Abstract
Reductions in adult neurogenesis have been documented in the original 3xTg mouse model of Alzheimer’s disease (AD), notably occurring at the same age when spatial memory deficits and amyloid plaque pathology appeared. As this suggested reduced neurogenesis was associated with behavioral deficits, we tested whether activity and pharmacological stimulation could prevent memory deficits and modify neurogenesis and/or neuropathology in the 3xTg model backcrossed to the C57Bl/6 strain. We chronically administered the antidepressant fluoxetine to one group of mice, allowed access to a running wheel in another, and combined both treatments in a third cohort. All treatments lasted for 11 months. The female 3xTg mice failed to exhibit any deficits in spatial learning and memory as measured in the Morris water maze, indicating that when backcrossed to the C57Bl/6 strain, the 3xTg mice lost the behavioral phenotype that was present in the original 3xTg mouse maintained on a hybrid background. Despite this, the backcrossed 3xTg mice expressed prominent intraneuronal amyloid beta (Aβ) levels in the cortex and amygdala, with lower levels in the CA1 area of the hippocampus. In the combined cohort, fluoxetine treatment interfered with exercise and reduced the total distance run. The extent of Aβ neuropathology, the tau accumulations, or BDNF levels, were not altered by prolonged exercise. Thus, neuropathology was present but not paralleled by spatial memory deficits in the backcrossed 3xTg mouse model of AD. Prolonged exercise for 11 months did improve the long-term survival of newborn neurons generated during middle-age, whereas fluoxetine had no effect. We further review and discuss the relevant literature in this respect.
Keywords: Adult neurogenesis, Alzheimer’s disease, Antidepressant, Exercise, Hippocampus
1 Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive deficits and abundant deposition of amyloid beta (Aβ) plaques and neurofibrillary tangles in selected brain regions. The etiology and progression of the disorder can be studied in transgenic mice that carry one or multiple mutated copies of the genes that are known to, at least partially, underlie aspects of the disease. The popular 3xTg mouse model for AD was first described in 2003 and contains human mutations in amyloid precursor protein APP, tau, and presenilin 1 that cause it to display both Aβ and tau accumulations. These mice are an attractive model to study AD in view of their robust behavioral and neuropathological phenotype that recapitulates several aspects of the disorder in humans (Oddo et al. 2003a, b). Initial findings from these mice demonstrated that intraneuronal Aβ accumulations not only preceded tau pathology but also correlated with behavioral deficits observed already during early phases of the neuropathology. The increasing levels of intraneuronal Aβ disrupted neuronal integrity and induced cognitive deficits in the mice in the Morris water maze (Billings et al. 2005). The authors further showed that vaccination with an anti-Aβ antibody reversed amyloid accumulation, but not tau neuropathology in these mice, in parallel to the behavioral changes (Billings et al. 2005).
Adult neurogenesis is a form of brain plasticity that refers to the birth of new neurons in adult and aging brains. The extent of neurogenesis is thought to influence vulnerability to accumulating deleterious events during the aging process (Kempermann 2008) and may reflect susceptibility to certain diseases. In the original 3xTg mice, sex and age-dependent deficits in adult neurogenesis were first described in 2008. Female 3xTg mice were shown to have reduced neurogenesis in the dorsal hippocampus starting at 4 months of age and a significant correlation between intraneuronal Aβ and reduced neurogenesis at 12 months of age (Rodriguez et al. 2008). This suggested that a decline in neurogenesis precedes later AD pathology and may correlate with behavioral deficits in the animals. This study was instrumental in stimulating interest in neurogenesis as a therapeutic target to reverse behavioral deficits associated with AD.
Adult neurogenesis is positively regulated by activity in adult rodents (van Praag et al. 1999a, b, 2005; Brown et al. 2003; van der Borght et al. 2009; Kempermann et al. 2010; Marlatt et al. 2010; Lucassen et al. 2010). Running conditions can reverse the decline in hippocampal neurogenesis and improve spatial memory in standard rodent models. For example, aged running mice learn the location of a submerged platform during a spatial memory task similar to young sedentary mice (van Praag et al. 2005).
