Abstract
Background
The diagnosis of hepatic sinusoidal obstruction syndrome (HSOS) induced by pyrrolizidine alkaloids is mainly based on clinical investigation. There is currently no prognostic index. This study evaluated the quantitative measurement of blood pyrrole-protein adducts (PPAs) as a diagnostic and prognostic index for pyrrolizidine alkaloid-induced HSOS.
Methods
Suspected drug-induced liver injury patients were prospectively recruited. Blood PPAs were quantitatively measured using ultra-performance liquid chromatography-tandem mass spectrometry. Patients’ age, sex, biochemistry test results, and a detailed drug history were recorded. The patients were divided into two groups, ie, those with HSOS induced by pyrrolizidine alkaloid-containing drugs and those with liver injury induced by drugs without pyrrolizidine alkaloids. The relationship between herb administration, clinical outcomes, blood sampling time, and blood PPA concentration in pyrrolizidine alkaloid-associated HSOS patients was analyzed using multiple linear regression analysis.
Results
Forty patients met the entry criteria, among whom 23 had pyrrolizidine alkaloid-associated HSOS and 17 had liver injury caused by drugs without pyrrolizidine alkaloids. Among the 23 patients with pyrrolizidine alkaloid-associated HSOS, ten recovered, four developed chronic disease, eight died, and one underwent liver transplantation within 6 months after onset. Blood PPAs were detectable in 24 of 40 patients with concentrations from 0.05 to 74.4 nM. Sensitivity and specificity of the test for diagnosis of pyrrolizidine alkaloid-associated HSOS were 100% (23/23) and 94.1% (23/24), respectively. The positive predictive value was 95.8% and the negative predictive value was 100%, whereas the positive likelihood ratio was 23.81. The level of blood PPAs in the severe group (died or received liver transplantation) was significantly higher than that in the recovery/chronicity group (P=0.004).
Conclusion
Blood PPAs measured by ultra-performance liquid chromatography-tandem mass spectrometry are highly sensitive and specific for pyrrolizidine alkaloid-associated HSOS. The blood PPA concentration is related to the severity and clinical outcome of pyrrolizidine alkaloid-associated HSOS.
Keywords: drug-induced liver injury, herb, blood protein adducts, prognostic marker
Introduction
Herbal medicine-induced or dietary supplement-induced liver injury has increased worldwide.1 Herb-induced liver injury occurs in a few susceptible individuals, and has characteristics similar to many other liver diseases that are unrelated to herbs or drugs.2 Determining the causality of drug-induced liver injury (DILI) is a major clinical challenge because there is no standard diagnostic approach. In addition, inconsistent documentation makes it difficult to clarify the drugs that were taken by these patients. In patients who have liver injury without a definite cause, herb-induced and supplement-induced liver injury should be considered.
Herbs containing pyrrolidizine alkaloids (PAs) cause hepatic sinusoidal obstruction syndrome (HSOS), which is severe, and typically manifested as abdominal distention, abdominal pain, ascites, malaise, hepatomegaly, jaundice, and edema.3,4 The diagnosis of PA-induced HSOS usually depends on a history of taking herbs that contain PAs, and a classical triad of weight gain, painful hepatomegaly, and jaundice.4,5 Histological liver biopsy is the gold standard for diagnosing HSOS.6
PAs and their N-oxides occur in an estimated 3% of flowering plants,6 and there are at least 6,000 plants worldwide that contain PAs. A total of 60% of bee pollen samples have been reported to be positive for PAs.7 Ingestion of PA-contaminated foods, herbal medicines, or dietary supplements can cause hepatotoxicity, genotoxicity, and pulmonary arterial hypertension.3,8 PAs themselves are not toxic but can be converted by cytochrome P450 monooxygenases, which are located primarily in hepatocytes, to 6,7-dihydropyrrolizine (pyrrolic) ester metabolites with toxic properties.9,10
Electrophilic pyrrolic metabolites can react with multiple proteins, forming pyrrole-protein adducts (PPAs). In our previous study, PPAs were found in blood samples from patients who had typical clinical manifestations of HSOS and a history of taking PA-containing herbs,11,12 which suggests that blood PPAs could be used as a potential diagnostic marker. However, the role of PPA quantitation in the diagnosis of PA-induced HSOS is not clear. Because PAs are exogenous, their metabolites in the body theoretically vary over time, but the pattern of dynamic blood PPA levels over time is unknown.
