Abstract
Purpose/Introduction
Changes in the quality of bone material contribute significantly to bone fragility. In order to establish a better understanding of the interaction of the different components of bone quality and their influence on bone fragility we investigated the relationship between non-enzymatic glycation, resorption, and microdamage generated in vivo in cortical bone using bone specimens from the same donors.
Methods
Total fluorescent advanced glycation end-products (AGEs) were measured in 96 human cortical bone samples from 83 donors. Resorption pit density, average resorption pit area, and percent resorption area were quantified in samples from 48 common donors with AGE measurements. Linear microcrack density and diffuse damage were measured in 21 common donors with AGE and resorption measurements. Correlation analyses were performed between all measured variables to establish the relationships among them and their variation with age.
Results
We found that average resorption pit area and percent resorption area decreased with increasing AGEs independently of age. Resorption pit density and percent resorption area demonstrated negative age-adjusted correlation with diffuse damage. Furthermore, average resorption pit area, resorption pit density, and percent resorption area were found to decrease significantly with age.
Conclusions
The current study demonstrated the in vivo interrelationship between the organic constituents, remodeling, and damage formation in cortical bone. In addition to the age-related reduction in resorption, there is a negative correlation between AGEs and resorption independent of age. This inverse relationship indicates that AGEs alter the resorption process and/or accumulate in the tissue as a result of reduced resorption and may lead to bone fragility by adversely affecting fracture resistance through altered bone matrix properties.
Keywords: Non-enzymatic glycation, Advanced glycation end-products, Bone resorption, Microdamage, Cortical bone
INTRODUCTION
Changes in the quality of bone material contribute significantly to bone fragility [1] and provide critical information that cannot be assessed by traditional fracture risk assessment techniques based on bone mineral density [1,2].
Bone quality is influenced by all hierarchical levels of bone including composition and arrangement of mineral and collagen phases, microstructural features, and cellular mechanisms such as bone remodeling [1,3]. Changes that occur in any of the material length scales and cellular mechanisms alter bone’s material properties and fracture resistance and may lead to increased bone fragility. As a result, establishing the interrelationship between parameters that influence bone quality is important to gain a better understanding of fracture risk and prevention. In order to achieve this, in the current study, we investigated the relationships between non-enzymatic glycation (NEG), resorption, and microdamage generated in vivo in cortical bone using bone specimens from the same donors.
Non-enzymatic glycation, one of the processes investigated in this study, leads to the formation of advanced glycation end-products (AGEs) which negatively affect the mechanical properties of bone [4,5]. AGEs also alter the bone remodeling process by inhibiting osteoblast proliferation and differentiation [6]. On the other hand, there have been contradictory findings about the relationship between osteoclastic resorption and AGEs. In vitro studies have demonstrated both increasing [7,8] and decreasing [9] trends in osteoclastic resorption with increasing AGEs. In addition, AGEs have been shown to reduce the effectiveness of the energy dissipation mechanisms in both cancellous and cortical bone by influencing the damage formation process and morphology [4,10,11]. Microdamage may also be affected by changes in bone resorption. Previous studies have demonstrated an increase in the number of linear microcracks with reduced bone turnover [12-14]. Microdamage influences the fracture propensity of bone. Diffuse damage preferentially forms in young donor bone and is a more effective energy dissipation mechanism than linear microcracks [15,16]. Although these observations provide insight into the relationship between NEG, bone remodeling, and microdamage, a study that evaluates the levels of these parameters generated in vivo and their association on the same set of donors has not been reported. A combined assessment of NEG, resorption, and microdamage occurrence in vivo will provide valuable information on the interaction and interrelationships between composition, cellular processes, and damage formation which directly affect the fracture propensity of bone.
The goal of this study is to derive the relationships between factors that affect bone quality including NEG, resorption, and microdamage in cortical bone. The results will elucidate the interaction and association between in vivo accumulation of AGEs, osteoclastic bone resorption, and microdamage and provide insight into how these changes ultimately influence the fracture propensity of bone.
METHODS
The current study is based on investigations performed on cortical bone specimens from the same donor inventory that evaluated the non-enzymatic glycation content, resorption, and microdamage (Figure 1a). In order to investigate the relationship between all parameters, common donors in each study were identified resulting in 96 samples from 83 donors for non-enzymatic glycation, 48 common donors between non-enzymatic glycation and resorption, and 21 common donors between non-enzymatic glycation, resorption, and microdamage measurements (Table 1). Donors for each study were selected such that samples from a wide age range were included in all analyses. The number of male donors was 2 to 3 times more than the female donors in each study (Table 1). All donors tested negative for hepatitis B and HIV. None of the donors had known history of osteoarthritis or diabetes or metabolic bone diseases.
