Abstract
To clarify the significance of immunoglobulin G autoantibody specific for the astrocyte water channel aquaporin-4 in cerebrospinal fluid, aquaporin-4-immunoglobulin G from a neuromyelitis optica patient was administered intrathecally to naïve mice, and the distribution and pathogenic impact was evaluated. A distinct distribution pattern of aquaporin-4-immunoglobulin G deposition was observed in the subarachnoid and subpial spaces where vessels penetrate the brain parenchyma, via a paravascular route with intraparenchymal perivascular deposition. Perivascular astrocyte-destructive lesions were associated with blood-borne horseradish peroxidase leakage indicating blood-brain barrier breakdown. The cerebrospinal fluid aquaporin-4-immunoglobulin G therefore distributes widely in brain to initiate astrocytopathy and blood-brain barrier breakdown.
Introduction
Neuromyelitis optica (NMO) is an autoimmune inflammatory disease of the central nervous system (CNS),1 associated with serum immunoglobulin G autoantibody specific for water channel aquaporin-4 (AQP4-IgG).2 AQP4-IgG is thought to mediate pathogenesis by binding selectively to AQP4 on astrocytes, causing complement-dependent injury.3 AQP4 is densely localized on ependymal cells and astrocytes, forming the glia limitans of the blood-brain barrier (BBB).2 Ependymal cells do not have a basement membrane and do not express tight junctions, thus AQP4-IgG in the cerebrospinal fluid (CSF) would have relatively free access to their target.4 AQP4-IgG is pathogenic only when reaching the CNS parenchyma as demonstrated in experimental animals where direct administration of AQP4-IgG into the CNS or with preestablished CNS inflammation induced NMO-like histopathology, whereas peripheral administration into naïve animals had no effect.5 NMO patients can be AQP4-IgG positive in serum for many years prior to the onset of disease.6,7 Gadolinium-enhanced magnetic resonance imaging (MRI) shows acute lesions in CNS including meningeal enhancement and markers of BBB permeability are significantly elevated during disease activity indicating BBB dysfunction.8,9 NMO lesions often have association to blood vessels as though the pathogenic mediator entered from blood.3 AQP4-IgG is detectable in the CSF of most AQP4-IgG seropositive NMO patients with AQP4-IgG serum titers >1:250 during acute disease relapse.10 Since intrathecal IgG synthesis in NMO only occurs rarely and does not persist over time, CSF AQP4-IgG likely derives from serum, but how it contributes to pathogenesis and BBB breakdown is poorly understood.1
We have previously reported that intrathecal injection of AQP4-IgG together with complement into the CSF of mice induced NMO-like periventricular lesions, correlating with ependymal damage.11 We also observed parenchymal NMO-like lesions that did not always co-localize with ependymal damage, suggesting entry by other route(s). We here show that AQP4-IgG in CSF can access the brain parenchyma and further induce perivascular lesions and BBB breakdown.
Materials and Methods
Experimental design
AQP4-IgG or control human IgG (HuIgG) and human complement were prepared as described11 and administered to adult female C57BL/6 mice as a single injection (6 μL AQP4-IgG + 4 μL complement) into CSF at the cisterna magna.11 Mice were sacrificed after 2 days.
The use of human material was approved by The Committee on Biomedical Research Ethics for the Region of Southern Denmark (ref. no. S-20080142). Mice were housed and experiments performed in accordance with the Danish Animal Research Committee (approval number 2012−15−2934−00110).
Tissue processing, histochemistry and immunohistochemistry
Tissue preparations were made.11 BBB breakdown was examined by injecting horseradish peroxidase (HRP) as a tracer intravenously 15 min before perfusion, then staining in tissue. Mouse IgG was detected by HRP-conjugated goat anti-mouse-IgG (A4416; Sigma, Sigma-Aldrich, Brøndby, Denmark). Single and double immunostaining were performed as described.11
Histopathology
Evaluation of histological changes was performed in a blinded manner and semiquantitatively using a scale from 0 to 3 (+ to +++) as described.11 Results were analyzed by one-way analysis of variance (ANOVA) test and Mann–Whitney U-Test. A P < 0.05 was considered statistically significant.
Animal assessment
Animals were assessed by measurement of whole body weight and gross evaluation of well-being. Assessment of behavioral or motor changes was not part of the study design. There was no difference between body weight of mice that received AQP4-IgG + complement and controls.