To date, there is no specific prognostic index for PA-induced HSOS. In this prospective cohort study, we recruited patients with potential HSOS caused by PA-containing herbs and those with DILI caused by other drugs that do not contain PAs. We evaluated the sensitivity and specificity of PPA measurement and investigated the potential for using blood PPA quantitation as a prognostic index for PA-associated HSOS.
Methods
Patients
This study was approved by the local ethics committee and informed consent was obtained from each patient. DILI patients were prospectively recruited at Zhongshan Hospital, Fudan University. Inclusion criteria were as follows: patients with new onset of liver injury (alanine transaminase >40 U/L, aspartate transaminase >40 U/L, or total bilirubin >34 μmol/L) within the past 3 months; presence of a history of taking drugs before the onset of liver injury; and a Roussel Uclaf Causality Assessment Method (RUCAM) score13 >3, which means that DILI is possible or probable. Exclusion criteria were as follows: other causes of hepatic injury (for example, a virus, autoimmune disease, alcohol abuse, fatty liver, heart failure, Budd-Chiari syndrome, congenital liver disease, or liver carcinoma); and liver injury without detailed drug information, such as the name, duration of taking drugs, and withdrawal time.
Diagnostic criteria for PA-associated HSOS were as follows: meeting the criteria for DILI; meeting the modified Seattle criteria for HSOS;5 and a previous history of taking tusanqi (a herb containing PAs) or other PA-containing herbs, as determined by ultra-performance liquid chromatography/tandem mass spectrometry (UPLC-MS). Exclusion criteria for PA-associated HSOS were as follows: no history of taking PA-containing herbs or PAs were undetectable in the herbs supplied; and liver injury had other causes, including a virus, autoimmune disease, alcohol abuse, fatty liver, heart failure, Budd-Chiari syndrome, congenital liver disease, or liver malignancy during hospitalization or follow-up.
Detailed data including patients’ age, sex, duration of taking the drug, withdrawal time, blood sampling time, liver function test results, imaging results, and follow-up information were recorded. The patients were classified into hepato-cellular liver injury, cholestatic liver injury, or mixed liver injury according to an algorithm proposed by the American College of Gastroenterology guidelines.13
All the patients were monitored for 6 months. Patients were divided into three groups (mild, moderate, and severe) based on the clinical outcome at 6 months. Clinical outcome was classified into recovery (signs and symptoms disappeared and therapeutic drugs were unnecessary at 6 months after onset), chronicity (signs and symptoms remained for 6 months and therapeutic drugs, such as diuretics, were needed) and severe outcome (liver injury caused the patient’s death or liver transplantation was performed within 6 months after the onset).
Quantitation of PPAs in blood
At least 250 μL of serum was taken from each patient. Blood PPA levels were determined using a modified UPLC-MS method.11 7,9-Diglutathionyl dehydroretronecine, a pyrrole–glutathione conjugate synthesized via reaction of dehydromonocrotaline with glutathione,14 was used to construct a calibration curve for the pyrrole-derived adducts. The quantity obtained is considered to be the concentration of the pyrrole moiety released from all possible PPAs. The technician in charge of assaying for PPAs was blinded to the clinical data and the history of drug intake.
Statistical analysis
The data were analyzed using Statistical Package for the Social Sciences version 18.0 software (SPSS Inc, Chicago, IL, USA). Continuous variables were reported as the mean and standard deviation, or the median, 25th and 75th percentiles. A normal distribution was found for the log of the blood PPA concentration. A homogeneity test of variance was performed. Means were compared using the unpaired t-test and one-way analysis of variance. Multiple linear regression analysis was also performed to determine the prognostic factors. A two-sided P-value <0.05 was considered to be statistically significant.
Results
General characteristics of all DILI patients
Between June 2008 and March 2014, 49 consecutive patients with suspected DILI were recruited according to the inclusion criteria in Zhongshan Hospital, Fudan University. Nine patients were excluded because they had another cause of liver impairment, including porphyria, autoimmune hepatitis, cavernous transformation of the portal vein, familial intra-hepatic cholestasis, hepatitis B, and hepatic carcinoma, or insufficient information on drug history (Figure 1).