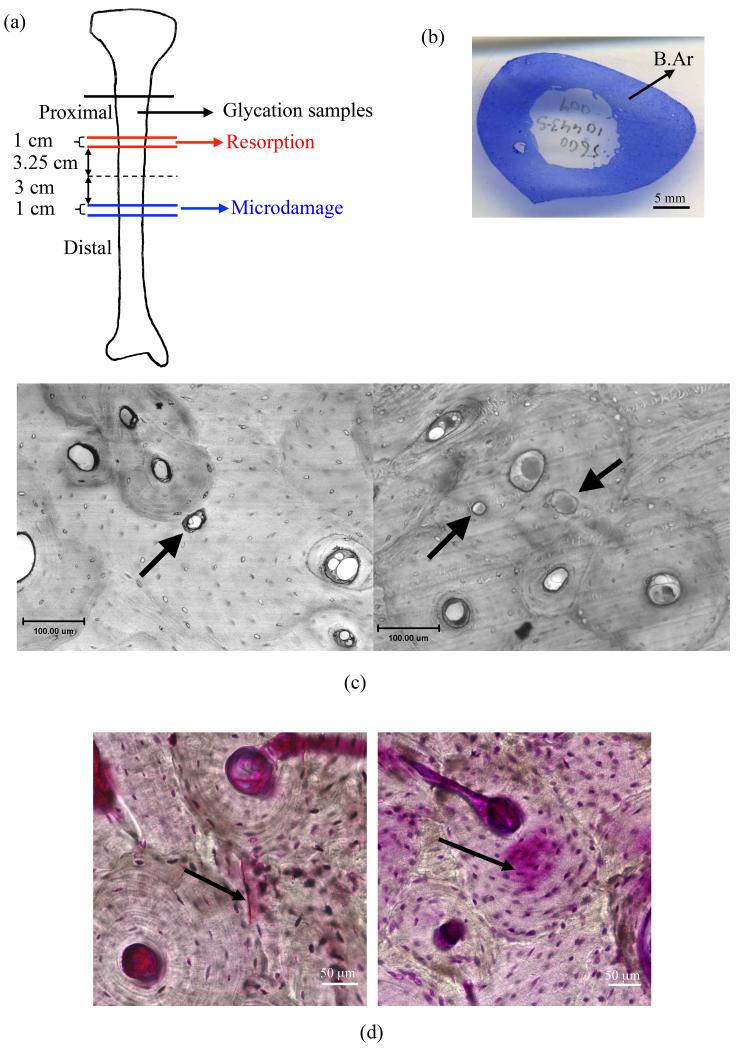
Figure 1.
(a) Schematics of the locations where bone samples for different types of analyses were taken from each tibia. (b) A sample cross-sectional area of bone slice (B.Ar) used in resorption analyses. In vivo generated (c) resorption pits in interstitial (left panel) and osteonal (right panel) bone marked by arrows (d) linear microcracks (left panel) and diffuse damage (right panel) marked by arrows (Adapted from [15]).
Table 1.
Summary of correlations investigated and the number of common samples available for each analysis including the age range of donors. Note that the first line in the second column is the number of samples and second line is the number of donors from which the samples were obtained. M and F refer to male and female donors.
| Correlations | Number of Samples and Donors |
Age |
|---|---|---|
| Non-enzymatic Glycation vs. Age | 96 (83: 57M, 26F) |
Total: 19-97 years (Average: 60.6 ± 21.0 years) |
| Male: 19-85 years (Average: 60.4 ± 20.2 years) |
||
| Female: 19-97 years (Average: 61.1 ± 23.0 years) |
||
| Resorption vs. Age | 48 (48: 36M, 12F) |
Total: 19-97 years (Average: 61.1 ± 20.2 years) |
| Male: 19-85 years (Average: 63.4 ± 19.4 years) |
||
| Female: 19-97 years (Average: 64.4 ± 24.9 years) |
||
| Non-enzymatic Glycation vs. Resorption |
48 (48: 36M, 12F) |
Total: 19-97 years (Average: 61.1 ± 20.2 years) |
| Male: 19-85 years (Average: 63.4 ± 19.4 years) |
||
| Female: 19-97 years (Average: 64.4 ± 24.9 years) |
||
| Non-enzymatic Glycation and Resorption vs. Microdamage |
21 (21: 14M, 7F) |
Total: 25-89 years (Average: 59.9 ± 21.5 years) |
| Male: 25-85 years (Average: 65.6 ± 22.0 years) |
||
| Female: 34-89 years (Average: 60.7 ± 22.7 years) |
Measurement of Total Fluorescent Advanced Glycation End-Products
AGEs generated in vivo were measured in a total of 96 cortical bone specimens that were obtained from the posterior quadrant of the proximal end of human tibiae (n = 83) of both male and female donors (age range: 19 to 97) (Table 1). The bone samples were lyophilized overnight using a freeze dry system (Labconco), and hydrolyzed according to dry mass in 6N HCl (10 uL HCl per 1 mg bone) for 20 hours at 110°C in a vacuum oven. The centrifuged hydrosylates were used to quantify total fluorescent advanced glycation end-products via a fluorometric assay. All centrifuged hydrosylates were stored at −80°C in complete darkness until use.
