemorrhage, these results provide added rationale for glucose control in hyperglycemic stroke patients treated with tPA. These results likewise provide support for consideration of blood glucose values in clinical guidelines for tPA use in stroke. The results obtained with apocynin in these studies additionally suggest that treatment approaches targeting NADPH oxidase could negate the deleterious effects of hyperglycemia in this setting.
Supplementary Material
Acknowledgments
This work was supported by the U.S. Department of Veterans Affairs and National Institutes of Health (P50 NS14543; R.A.S. and M.A.Y.).
Footnotes
POTENTIAL CONFLICTS OF INTEREST: Nothing to report.
References
- 1.NINDS rt-PA Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Demchuk AM, Morgenstern LB, Krieger DW, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- 3.Bruno A, Levine SR, Frankel MR, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669–674. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Sabin J, Molina CA, Montaner J, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator--treated patients. Stroke. 2003;34:1235–1241. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- 5.Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57:1603–1610. doi: 10.1212/wnl.57.9.1603. [DOI] [PubMed] [Google Scholar]
- 6.Hill MD, Buchan AM. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172:1307–1312. doi: 10.1503/cmaj.1041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Silva DA, Ebinger M, Christensen S, et al. Baseline diabetic status and admission blood glucose were poor prognostic factors in the EPITHET trial. Cerebrovasc Dis. 2010;29:14–21. doi: 10.1159/000255969. [DOI] [PubMed] [Google Scholar]
- 8.Simpson MA, Dewey HM, Churilov L, et al. Thrombolysis for acute stroke in Australia: outcomes from the Safe Implementation of Thrombolysis in Stroke registry (2002–2008) Med J Aust. 2010;193:439–443. doi: 10.5694/j.1326-5377.2010.tb03996.x. [DOI] [PubMed] [Google Scholar]
- 9.Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24:1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 10.Muranyi M, Li PA. Hyperglycemia increases superoxide production in the CA1 pyramidal neurons after global cerebral ischemia. Neurosci Lett. 2006;393:119–121. doi: 10.1016/j.neulet.2005.09.079. [DOI] [PubMed] [Google Scholar]
- 11.Suh SW, Shin BS, Ma H, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehncrona S, Rosen I, Siesjo BK. Brain lactic acidosis and ischemic cell damage: 1. Biochemistry and neurophysiology. J Cereb Blood Flow Metab. 1981;1:297–311. doi: 10.1038/jcbfm.1981.34. [DOI] [PubMed] [Google Scholar]
- 13.Paljarvi L, Rehncrona S, Soderfeldt B, et al. Brain lactic acidosis and ischemic cell damage: quantitative ultrastructural changes in capillaries of rat cerebral cortex. Acta Neuropathol. 1983;60:232–240. doi: 10.1007/BF00691871. [DOI] [PubMed] [Google Scholar]
- 14.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 15.Gobbel GT, Chan TY, Chan PH. Nitric oxide- and superoxide-mediated toxicity in cerebral endothelial cells. J Pharmacol Exp Ther. 1997;282:1600–1607. [PubMed] [Google Scholar]
- 16.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 17.Suh SW, Gum ET, Hamby AM, et al. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupte SA, Levine RJ, Gupte RS, et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Decoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang J, Liu J, Zhou C, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 21.Kahles T, Luedike P, Endres M, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 22.Johnston KC, Hall CE, Kissela BM, et al. Glucose Regulation in Acute Stroke Patients (GRASP) trial: a randomized pilot trial. Stroke. 2009;40:3804–3809. doi: 10.1161/STROKEAHA.109.561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 24.Bruno A, Liebeskind D, Hao Q, Raychev R. Diabetes Mellitus, Acute Hyperglycemia, and Ischemic Stroke. Curr Treat Options Neurol. 2010 doi: 10.1007/s11940-010-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Won SJ, Xie L, Kim SH, et al. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res. 2006;1123:237–244. doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang XN, Berman AE, Swanson RA, Yenari MA. Digitally quantifying cerebral hemorrhage using Photoshop and Image J. J Neurosci Methods. 2010;190:240–243. doi: 10.1016/j.jneumeth.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier CM, Sun GH, Kunis D, et al. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Jadhav V, Tang J, Zhang JH. HIF-1 alpha inhibition ameliorates neonatal brain damage after hypoxic-ischemic injury. Acta Neurochir Suppl. 2008;102:395–399. doi: 10.1007/978-3-211-85578-2_77. [DOI] [PubMed] [Google Scholar]
- 30.Chan PH, Kawase M, Murakami K, et al. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi T, Saito A, Okuno S, et al. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab. 2005;25:41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- 32.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 33.Yip PK, He YY, Hsu CY, et al. Effect of plasma glucose on infarct size in focal cerebral ischemia-reperfusion. Neurology. 1991;41:899–905. doi: 10.1212/wnl.41.6.899. [DOI] [PubMed] [Google Scholar]
- 34.Plum F. What causes infarction in ischemic brain?: The Robert Wartenberg Lecture. Neurology. 1983;33:222–223. doi: 10.1212/wnl.33.2.222. [DOI] [PubMed] [Google Scholar]
- 35.Jian Liu K, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39:71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Tsuji K, Lee SR, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 37.Brennan AM, Suh SW, Won SJ, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nature Neuroscience. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omori K, Ohira T, Uchida Y, et al. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol. 2008;84:292–301. doi: 10.1189/jlb.1207832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bang OY, Saver JL, Alger JR, et al. Patterns and predictors of blood-brain barrier permeability derangements in acute ischemic stroke. Stroke. 2009;40:454–461. doi: 10.1161/STROKEAHA.108.522847. [DOI] [PubMed] [Google Scholar]
- 40.Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2007;27:1573–1582. doi: 10.1038/sj.jcbfm.9600454. [DOI] [PubMed] [Google Scholar]
- 41.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 43.Sacco RL, Shi T, Zamanillo MC, Kargman DE. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology. 1994;44:626–634. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Zhang ZG, Zhang C, et al. Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–2895. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- 45.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- 46.Dzialowski I, Pexman JH, Barber PA, et al. Asymptomatic hemorrhage after thrombolysis may not be benign: prognosis by hemorrhage type in the Canadian alteplase for stroke effectiveness study registry. Stroke. 2007;38:75–79. doi: 10.1161/01.STR.0000251644.76546.62. [DOI] [PubMed] [Google Scholar]
- 47.Kimura K, Iguchi Y, Shibazaki K, et al. Hemorrhagic transformation of ischemic brain tissue after t-PA thrombolysis as detected by MRI may be asymptomatic, but impair neurological recovery. J Neurol Sci. 2008;272:136–142. doi: 10.1016/j.jns.2008.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.