Figure 1.

Flowchart of inclusion and diagnostic process in DILI patients.
Note: All numerals in the boxes indicate number of cases.
Abbreviation: DILI, drug-induced liver injury.
Among the 40 enrolled patients, 19 were females and 21 were males. The average age was 53.75 years. Four patients were classified as having hepatocellular liver injury, 21 patients had cholestatic liver injury, and 15 patients had mixed liver injury. RUCAM scores were 5–7, which indicated that the causality of DILI was possible or probable. General information for the 40 patients enrolled is listed in Table 1.
Table 1.
Demographics of patients with DILI
| PA-HSOS patients (n=23) | Other DILI patients (n=17) | P-value | |
|---|---|---|---|
| Age, year | 54.21±11.10 | 53.11±17.71 | 0.811 |
| Sex (male/total) | 13/23 | 8/17 | 0.520 |
| Drug type (1/2/3)* | 23/0/0 | 10/3/4 | 0.003 |
| RUCAM score | 5.52±0.67 | 5.82±0.64 | 0.157 |
| Alanine transferase | 243.4±60.0 | 385.1±77.8 | 0.151 |
| Aspartate transferase | 259.9±62.7 | 455.9±27.2 | 0.195 |
| Alkaline phosphatase | 161.7±12.2 | 171.4±19.1 | 0.657 |
| Total bilirubin | 66.1±19.5 | 156.9±29.1 | 0.010 |
| DILI type (hepatocellular/cholestatic/mixed liver injury) | 2/14/7 | 2/7/8 | 0.464 |
Notes:
Drug type: 1, herb; 2, synthetic drug; 3, herb/synthetic drug.
Abbreviations: DILI, drug-induced liver injury; RUCAM, Roussel Uclaf Causality Assessment Method; PA-HSOS, pyrrolizidine alkaloid-associated hepatic sinusoidal obstruction syndrome.
Twenty-three patients (23/40, 57.5%) were diagnosed with PA-associated HSOS. Ten patients completely recovered in 6 months. Four patients relied on diuretics, had symptoms, or their imaging examination showed chronic lesions or liver cirrhosis. Eight patients died from liver injury and one patient underwent liver transplantation. Among the 23 patients, 18 patients took tusanqi, two patients took wild sanqi, one patient took jinjiu, and two patients could not supply the names of the herbs that the patients took (Table 2). The duration of herb intake was 4–730 days, with a mean of 70.2 days (25th and 75th percentiles, 7.0 and 77.0 days; median 26.0 days). The onset time (from the time that the patient started to take drug to the time that symptoms appeared) was 5–730 days, with a mean of 88.8 days (25th and 75th percentiles, 21.5 and 105.5 days; median, 30.5 days).
Table 2.
Information on herbs taken by patients
| Items | Results |
|---|---|
| Herbs taken | 18 tusanqi; 2 wild sanqi; 1 jinjiu; 2 with name of herb unavailable |
| Herb intake period (days) | |
| Median (25th, 75th) | 22.0 (7.0, 72.0) |
| Mean (range) | 70.2 (4.0–730.0) |
| Time from drug intake until reaction onset (days) | |
| Median (25th, 75th) | 30.0 (20.0, 105) |
| Mean (range) | 85.57 (4–730) |
| Method of taking herbs | 7 with wine; 5 with herbal tea followed by direct ingestion of herb; 3 with direct ingestion of herbal powder (followed by water); 8 as herbal tea |
Analysis of blood PPA concentration and its risk factors
Blood PPA was detected in 24 of the 40 patients. Blood PPA levels varied from 0.05 to 74.4 nM, with a mean value of 10.50 nM. The median value was 6.70 nM (25th and 75th percentiles, 0.33 nM and 11.80 nM).
Blood PPA levels in patients with DILI caused by herbs and DILI caused by synthetic drugs were assessed. Blood PPA levels of patients with DILI caused by herbs, DILI caused by synthetic drugs, and DILI caused by synthetic drugs and herbs were different. The positive proportions of blood PPAs were 23/33, 0/3, and 1/4, respectively (Figure 1 and Table 3).