Total fluorescent AGEs were quantified using protocols from previous studies [17-19]. Fluorescence was measured for quinine standards (stock solution: 10 μg/mL quinine in 0.1 N sulfuric acid) and hydrosylates at 360/460 nm excitation/emission using an Infinite 200 microplate reader (Tecan). A chloramine-T solution was added to hydroxyproline standards (stock solution: 2000 μg/mL L-hydroxyproline in 0.001 N HCl) and sample hydrosylates. The resulting solution was incubated at room temperature to oxidize hydroxyproline. To quench residual chloramine-T, 3.15 M perchloric acid was added and incubated at room temperature. Finally, a p-dimethylaminobenzaldehyde solution was added and incubated at 60°C in a water bath. All specimens and standards were cooled at room temperature in complete darkness. The absorbance was measured at 570 nm using the same microplate reader used for fluorescence measurements. The amount of collagen per sample was determined based on hydroxyproline quantity measured [20], and AGE content was normalized to the amount of collagen per specimen. Total fluorescent AGEs were quantified in terms of ng quinine fluorescence per mg collagen (Table 2). AGEs were measured in one specimen per individual for the majority of the donors (72 donors) with the exception of 9 donors for which two specimens were utilized and 2 donors for which three specimens were measured. AGE measurements made on multiple specimens from the same donor were averaged for all correlation analyses.
Table 2.
List of investigated parameters associated with non-enzymatic glycation, resorption, and microdamage.
| Variables | Abbreviation | Unit |
|---|---|---|
|
Non-enzymatic Glycation Parameters
| ||
| Total Fluorescent Advanced | AGEs | ng quinine |
| Glycation End-Products | fluorescence/ mg collagen |
|
|
| ||
|
Resorption Parameters
| ||
| Average Resorption Pit Area | Av.Rs.Ar | μm2 |
| Resorption Pit Density (Number of resorption pits/Cross-sectional area of bone slice) |
Rs.Dn | #/cm2 |
| Percent Resorption Area (Resorption area/Cross-sectional area of bone slice) ×100 |
Rs.Ar/B.Ar | % |
|
| ||
|
Microdamage Parameters
| ||
| Linear Microcrack Density (Number of linear microcracks/Total bone area) |
Cr.Dn | (#/mm2) |
| Diffuse Damage (Diffuse damage area/Total bone area) |
Df.Dx | (μm2/μm2) |
Measurement of Resorption Parameters
Resorption related parameters generated in vivo were measured on transverse histological sections of human tibiae obtained from 48 male and female donors (age range: 19 to 97 years) (Table 1). Transverse histological sections were prepared from 1 cm long cortical bone segments from the proximal mid-diaphysis of each donor tibia. The center of each of these segments was located 3.75 cm from the middle of the diaphysis. The bone length was measured from the extreme points of the proximal and distal epiphysis. The proximal 1-cm sections were embedded in polymethyl methacrylate (PMMA), stained with toluidine blue, sectioned using a microtome, and mounted on glass slides.
On each slide the number of resorption pits generated in vivo was counted over the entire bone cross section using a Nikon Eclipse E600 microscope (Nikon, Melville, NY, USA) (Figure 1b). Resorption pits, reflecting the resorption stage of a current remodeling cycle, were identified based on scalloped edges (Figure 1c). The areas of resorption pits were identified using Spot Imaging Software (version 4.5, Spot Imaging Solutions, Sterling Height, MI). The area of each bone slice was calculated using ImageJ (http://rsb.info.nih.gov/ij/) based on the cross-sectional images of the tibiae. The measured bone area included both mineralized bone and osteoid. The cross-sectional area of each tibia varied from donor-to-donor within 1.99 to 5.43 cm2 with a mean value of 3.47 (±0.73) cm2. The results were expressed as resorption pit density (#/cm2), average resorption pit area (μm2), and percent resorption area (%) (Table 2).
Measurement of Microdamage
Microdamage measurements were performed on human tibiae obtained from 21 male donors (age range: 25 to 89 years) (Table 1). Transverse 1 cm long cortical bone sections were extracted from the distal diaphysis of tibia. The center of each of these segments was located 3.5 cm below the middle of the diaphysis. The bone segments were subjected to en bloc staining in 1% basic fuchsin. This protocol captures microdamage generated in vivo as it only marks the damage present at the time of donor’s death [21]. The bone sections were then embedded in PMMA and sectioned into 100 μm thick serial transverse slices.
Microdamage measurements were performed on the anterior and posterior cortices at 200× magnification using bright-field microscopy (IX81, Olympus, Melville, NY). Due to the basic fuchsin staining, linear microcracks appeared as sharply defined lines (Figure 1d) and were reported as number of microcracks per given area (#/mm2) whereas diffuse damage appeared as an area of pooled staining (Figure 1d) and was reported as damaged area per total area (μm2/μm2) [15,22] (Table 2). The details of the specimen preparation and measurement can be found in a previous study [15]. In the current study, the average of the anterior and posterior diffuse damage and linear microcrack density were utilized in the correlation analyses.
Statistical Analysis
Correlation analyses were carried out between the parameters associated with non-enzymatic glycation, resorption, and microdamage (Table 2). Age-related changes in resorption parameters, AGEs and microdamage were also assessed. Outliers were identified as data points that were beyond three standard deviations from the mean of each variable. Kolmogorov-Smirnov normality tests showed that several data sets were not normally distributed. As a result, the nonparametric Spearman correlation coefficients (r) and their statistical significance (p < 0.05) were calculated to determine the variation of resorption, non-enzymatic glycation and microdamage parameters with age. In addition, age-adjusted partial Spearman correlation coefficients were determined between parameters associated with non-enzymatic glycation, resorption, and microdamage. In order to evaluate the dependence of the results on gender, all correlation analyses were repeated adjusting for gender. All statistical analyses were performed using MATLAB (MathWorks, Natick, MA).