Results
Entry of AQP4-IgG from CSF into the brain parenchyma
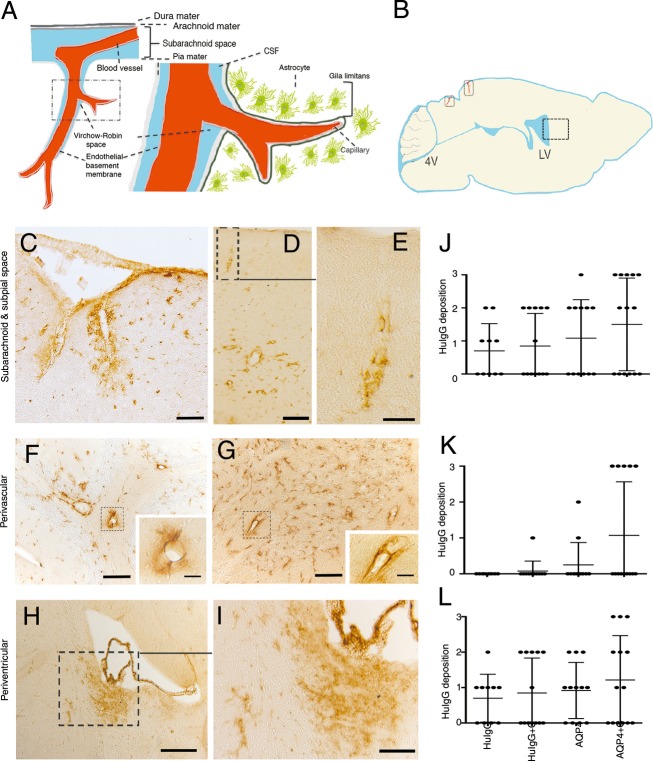
We examined parenchymal distribution patterns of AQP4-IgG in brain parenchyma and pathologic effects, following intrathecal injection. Injection of AQP4-IgG + complement into the CSF was followed by deposition of HuIgG along the subarachnoid space and the subpial spaces where vessels penetrate the brain parenchyma in 8/14 mice, severe in five, localized in the cerebellum, brainstem and to a lesser degree in the cortex (Fig.1A–E and J). This deposition was for AQP4-IgG + complement associated with an intense perivascular deposition at brain parenchymal vessels distal from the site of parenchymal entry of pial vessels (Fig.1F, G, and K) in five mice. In this area no deposition was observed for control HuIgG (0/10), and apart from one animal (1/12) with a moderate score in the cerebellum, AQP4-IgG deposition was only notable when co-injected with complement. For sites close to the brain surface where pial vessels penetrate there was some deposition of AQP4-IgG alone (6/12) and of control IgG + complement (6/13) and – complement (5/10) in the cerebellum.
Figure 1.
Distribution of AQP4-IgG from cerebrospinal fluid (CSF) into the brain parenchyma. (A) Subpial vasculature in relation to subarachnoid space and brain parenchyma, showing relevant anatomical structures including the pial vessel, subarachnoid space, the Virchow-Robin space and the subpial glia limitans surrounding penetrating vessels into the brain, in schematic form. (B) A mouse brain in sagittal section showing a diagram of the intrathecal distribution of human IgG in this study. Areas of penetrating vessels into the brain are marked with rectangles. (C–K) Sagittal sections of the brain of animals after intrathecal injection of AQP4-IgG + complement into the cisterna magna. Sections were stained for human IgG. (C) Micrographs show distribution of AQP4-IgG in the brain parenchyma around surface pial vessels in subarachnoid and subpial spaces, surrounding penetrating vessels into the brain in midbrain (superior colliculus, midbrain), (D) and in a cortical region proximal to the hippocampus, (E) shows a magnified view of an area in (D) with a penetrating vessel into the brain. (F) Perivascular deposition of AQP4-IgG at brain parenchymal vessels distal from the site of parenchymal entry of pial vessels in cerebellum and (G) midbrain. (H) periventricular deposition of AQP4-IgG at the lateral ventricles and (I) Magnified view of an area from (H). (J–L) HuIgG deposition of varying intensity from groups of mice treated 2 days previously by intrathecal injection of AQP4-IgG + complement (n = 14), AQP4-IgG alone (n = 12), control HuIgG (n = 10), control HuIgG + complement (n = 13). Histological grading as mild (+ = 1), moderate (++ = 2) or intense (+++ = 3). Data are presented as mean ± SD, (J) Paravascular route deposition (K) Perivascular deposition at brain parenchymal vessels distant from the site of parenchymal entry of pial vessels (L) Periventricular deposition. Bar 50 μm (C, F, and G), 20 μm (inserts F, G, and E), 100 μm (D and I), 200 μm (H).