Table 3.
Blood pyrrole-protein adduct positivity in patients with DILI caused by different types of drugs
| PA-HSOS | DILI caused by other drugs
|
All DILI | |||
|---|---|---|---|---|---|
| Herb group | Synthetic drug group | Herb and synthetic drug group | |||
| Positive (no. of cases) | 23 | 0 | 0 | 1 | 24 |
| Negative (no. of cases) | 0 | 10 | 3 | 3 | 16 |
Abbreviations: DILI, drug-induced liver injury; PA-HSOS, pyrrolizidine alkaloid-associated hepatic sinusoidal obstruction syndrome.
Blood PPA concentrations were positive in all 23 patients with HSOS caused by PA-containing herbs and one of 17 DILI patients caused by drugs without PAs. Sensitivity of the test was 100% and specificity was 16/17×100% =94.1%. The positive predictive value was 23/24×100% =95.8%, the negative predictive value was 100%, and the positive likelihood ratio was 23.81.
Logarithmic values of the concentrations were calculated and the distribution of the transformed variable was normal. The blood PPA level tended to decrease quickly within 40 days and decreased slowly until it was undetectable within 300 days (Figure 2). Time from withdrawal of herb intake tended to be negatively associated with the blood PPA concentration as follows: the longer the sampling time, the lower the blood PPA concentration. However, the trend showed a slowly decreasing concentration over time (B=−0.016, Figure 2). There was no significant relationship between the blood PPA concentration and sampling time (P=0.081).
Figure 2.
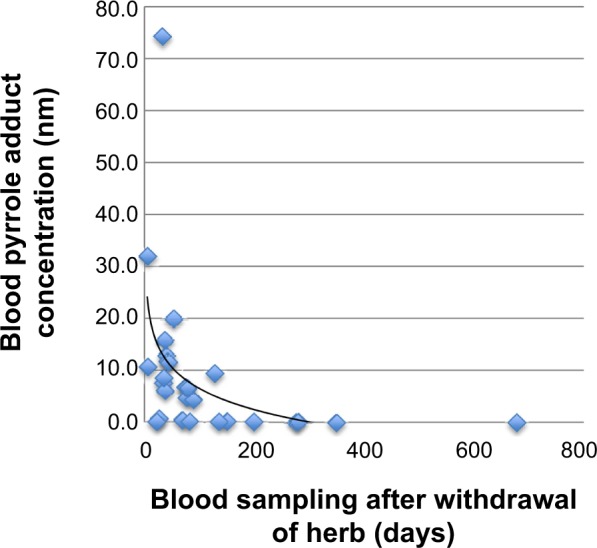
Distribution of blood pyrrole-protein adduct concentration over time for PA-associated HSOS patients.
Abbreviations: HSOS, hepatic sinusoidal obstruction syndrome; PA, pyrrolidizine alkaloid.
Disease outcome and the blood concentration of PPAs
PA-associated HSOS patients were classified into a mild (recovery) group, a moderate (chronicity) group, and a severe (died or received liver transplantation) group according to the outcome within 6 months. Biochemistry test results and PPA blood levels were compared. Data analysis showed that the blood PPA concentration was significantly associated with the clinical outcome within 6 months in PA-associated HSOS patients (P=0.045; Table 4).
Table 4.
Blood concentration of PPAs in groups with different outcomes
| Groups according to clinical outcomes | Patients (n) | In (blood concentration of PPAs, mol/L)
|
P-value | |
|---|---|---|---|---|
| Mean | SE | |||
| Recovery group | 10 | −20.69 | 0.67 | – |
| Chronicity group | 4 | −20.60 | 1.80 | 0.946* |
| Severe group (death or | 9 | −18.29 | 0.27 | 0.020* |
| liver transplantation) | ||||
| Total | 23 | −19.74 | 0.48 | 0.045# |
Notes:
Versus recovery group;
significant difference among the three groups.
Abbreviations: PPAs, pyrrole-protein adducts; SE, standard error; ln, natural logarithm function.