RESULTS
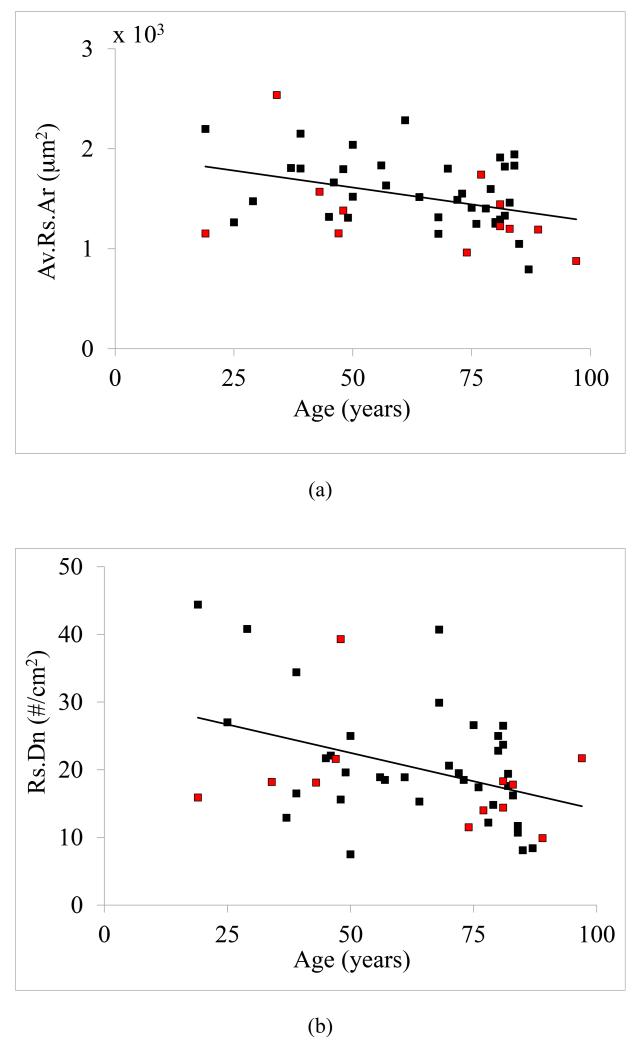
All the measured resorption parameters, including average resorption pit area (r = −0.33, p < 0.05), resorption pit density (r = −0.38, p < 0.01), and percent resorption area (r = −0.46, p = 0.001), decreased significantly with age (Figure 2a-2c). On the other hand, AGEs did not demonstrate a significant positive correlation with age (r = 0.14, p = 0.22). Linear microcrack density increased with age (r = 0.67, p < 0.001) whereas diffuse damage decreased with age (r = −0.45, p < 0.05).
Figure 2.
Correlations between age and resorption. Statistically significant (p < 0.05) correlations were observed between age and (a) average resorption pit area (r = −0.33), (b) resorption pit density (r = −0.38), and (c) percent resorption area (r = −0.46). Note that the black and red data points correspond to male and female donors, respectively.
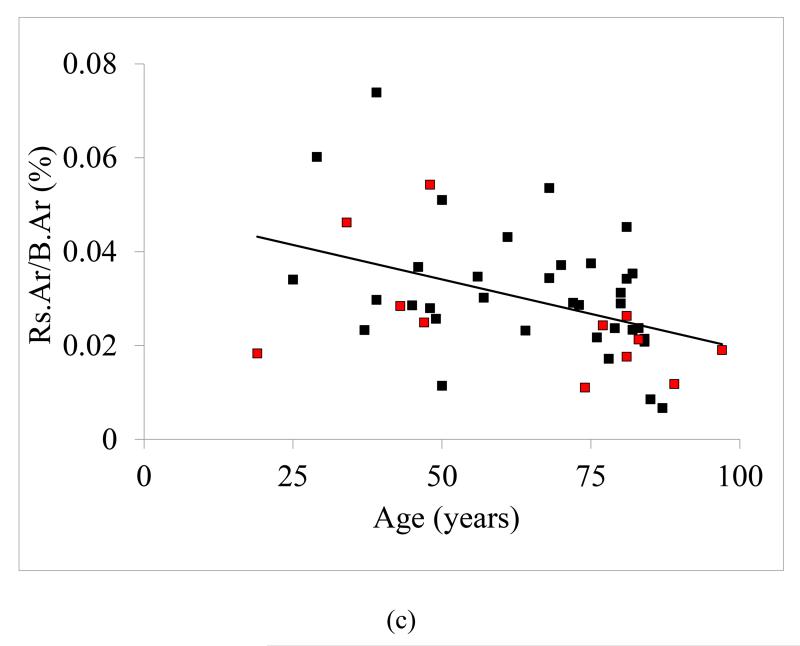
The statistical analyses performed on the data sets identified statistically significant correlations between parameters associated with non-enzymatic glycation, resorption, and microdamage (Table 3). The investigation of the correlation between non-enzymatic glycation and resorption parameters showed that the average resorption pit area and percent resorption area decreased significantly with increasing AGEs independent of age (Table 3, Figure 3a and 3b). AGE content did not show any statistically significant age-adjusted correlation with microdamage parameters.