In addition, AQP4-IgG from CSF was distributed to the periventricular region as described previously11 (Fig.1H, I, and L). Co-injection of complement led to intense deposition in three mice and IgG deposition in 8/14 mice. Deposition was most intense at the fourth and lateral ventricles. AQP4-IgG alone (deposition in 8/12) and control IgG (6/10) distributed similarly, localized at the fourth ventricle, and deposition of control IgG was not influenced by complement (6/13).
The distribution patterns J and L did not show any significant difference between the groups. AQP4-IgG + complement-treated mice presented a significantly more intense perivascular deposition of HuIgG (pattern K) as compared to controls HuIgG and HuIgG + complement (P < 0.0001). These data indicate that pattern K is specific for AQP4-IgG.
AQP4-IgG in the CSF induces perivascular astrocyte-destructive lesions
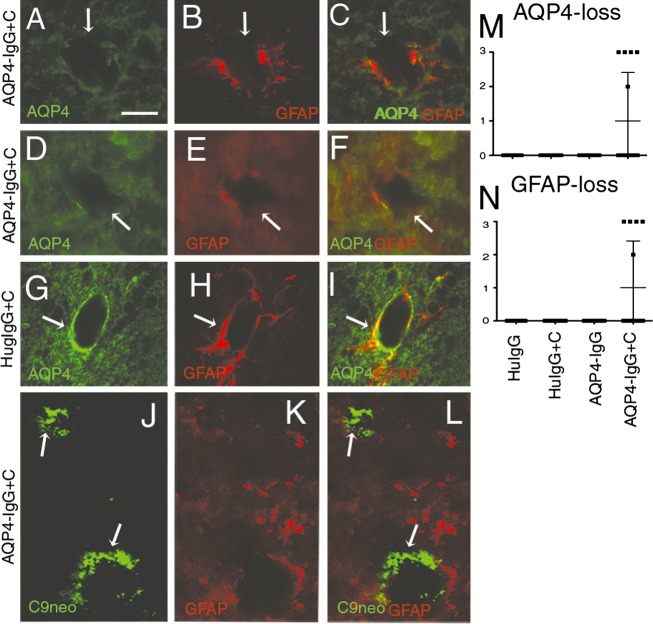
The perivascular deposition of HuIgG, observed at brain parenchymal vessels distant from the site of pial vessels in mice that received AQP4-IgG + complement, raised the question whether AQP4-IgG would achieve sufficient concentrations at brain parenchymal vessels to initiate astrocyte-destructive lesions. Perivascular leakage of HuIgG co-localized with deposition of C9neo (a marker of the membrane attack complex, i.e., activated complement) and astrocytopathy (loss of AQP4 and glial fibrillary acidic protein [GFAP]) in the five AQP4-IgG + complement-treated mice, with perivascular and subpial deposition of HuIgG in cerebellum (+++), brainstem (++) and to a lesser degree in cortex (+) (Fig.2A–F and M, N). In addition, similar NMO-like lesions were induced by AQP4-IgG + complement in subpial space and surrounding the ventricular system as described previously.11 These histopathological changes were not seen in control mice (Fig.2G–I).
Figure 2.
AQP4-IgG in the CSF induces perivascular complement-dependent astrocyte injury. Micrographs show sagittal sections of the brain of animals that had received intrathecal injection of AQP4-IgG + complement (A–F) or control IgG + complement (G–I). Sections were stained for AQP4 (A, D, and G), GFAP (B, E, H, and K) and C9neo (J). Areas of histopathological changes are marked with white arrowheads. (A and B) Loss of AQP4, (D and E) Loss of GFAP staining in midbrain and in cerebellum. This astropathy coincided with perivascular deposition of HuIgG at brain parenchymal vessels distant from the site of pial vessels in mice that received AQP4-IgG + complement. (C and F) Merge of (A and B), and (D and E) respectively, showing that loss of AQP4 coincided with loss of GFAP. Such pathology was not seen in control mice (G–I). (J–L) Deposition of C9neo at the same locations as loss of AQP4 and GFAP, denoted by white arrows. (M and N) Semi-quantitative estimate of loss of AQP4 (M) and GFAP immunoreactivity. Data are presented as mean ± SD (N). Bar 15 μm (J–L), Bar 20 μm (A–I). CSF, cerebrospinal fluid; GFAP, glial fibrillary acidic protein.