Blood PPA levels in the mild group were significantly different from those in the severe group (P=0.020) but not different from those in the moderate group (P=0.946). The mild and moderate groups were combined and compared with the severe group. The blood PPA concentrations in the severe group were significantly higher than those in the mild/moderate group (P=0.004; Table 5). Other factors such as age, sex, herb intake duration, herb intake method, onset time, and sampling time were not significantly associated with the blood PPA concentration using multiple linear regression analysis (P>0.05).
Table 5.
Characteristics of the mild/moderate and severe drug-induced liver injury groups
| Mild/moderate (recovery/chronicity) group (n=14) | Severe group (death or liver transplantation) (n=9) | P-value | |
|---|---|---|---|
| Sex (male/female) | 8/6 | 5/4 | 0.944 |
| Age, years | 54.57±11.46 | 53.67±11.20 | 0.854 |
| Alanine transferase | 152.29±45.61 | 385±126.35 | 0.114 |
| Aspartate transferase | 153.57±41.76 | 425±132.72 | 0.08 |
| Alkaline phosphatase | 165.43±18.42 | 155.89±13.78 | 0.713 |
| Total bilirubin | 66.14±29.87 | 66.00±20.32 | 0.997 |
| Herb intake period (days; median: 25th, 75th) | 21.50 (9.25, 77.00) | 22.00 (7.00, 117.50) | 0.364 |
| Onset time (days; median: 25th, 75th) | 35.00 (16.50, 105.50) | 28.00 (22.00, 143.50) | 0.402 |
| Sampling time after onset (days) | 61.57±41.79 | 37.33±17.61 | 0.070 |
| Sampling time after withdrawal (days) | 79.14±65.38 | 46.89±16.01 | 0.164 |
| ln (blood PPA level, mol/L) | −20.66±2.47 | −18.29±0.81 | 0.004* |
Note:
Significant difference between the two groups.
Abbreviation: PPA, pyrrole-protein adduct; ln, natural logarithm function.
Discussion
Blood PPA concentrations varied from 0.05 to 74.4 nM in PA-associated HSOS patients and the test differentiated well between patients with PA-associated HSOS (23/23) and DILI patients caused by other drugs (1/17). Blood PPA levels had a high positive predictive value (95.8%) and negative predictive value (100%) for the diagnosis of PA-associated HSOS. Therefore, blood PPA detection can be a useful and reliable diagnostic index for PA-associated HSOS. Because the diagnosis for most patients with DILI is based on exclusion of other causes and drug history, blood PPA detection can be a good supplemental test, especially for those who have an ambiguous history of drug use.
One patient who was classified as DILI caused by drugs without PAs was found to be positive for blood PPAs (0.05 nM, which was the lower limit of detection). After onset of liver injury, the patient ingested some herbs in an effort to protect his liver. We inferred that the herbs might contain PAs. It is reasonable that the original cause of liver injury might be the synthetic drugs that the patient had used before onset of the disorder, and the subsequent ingestion of PA-containing herbs caused the positive blood PPA results.
The recent American College of Gastroenterology guidelines for the diagnosis and management of patients with DILI suggest the need to consider drug-induced causes in patients with what appears to be acute viral hepatitis or other types of chronic liver disease, and the importance of taking a careful patient history with respect to the use of medications or herbal supplements in those with recent-onset liver diseases.13 A lack of information or ambiguity about the suspected drugs causes difficulty in making an accurate diagnosis.
Analysis of drug metabolite biomarkers in samples from high-risk subjects will lead to improved understanding, diagnosis, differential diagnosis, therapeutic evaluation, and prognostic evaluation of DILI. Current biomarkers for DILI are essential to diagnose severe DILI, but their ability to differentiate between patients with severe DILI and those with mild DILI at the onset of DILI and/or to predict an outcome following liver injury is limited.15
Blood PPAs are derived from toxic PA metabolites. They are detectable using UPLC-MS and a qualitative study suggested that blood PPA determination may be used as a diagnostic marker.11 The current study is the first investigation to quantify blood PPA levels in PA-associated HSOS patients and to evaluate its role as a diagnostic marker and prognostic index. There is currently no appropriate prognostic index for PA-associated HSOS.