Table 3.
Statistically significant age-adjusted partial Spearman correlation coefficients between non-enzymatic glycation, resorption, and microdamage parameters for human cortical bone.
| AGEs | Av.Rs.Ar | Rs.Dn | Rs.Ar/B.Ar | Cr.Dn | Df.Dx | |
|---|---|---|---|---|---|---|
| AGEs | - | − 0.35a | NS | − 0.35a | NS | NS |
| Av.Rs.Ar | - | - | NS | 0.43a | NS | NS |
| Rs.Dn | - | - | - | 0.82c | NS | − 0.70b |
| Rs.Ar/B.Ar | - | - | - | - | NS | − 0.57a |
| Cr.Dn | - | - | - | - | - | NS |
| Df.Dx | - | - | - | - | - | - |
p < 0.05,
p < 0.001,
p < 0.0001,
NS = not significant (p > 0.05)
Figure 3.
Correlations between total fluorescent advanced glycation end-products (AGEs) and resorption. Statistically significant (p < 0.05) correlations were observed between AGEs and (a) average resorption pit area (r = −0.35) and (b) percent resorption area (r = −0.35). Note that the black and red data points correspond to male and female donors, respectively.
The evaluation of the age-adjusted correlation between resorption and microdamage parameters revealed a reduction in diffuse damage with increasing resorption pit density and percent resorption area (Table 3). Percent resorption area demonstrated significant positive correlation with average resorption area and resorption density independent of age whereas the age-adjusted correlation between resorption density and average resorption area was not statistically significant (Table 3).
When the above correlation analyses were repeated adjusting for both age and gender the significance of the Spearman correlation coefficients between non-enzymatic glycation, resorption and microdamage parameters did not change (Table S1 in Supplementary Material). Additionally, the significance of age-related changes in non-enzymatic glycation, resorption and microdamage did not show any differences when adjusted for gender with the exception of diffuse damage which demonstrated only near significance (r = −0.45, p = 0.056) (Table S2 in Supplementary Material).
DISCUSSION
In the current study, we investigated the relationship between non-enzymatic glycation, resorption, and microdamage generated in vivo in human cortical bone using bone specimens from the same donors. The results showed evidence of interrelationship between the parameters associated with each category as well as their age-related change. The strength of our study comes from the use of the same donors for the direct measurement of in vivo generated levels of resorption, non-enzymatic glycation and microdamage in human cortical bone. As a result, our study eliminates the effect of testing conditions and variability between different donor groups and provides a robust evaluation of the in vivo processes that relate to osteoclastic bone resorption, non-enzymatic glycation, and microdamage.
Our study demonstrated that percent resorption area and average resorption pit size generated in vivo decreased with increasing AGEs independently of age. Previous studies in the literature reported conflicting data on the interaction between AGEs and osteoclastic bone resorption. An in vitro study by Valcourt et al. [9] demonstrated a reduction in resorption area and average resorption pit area with increasing AGEs. Similarly, a negative relationship between bone turnover and AGEs was observed during bisphosphonate treatment [23]. However, other studies [7,8] reported an increase in resorption with increased amount of AGEs. The discrepancy between the results from different studies is most likely due to the variation in the types of bone slices and procedures used in each study including the duration of culture which has been shown to have a significant influence [9]. Furthermore in one of the in vitro studies [8], only positive relationships between resorption pit number and AGEs was reported without any measure of the total resorption area, therefore, the results do not give a measure of the extent of bone resorption at each location. Our results capture the in vivo generated resorption related parameters and are consistent with Valcourt et al. [9]. These results provide important new information that reflect the in vivo generated levels of AGEs and resorption parameters on human bone at the time of the donor’s death and are not affected by testing conditions.
Based on the above results it is difficult to determine whether AGEs accumulate first and cause reduced resorption and formation or that reduced resorption leads to accumulation of AGEs in bone. Previous studies have demonstrated that both of the above situations may exist in vivo. Reduced bone resorption has been shown to lead to accumulation of AGEs in bone treated with bisphosphonates, in knockout mice models for AGEs, and in altered glycemic controls [23,24]. Similarly, AGEs can accumulate in tissues including bone due to dietary intake, increased oxidative stress, collagen crosslinking and result in reduced bone resorption, osteopenia, and aging phenotype [25,26]. Furthermore, previous studies have identified the interaction between AGEs and receptor of AGEs (RAGE) as one of the causes that alter bone remodeling [27,28]. The activation of AGE-RAGE pathway was shown to negatively influence osteoblasts and therefore the bone formation process [29-31]. On the other hand, the activated AGE-RAGE pathway was found to enhance [32], have no significant influence [29] or reduce resorption [9]. The observed differences in bone turnover response based on activation of AGE-RAGE pathway have been attributed to the specificity of the generated AGEs [27].