Effects of intrathecal AQP4-IgG on the integrity of the BBB
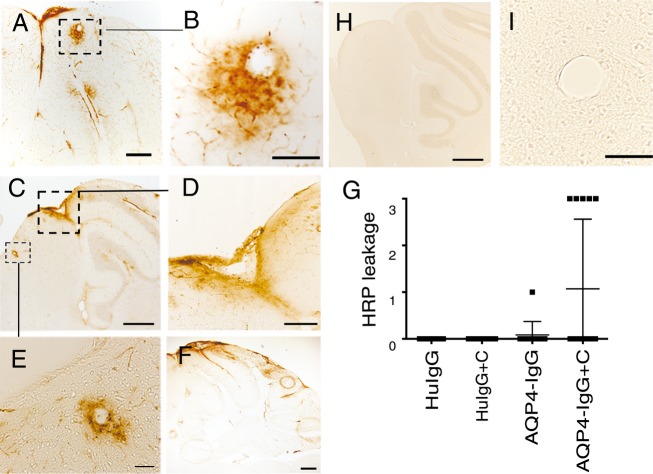
Perivascular astrocyte-destructive lesions were associated with disruption of the BBB. HRP from peripheral blood leaked into the brain parenchyma in cerebellum and brainstem and to a lesser degree in cortex in the same five AQP4-IgG + complement-treated mice that exhibited perivascular astrocyte pathology (5/14) (Fig.3A–G). Staining for mouse IgG confirmed the HRP results, and verified that parenchymal diffusion of CSF-derived control HuIgG was not accompanied by endogenous mouse IgG (data not shown). BBB breakdown without AQP4-IgG-mediated immunopathology was not observed.
Figure 3.
BBB breakdown associates with CSF-derived AQP4-IgG + complement and perivascular astrocyte pathology. Micrographs show sagittal sections of the brain of animals injected with AQP4-IgG + complement. BBB breakdown was visualized by leakage of intravenously injected HRP as tracer (brown). (A–F) Micrographs show HRP leakage into the brain parenchyma in cerebellum and midbrain, (D) shows a magnified view of an area in (C) in midbrain. (B and E) show magnified views of perivascular HRP leakage shown in (A and C) respectively, as wide halos around blood vessels. Such pathology was not seen in controls mice (H and I). (G) Quantitation of HRP leakage into the brain parenchyma in animals injected with AQP4-IgG + complement (n = 14), AQP4-IgG alone (n = 12), control HuIgG (n = 10), control HuIgG + complement (n = 13), expressed on a scale of 0–3. Data are presented as mean ± SD. Data indicate BBB breakdown in AQP4-IgG + complement-treated mice. Bar 50 μm (B and D), 20 μm (E), 100 μm (A and F), 200 μm (C, F, and H). BBB, blood-brain barrier; CSF, cerebrospinal fluid; HRP, horseradish peroxidase.
In the control group one mouse receiving AQP4-IgG alone (1/12) showed faint HRP staining in the cerebellum. The HRP staining in the brain was not seen in control mice (Fig.3H and I).
Discussion
In this study the anatomical basis of distribution of IgG from CSF to brain parenchyma was investigated. A specific distribution pattern of AQP4-IgG was identified along subarachnoid and subpial spaces, where vessels penetrate the brain parenchyma, with pronounced intraparenchymal perivascular deposition compared to control IgG. These changes were accompanied by perivascular astrocyte-destructive lesions and by leakage of the blood-derived tracer HRP and serum-IgG into brain parenchyma, reflecting BBB breakdown.
The subpial space surrounds penetrating vessels,12 and is completely ensheathed by astrocyte end-feet. The pial cell layer is fenestrated at the level of the arterioles and widely lacking at the level of capillaries and venules.13 In these areas, the AQP4-expressing astrocytic endfeet are directly exposed to CSF AQP4-IgG. Recently, an anatomically distinct clearing system in the brain was described, whereby a large proportion of subarachnoid CSF circulates through the brain parenchyma via the so-called paravascular route and is exchanged with the interstitial fluid.14 This observation was substantiated by studies in which fluorescent tracers and a MRI contrast agent could be tracked from CSF to brain parenchyma via this route.15 This pathway comprises a physically and functionally distinct sub-compartment of the brain circulation. Interestingly, the glia limitans-associated water pump AQP4 drove the flow of fluid along this route and through the interstitium.15 Consistent with these observations our data suggest that AQP4-IgG in CSF can achieve widespread distribution within the brain via this paravascular pathway to exert pathologic effects by selective binding to astrocytes with subsequent complement-dependent cytotoxicity that includes parenchymal BBB disruption.
For several reasons not all mice showed the same distribution of pathology. Firstly, we intrathecally injected only a single bolus of AQP4-IgG plus complement. Secondly, recirculation of the CSF may lead to a further dilution of the preparation. Thirdly, only a proportion of the subarachnoid CSF enters the brain parenchyma along paravascular channels surrounding penetrating arteries. Furthermore, astrocytopathy and BBB disruption require even deeper penetration of IgG distant from the site of parenchymal entry of pial vessels.