Hyperbilirubinemia, significant fluid retention, and a hepatic venous pressure gradient greater than 20 mmHg have been reported to indicate a poor prognosis, but these indicators lack specificity or feasibility.15 Patients who met the criteria for an HSOS diagnosis after bone marrow transplantation were retrospectively assessed based on their outcome within 100 days.6,16 However, this assessment has not been used in patients with HSOS caused by PAs.
Our data showed that blood PPA levels in PA-associated HSOS patients who died from liver injury within 6 months or who underwent liver transplantation were significantly higher than in those who recovered completely or manifested a chronic process (P=0.004). This suggests that blood PPA levels may be a prognostic index for PA-associated HSOS: the higher the blood PPA concentration, the worse the patient’s prognosis. Therefore, in addition to a qualitative measure, quantification of blood PPA levels is necessary and meaningful.
After 40 days, blood PPA concentrations tended to decrease slowly, which indicates that PA metabolites can remain in the blood for a relatively long period of time. This suggests that the blood index is a potential prognostic variable and a diagnostic method for PA-associated HSOS within a certain time frame. The 6-month outcome for patients with PA-associated HSOS is significantly associated with blood PPA concentrations, but the relationship is not well established within 100 days. Therefore, we recommend that patients with PA-associated HSOS be followed up for at least 6 months.
Existing DILI scoring systems such as the Council of International Organizations of Medical Scientists (CIOMS)/RUCAM scale17,18 take into account the onset time after drug administration and the reaction after withdrawal of drugs (whether liver function improves or not). However, PA-associated HSOS can have a long latent period,13 and improvement of signs and symptoms is rare in PA-associated HSOS patients. Ascites, hepatomegaly, and severe liver injury can remain and worsen after withdrawal of the herbs. Scores using the CIOMS/RUCAM scale are relatively low compared with DILI caused by other drugs. PA-associated HSOS is a special type of DILI, and the assessment scale should be carefully selected and explained.
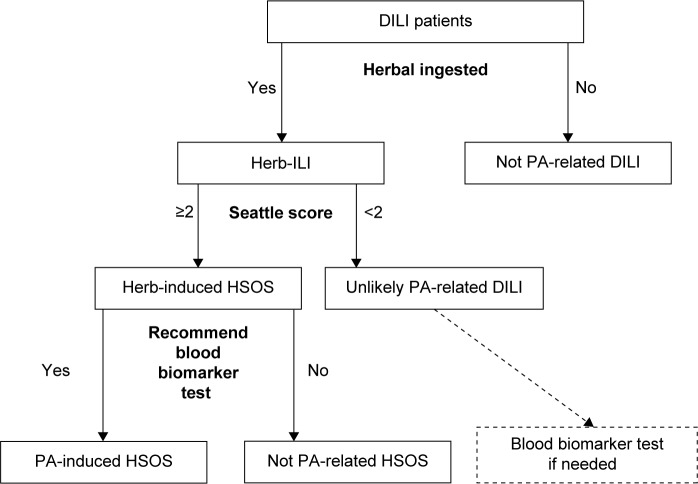
Although the Seattle criteria were based on patients who underwent bone marrow transplantation, they were shown to be useful for diagnosis in patients with HSOS caused by PA-containing herbs. A detailed history of herb ingestion, modified Seattle criteria, and blood PPA levels are key points for the diagnosis of PA-associated HSOS. We suggest using the diagnostic flow chart for PA-associated HSOS, as illustrated in Figure 3.
Figure 3.
Suggested procedures for the diagnosis of PA-induced HSOS.
Abbreviations: HSOS, hepatic sinusoidal obstruction syndrome; ILI, induced liver injury; DILI, drug-induced liver injury; PA, pyrrolidizine alkaloid.
Because we did not enroll all DILI patients in this study, it is difficult to determine the proportion of PA-induced liver injury in all the DILI patients. Because no other researchers in the People’s Republic of China reported identifying PPAs in blood samples, many patients with PA-associated HSOS were referred to our hospital from other hospitals in the People’s Republic of China, which resulted in a high positive proportion of blood PPAs for DILI patients in this study; thus, the percentage of 60% (24/40) for blood PPA levels might be overestimated in DILI patients in the People’s Republic of China.