The current study also found that both the number and size of resorption pits decreased with age resulting in a reduction in the percentage of resorption area compared to the total cortical bone area. Previous studies performed on bone specimens from male donors showed a reduction in bone resorption [33-36] whereas bone resorption increased with age for women [34,35]. In addition, Martin et al. [37] provided evidence of reduced osteoclast and osteoblast activity in elderly men with reduced resorption pit size. Measurements using micro-computed tomography also showed decreasing resorption pit density with age for a pooled group of bone specimens from men and women [38]. The bone specimens used in our study were obtained predominantly from men (Table 1), therefore, the observed trends are expected to agree particularly with previous studies performed on specimens from male donors. These results indicate that reduction in osteoblast activity with age may be coupled with a reduction in bone resorption and the combination of these two changes may help preserve the bone remodeling balance in men.
An indirect evidence of reduction in resorption pit size with age is provided by previous studies that reported a decrease in osteon size with advancing age in both women [39] and men [40]. The size of osteons reflects the amount of bone resorption, therefore, smaller osteons are associated with reduced resorption pit size [39,41]. The reduction in the resorption pit size with age have been hypothesized to be caused by a decrease in the number of osteoclasts attracted to bone remodeling sites due to spatially limited osteocyctic function [39].
The assessment of the age-adjusted relationship among the resorption parameters showed that a reduction in percent resorption area was associated with both smaller and fewer resorption pits. No correlation was found between resorption density and average resorption pit area independent of age. The lack of correlation between the size and density of resorption pits may indicate that the activation of resorption process and the extent of resorption after activation are determined by separate mechanisms.
Resorption pit density and percent resorption area were negatively correlated with diffuse damage independent of age. This trend may be a result of the removal of diffuse damage areas with resorption. The relationship between diffuse damage and resorption has not been clearly identified in the literature. Both a trend in removal of diffuse damage with resorption [12] as well as no association [13] has been reported previously in rat bone. In addition, probability density distribution of total diffuse damage in cortical bone was found to decrease with an increase in the size of the area of diffuse damage [42]. This observation indicates that excessive accumulation of diffuse damage is prevented as a result of the remodeling process. No age-adjusted correlation was found between AGEs and microdamage. A previous study on in vitro ribosylated cancellous bone showed elevated levels of linear microcracks [10]. Cancellous bone has higher levels of non-enzymatic glycation compared to cortical bone [43] which may influence the level of microdamage accumulation in the bone tissue. The absence of a relationship between AGEs and linear microcrack density in this study may also be due to the limited size of the samples used in the correlation analysis between non-enzymatic glycation and microdamage.
Unlike previous reports [5, 43, 44], AGEs did not show a positive relationship with age. This difference can be explained by the presence of larger variations in AGE values and smaller samples in our study compared to previous studies. We have previously reported an increase in linear microcrack density and reduction in diffuse damage with age using a larger sample group [45]. The results reported here agree with our previous study as well as other studies that showed accumulation of linear microcracks with age [46,47].
The adjustment of the results for gender did not change the results with the exception of near significant negative correlation of diffuse damage with age. It should be noted that the donor bones used in the current study were obtained predominantly from men. The use of equal number of specimens from both women and men may provide a better insight into the influence of gender on the correlations investigated. Based on previous studies, as outlined above, the variation of parameters associated with bone resorption may be expected to increase with age for women especially due to postmenopausal osteoporosis [34,35]. AGEs have been shown to accumulate with age in both men and women and no differences have been observed between the two groups [44]. Linear microcrack density was shown to be higher in women than men with a higher rate of increase for women with age [46,47]. Diffuse damage was found to be higher in trabecular bone obtained from men [42] whereas another study found no significant differences in diffuse damage between men and women [48]. Based on these observations, the majority of the correlations reported in this study are expected to be valid for both men and women with the possible exception of age-related changes in resorption particularly if postmenopausal women with high bone turnover are included in the study.
As highlighted above, the main limitation of our study is the relatively small sample size for the microdamage measurements. A larger sample size would provide a more thorough assessment of the relationship of microdamage to non-enzymatic glycation and resorption and may unveil relationships that may have not reached statistical significance because of sample size. Another limitation that may influence the results is the disease and treatment states of the donors. Although, the donors did not have any basic bone metabolic diseases, there is not sufficient information in the donor registries to rule out osteoporosis or the use of anti-resorptive agents. The existence of the above situations may affect the resorption, glycation and microdamage measures and could affect the interactions found in the current study. However, as our study contained a large age-range with predominantly male donors and found no gender differences, the influence of anti-resorptives and osteoporosis on the results is likely to be minimal. The use of one transverse bone cross-section per donor for measuring damage and resorption variables may also be a limitation of the study. The resorption measurements were performed over an entire cross-section of each tibia. Similarly, microdamage measurements were done on the anterior and posterior quadrants of a bone cross-section. As both measurements were performed over a large area they are expected to capture the possible local variation in resorption and microdamage variables.
In summary, the current study demonstrated the in vivo interrelationship between the organic constituents, remodeling, and damage formation in cortical bone. The results showed that the age-related reduction in resorption and increase in AGEs is accompanied by a negative correlation between AGEs and resorption independent of age. The inverse relationship between AGEs and bone resorption shown in this study combined with negative association of AGEs with bone formation reported in previous in vitro studies indicate that AGEs alter resorption and formation and/or accumulate in the bone tissue as a result of reduced resorption and formation. Such changes lead to bone fragility by adversely affecting fracture resistance through altered bone matrix properties. These findings, based on in vivo generated levels of resorption and non-enzymatic glycation parameters, provide additional insight into the age-related reduction in bone fracture toughness and increased bone fragility.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and National Institute on Aging (NIA), part of the National Institutes of Health, under Award Numbers AR49635 and AG020618. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge the use of core facilities at the Center for Biotechnology and Interdisciplinary Studies at Rensselaer Polytechnic Institute.