In NMO the presence and levels of AQP4-IgG in CSF are associated with high titers of AQP4-IgG in serum during disease attacks.10,16,17 Moreover, the amount of AQP4-IgG present in the CSF correlated with astrocyte damage, as reflected in elevated levels of GFAP in the CSF during relapse of NMO18 and BBB disruption.17,19 In the present study AQP4-IgG in CSF was observed to be a critical element to promote perivascular astrocyte pathology and consequently BBB disruption. These data suggest a model whereby a small amount of AQP4-IgG initially is spilled over to the CSF, and then initiates a pathogenic process,20 similar to the characteristic CSF data and radiological features of human NMO.8,17,21 This model may be further improved by repetitive administration of antibody.22
In conclusion we have utilized an intrathecal route for antibody administration, mimicking physiological presence of antibody in the CSF. We demonstrate that AQP4-IgG in CSF achieves widespread distribution to brain parenchyma via the paravascular route. Our observations that perivascular astrocyte-destructive lesions are formed in the brain parenchymal vessels associated with inside-out breakdown of the BBB indicate that AQP4-IgG in CSF can be a significant element in NMO pathogenicity.
Acknowledgments
The authors thank Soeren Lillevang (Clinical Immunology Department, Odense University Hospital) and Lars Vitved (Department of Cancer and Inflammation Research, IMM, SDU) for antibody characterization and purification. We thank Dina Draeby and Pia Nyborg Nielsen for excellent technical assistance. We acknowledge funding support from the Lundbeck Foundation, the Danish Multiple Sclerosis Society, The Region of Southern Denmark and The Danish Council for Independent Research. C. T. B. acknowledges a Ph.D. stipend from The University of Southern Denmark Health Sciences Faculty.
Conflict of Interest
Dr. Asgari reports grants from Merck Serono, Lundbeck Foundation, outside the submitted work.
References
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, et al. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Experimental models of neuromyelitis optica. Brain Pathol. 2014;24:74–82. doi: 10.1111/bpa.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama S, Ito T, Misu T, et al. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology. 2009;72:1960–1961. doi: 10.1212/WNL.0b013e3181a82621. [DOI] [PubMed] [Google Scholar]
- Asgari N, Henriksen TB, Petersen T, et al. Pregnancy outcomes in a woman with neuromyelitis optica. Neurology. 2014;83:1576–1577. doi: 10.1212/WNL.0000000000000911. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84:1165–1173. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa Y, Yokoyama K, Saiki S, et al. Blood-brain barrier disruption is more severe in neuromyelitis optica than in multiple sclerosis and correlates with clinical disability. J Int Med Res. 2012;40:1483–1491. doi: 10.1177/147323001204000427. [DOI] [PubMed] [Google Scholar]
- Jarius S, Franciotta D, Paul F, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation. 2010;7:52. doi: 10.1186/1742-2094-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari N, Khorooshi R, Lillevang ST, Owens T. Complement-dependent pathogenicity of brain-specific antibodies in cerebrospinal fluid. J Neuroimmunol. 2013;254:76–82. doi: 10.1016/j.jneuroim.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- Sato DK, Callegaro D, de Haidar Jorge FM, et al. Cerebrospinal fluid aquaporin-4 antibody levels in neuromyelitis optica attacks. Ann Neurol. 2014;76:305–309. doi: 10.1002/ana.24208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano R, Misu T, Takahashi T, et al. Astrocytic damage is far more severe than demyelination in NMO: a clinical CSF biomarker study. Neurology. 2010;75:208–216. doi: 10.1212/WNL.0b013e3181e2414b. [DOI] [PubMed] [Google Scholar]
- Bergamaschi R. Importance of cerebrospinal fluid examination in differential diagnosis of Devic’s neuromyelitis optica by multiple sclerosis. Neurol Sci. 2003;24:95–96. doi: 10.1007/s10072-003-0092-4. [DOI] [PubMed] [Google Scholar]
- Asgari N, Skejoe HP, Lennon VA. Evolution of longitudinally extensive transverse myelitis in an aquaporin-4 IgG-positive patient. Neurology. 2013;81:95–96. doi: 10.1212/WNL.0b013e318297ef07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Paul F, Franciotta D, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. 2011;306:82–90. doi: 10.1016/j.jns.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Geis C, Ritter C, Ruschil C, et al. The intrinsic pathogenic role of autoantibodies to aquaporin 4 mediating spinal cord disease in a rat passive-transfer model. Exp Neurol. 2015;265:8–21. doi: 10.1016/j.expneurol.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]