In our study, most of the patients were transferred from local hospitals. The blood sampling times were different, which complicates the analysis of blood metabolite concentrations. Further, the half-life of PPAs cannot be estimated based on the available data. These data were measured from different patients, and the initial PPA levels in different individuals varied significantly because of differences in the dose of PAs taken and the duration of herb use. More patients and multiple different sampling time points could provide more information for analyzing the relationship between the blood PPA concentration and the natural history of PA-associated HSOS.
PA metabolites are reactive and can bind with multiple proteins, and many factors might affect the blood PPA concentration. For example, the effect of serum albumin level on blood PPA concentration should be further investigated. It is noted that, although the detection of PPAs is useful for diagnosis and prognosis of PA-induced HSOS, the presently used UPLC-MS methodology is sophisticated and not commonly available in clinical practice, which may limit the application of this method.
In conclusion, blood PPA measurement using UPLC-MS is a highly sensitive and specific diagnostic indicator, and also a prognostic index for PA-associated HSOS. It can be used to predict outcomes for patients with PA-associated HSOS. We recommend that patients with suspected PA-associated HSOS undergo quantitative PPA concentration analysis using UPLC-MS.
Acknowledgments
This study was supported by the Shanghai Committee of Science and Technology, People’s Republic of China (grant 12401907400) and the Research Grant Council of Hong Kong SAR (GRF grants, 471013 and 469712).
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399–1408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teschke R, Frenzel C, Glass X, Schulze J, Eickhoff A. Herbal hepatotoxicity: a critical review. Br J Clin Pharmacol. 2013;75(3):630–636. doi: 10.1111/j.1365-2125.2012.04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JY, Gao H. Tusanqi and hepatic sinusoidal obstruction syndrome. J Dig Dis. 2014;15(3):105–107. doi: 10.1111/1751-2980.12112. [DOI] [PubMed] [Google Scholar]
- 4.Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10(6):451–462. doi: 10.1097/00062752-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther. 2006;23(1):11–25. doi: 10.1111/j.1365-2036.2006.02742.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Huo JR. Hepatic veno-occlusive disease associated with toxicity of pyrrolizidine alkaloids in herbal preparations. Neth J Med. 2010;68(6):252–260. [PubMed] [Google Scholar]
- 7.Dübecke A, Beckh G, Lüllmann C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(3):348–358. doi: 10.1080/19440049.2010.541594. [DOI] [PubMed] [Google Scholar]
- 8.Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine alkaloids. J Hepatol. 2003;39(3):437–446. doi: 10.1016/s0168-8278(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 9.Edgar JA, Colegate SM, Boppré M, Molyneux RJ. Pyrrolizidine alkaloids in food: a spectrum of potential health consequences. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(3):308–324. doi: 10.1080/19440049.2010.547520. [DOI] [PubMed] [Google Scholar]
- 10.Fu PP, Xia Q, Lin G, Chou MW. Pyrrolizidine alkaloids – genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev. 2004;36(1):1–55. doi: 10.1081/dmr-120028426. [DOI] [PubMed] [Google Scholar]
- 11.Lin G, Wang JY, Li N, et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J Hepatol. 2011;54(4):666–673. doi: 10.1016/j.jhep.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Gao H, Li N, Wang JY, Zhang SC, Lin G. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J Dig Dis. 2012;13(1):33–39. doi: 10.1111/j.1751-2980.2011.00552.x. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ, Practice Parameters Committee of the American College of Gastroenterology ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–966. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 14.Lin G, Cui YY, Hawes EM. Microsomal formation of a pyrrolic alcohol glutathione conjugate of clivorine. Firm evidence for the formation of a pyrrolic metabolite of an otonecine-type pyrrolizidine alkaloid. Drug Metab Dispos. 1998;26(2):181–184. [PubMed] [Google Scholar]
- 15.Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11(9):1729–1736. doi: 10.1200/JCO.1993.11.9.1729. [DOI] [PubMed] [Google Scholar]
- 16.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37(1):3–17. doi: 10.1111/apt.12109. [DOI] [PubMed] [Google Scholar]
- 18.Teschke R, Wolff A, Frenzel C, et al. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6(1):17–32. doi: 10.4254/wjh.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]