Footnotes
CONFLICT OF INTEREST
Ani Ural, Colleen Janeiro, Lamya Karim, Tamim Diab, and Deepak Vashishth declare no conflict of interest.
REFERENCES
- 1.Seeman E, Delmas PD. Bone Quality — The material and structural basis of bone strength and fragility. New England Journal of Medicine. 2006;354(21):2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 2.Hui SL, Slemenda CW, Johnston CC., Jr. Age and bone mass as predictors of fracture in a prospective study. Journal of Clinical Investigation. 1988;81(6):1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vashishth D. Age-dependent biomechanical modifications in bone. Critical Reviews in Eukaryotic Gene Expression. 2005;15(4):343–358. doi: 10.1615/critreveukargeneexpr.v15.i4.40. [DOI] [PubMed] [Google Scholar]
- 4.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Shen X, Li X, Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31(1):1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy AD, Etcheverry SB, Cortizo AM. Advanced glycation endproduct-specific receptors in rat and mouse osteoblast-like cells: regulation with stages of differentiation. Acta Diabetologica. 1999;36(1):45–52. doi: 10.1007/s005920050144. [DOI] [PubMed] [Google Scholar]
- 7.Dong XN, Qin A, Xu J, Wang X. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone. 2011;49(2):174–183. doi: 10.1016/j.bone.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyata T, Notoya K, Yoshida K, Horie K, Maeda K, Kurokawa K, Taketomi S. Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. Journal of the American Society of Nephrology. 1997;8(2):260–270. doi: 10.1681/ASN.V82260. [DOI] [PubMed] [Google Scholar]
- 9.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. Journal of Biological Chemistry. 2007;282(8):5691–5703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- 10.Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone. 2010;46(1):148–154. doi: 10.1016/j.bone.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40(4):1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23(3):275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 13.Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: Differential response to linear microcracks and diffuse damage. Bone. 2010;47(4):766–772. doi: 10.1016/j.bone.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. Journal of Bone and Mineral Research. 2000;15(4):613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 15.Diab T, Vashishth D. Morphology, localization and accumulation of in vivo microdamage in human cortical bone. Bone. 2007;40(3):612–618. doi: 10.1016/j.bone.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, Vashishth D. Dilatational band formation in bone. P Natl Acad Sci. 2012;109(47):19178–19183. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One. 2012;7(4):e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40(4):1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 20.Gross J. Studies on the formation of collagen. I. Properties and fractionation of neutral salt extracts of normal guinea pig connective tissue. The Journal of Experimental Medicine. 1958;107(2):247–263. doi: 10.1084/jem.107.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burr DB, Stafford T. Validity of the bulk-staining technique to separate artifactual from in vivo bone microdamage. Clinical Orthopaedics and Related Research. 1990;260:305–308. [PubMed] [Google Scholar]
- 22.Boyce TM, Fyhrie DP, Glotkowski MC, Radin EL, Schaffler MB. Damage type and strain mode associations in human compact bone bending fatigue. Journal of Orthopaedic Research. 1998;16(3):322–329. doi: 10.1002/jor.1100160308. [DOI] [PubMed] [Google Scholar]
- 23.Tang S, Allen M, Phipps R, Burr D, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporosis International. 2009;20(6):887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamagishi S-i. Role of Advanced Glycation End Products (AGEs) in Osteoporosis in Diabetes. Current Drug Targets. 2011;12(14):2096–2102. doi: 10.2174/138945011798829456. [DOI] [PubMed] [Google Scholar]
- 25.Semba RD, Nicklett EJ, Ferrucci L. Does Accumulation of Advanced Glycation End Products Contribute to the Aging Phenotype? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65A(9):963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg E, Maymon T, Weinreb M. AGEs induce caspase-mediated apoptosis of rat BMSCs via TNFα production and oxidative stress. Journal of Molecular Endocrinology. 2014;52(1):67–76. doi: 10.1530/JME-13-0229. [DOI] [PubMed] [Google Scholar]
- 27.Hein GE. Glycation endproducts in osteoporosis — Is there a pathophysiologic importance? Clinica Chimica Acta. 2006;371(1-2):32–36. doi: 10.1016/j.cca.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Saito M, Marumo K, Soshi S, Kida Y, Ushiku C, Shinohara A. Raloxifene ameliorates detrimental enzymatic and nonenzymatic collagen cross-links and bone strength in rabbits with hyperhomocysteinemia. Osteoporosis International. 2010;21(4):655–666. doi: 10.1007/s00198-009-0980-4. [DOI] [PubMed] [Google Scholar]
- 29.Hein G, Weiss C, Lehmann G, Niwa T, Stein G, Franke S. Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings. Annals of the Rheumatic Diseases. 2006;65(1):101–104. doi: 10.1136/ard.2004.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hein G, Wiegand R, Lehmann G, Stein G, Franke S. Advanced glycation end-products pentosidine and Nε-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology. 2003;42(10):1242–1246. doi: 10.1093/rheumatology/keg324. [DOI] [PubMed] [Google Scholar]
- 31.Franke S, Siggelkow H, Wolf G, Hein G. Advanced glycation endproducts influence the mRNA expression of RAGE, RANKL and various osteoblastic genes in human osteoblasts. Archives Of Physiology And Biochemistry. 2007;113(3):154–161. doi: 10.1080/13813450701602523. [DOI] [PubMed] [Google Scholar]
- 32.Ding K-H, Wang Z-Z, Hamrick MW, Deng Z-B, Zhou L, Kang B, Yan S-L, She J-X, Stern DM, Isales CM, Mi Q-S. Disordered osteoclast formation in RAGE-deficient mouse establishes an essential role for RAGE in diabetes related bone loss. Biochemical and Biophysical Research Communications. 2006;340(4):1091–1097. doi: 10.1016/j.bbrc.2005.12.107. [DOI] [PubMed] [Google Scholar]
- 33.Fatayerji D, Eastell R. Age-related changes in bone turnover in men. Journal of Bone and Mineral Research. 1999;14(7):1203–1210. doi: 10.1359/jbmr.1999.14.7.1203. [DOI] [PubMed] [Google Scholar]
- 34.Minisola S, Dionisi S, Pacitti MT, Paglia F, Carnevale V, Scillitani A, Mazzaferro S, De Geronimo S, Pepe J, D’Erasmo E, Romagnoli E. Gender Differences in Serum Markers of Bone Resorption in Healthy Subjects and Patients with Disorders Affecting Bone. Osteoporosis International. 2002;13(2):171–175. doi: 10.1007/s001980200009. [DOI] [PubMed] [Google Scholar]
- 35.Rehman MTA, Hoyland JA, Denton J, AJ Freemont. Age related histomorphometric changes in bone in normal British men and women. Journal of Clinical Pathology. 1994;47(6):529–534. doi: 10.1136/jcp.47.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wishart JM, Need AO, Horowitz M, Morris HA, Nordin BEC. Effect of age on bone density and bone turnover in men. Clinical Endocrinology. 1995;42(2):141–146. doi: 10.1111/j.1365-2265.1995.tb01854.x. [DOI] [PubMed] [Google Scholar]
- 37.Martin RB, Pickett JC, Zinaich S. Studies of Skeletal remodeling in aging men. Clinical Orthopaedics and Related Research. 1980;149:268–282. [PubMed] [Google Scholar]
- 38.Cooper DML, Thomas CDL, Clement JG, Hallgrímsson B. Three-dimensional microcomputed tomography imaging of basic multicellular unit-related resorption spaces in human cortical bone. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2006;288A(7):806–816. doi: 10.1002/ar.a.20344. [DOI] [PubMed] [Google Scholar]
- 39.Bernhard A, Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Krause M, Breer S, Püschel K, Djuric M, Amling M, Busse B. Micro-morphological properties of osteons reveal changes in cortical bone stability during aging, osteoporosis, and bisphosphonate treatment in women. Osteoporosis International. 2013;24(10):2671–2680. doi: 10.1007/s00198-013-2374-x. [DOI] [PubMed] [Google Scholar]
- 40.Ural A, Vashishth D. Interactions between microstructural and geometrical adaptation in human cortical bone. Journal of Orthopaedic Research. 2006;24(7):1489–1498. doi: 10.1002/jor.20159. [DOI] [PubMed] [Google Scholar]
- 41.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Dependence of bone yield (volume of bone formed per unit of cement surface area) on resorption cavity size during osteonal remodeling in human rib: implications for osteoblast function and the pathogenesis of age-related bone loss. Journal of Bone and Mineral Research. 2010;25(2):423–430. doi: 10.1359/jbmr.091003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, Schaffler MB, Fyhrie DP. In vivo diffuse damage in human vertebral trabecular bone. Bone. 2000;26(2):147–152. doi: 10.1016/s8756-3282(99)00253-7. [DOI] [PubMed] [Google Scholar]
- 43.Karim L, Tang S, Sroga G, Vashishth D. Differences in non-enzymatic glycation and collagen crosslinks between human cortical and cancellous bone. Osteoporosis International. 2013;24(9):2441–2447. doi: 10.1007/s00198-013-2319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Annals of the New York Academy of Sciences. 2005;1043(1):710–717. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 45.Diab T, Condon KW, Burr DB, Vashishth D. Age-related change in the damage morphology of human cortical bone and its role in bone fragility. Bone. 2006;38(3):427–431. doi: 10.1016/j.bone.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17(6):521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 47.Norman TL, Wang Z. Microdamage of human cortical bone: Incidence and morphology in long bones. Bone. 1997;20(4):375–379. doi: 10.1016/s8756-3282(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 48.Arlot ME, Burt-Pichat B, Roux J-P, Vashishth D, Bouxsein ML, Delmas PD. Microarchitecture influences microdamage accumulation in human vertebral trabecular bone. Journal of Bone and Mineral Research. 2008;23(10):1613–1618. doi: 10.1359/jbmr.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.