So far, however, very few studies have investigated effects of stimulating neurogenesis over longer timescales, or addressed its implications for learning and memory late in life. In regard to AD mouse models, wheel running was previously shown to increase neurogenesis in transgenic amyloid precursor protein (APP) mice with the transgenes expressed under the same promoter as that used in the present 3xTG mice (Wolf et al. 2006). Collectively, we predicted that (1) reduction in neurogenesis would relate to behavioral deficits and (2) induction of neurogenesis through activity and pharmacology-dependent mechanisms would rescue these functional deficits. Similar to our recent study in middle-aged wildtype mice (Marlatt et al. 2012), we sought to evaluate the effects of prolonged interventions starting in middle-age in the 3xTg AD mice. Such a design bears relevance for prevention in humans, where AD is often not discovered until late in the course of the disease when behavioral and neuropathological deficits have manifested already.
Running is a potent stimulus for different stages of the neurogenic process (Kempermann et al. 2010) as well as for spatial memory in mice (Brown et al. 2003; van Praag et al. 1999a, b). It also increases brain-derived neurotrophic factor (BDNF) and angiogenesis (Neeper et al. 1995; van der Borght et al. 2009). As we set out to correlate behavioral changes with neurogenesis, we focused particularly on the survival phase of the newborn cells. Furthermore, as combined running and fluoxetine treatment had previously potentiated the expression of BDNF mRNA in the brain, we expected that application of both running and SSRI treatment would have an aggregate effect greater than either treatment alone (Russo-Neustadt et al. 2000).
We further evaluated the ability of both interventions to impact the extent of neuropathology by measuring Aβ and tau pathology in the 3xTg mice. A relatively novel aspect of the biochemistry and neuropathology of Aβ is the accumulation of intraneuronal Aβ as observed in human brain and several studies now suggest it is the intraneuronal Aβ that forms one of the earliest pathological events in AD (Gouras et al. 2000; Wirths et al. 2004; Christensen et al. 2009, 2010). As synaptic pathology correlates better with cognitive dysfunction than the accumulation of amyloid plaques (Terry et al. 1991), many studies of Aβ seek to establish a mechanistic link between Aβ and the physical damage to synapses and neurons (Bayer and Wirths 2010; Gouras et al. 2010). We here show that despite the loss of the behavioral phenotype in these backcrossed animals, the 3xTg mice express prominent levels of intraneuronal Aβ in the cortex and amygdala, with lower levels in the CA1 area. Fluoxetine seemed to reduce Aβ accumulations. Although the overall extent of neuropathology and BDNF levels remained unaltered, prolonged exercise significantly increased neurogenesis. Possible explanations and similar correlations in related models will be discussed.
2 Materials and Methods
2.1 Mice
3xTg mice were first generated by Dr. LaFerla (University of California, Irvine, USA) and acquired by the National Institute on Aging (NIA, Baltimore, MD, USA). The 3xTg mice, harboring PS1M146 V, APPswe, and tauP301L transgenes were first backcrossed to the C57Bl6 strain for seven generations. All mice were maintained on a standard NIH-07 diet (Harlan-Tekland, Indianapolis, IN) with free access to water during a 12-h light/12-h dark cycle. Female mice were group housed until the start of the experiment at 9 months of age and then singly housed for the duration of the experiment. Female 3xTg mice (n = 10 per group) were randomly assigned to control (Con), fluoxetine (Flu), running (Run), and synergistic treatment with fluoxetine and running (FluxRun). Running mice (Run and FluxRun groups) were housed with a running wheel and distance run was recorded daily (Clocklab, Coulborn Instruments, Whitehall, PA).
Two cohorts of animals were used to evaluate acute and chronic responses to the described conditions. Cohort 1: Animals were chronically treated with the conditions listed above for 10 months, with behavioral testing at 1 and 10-month time points. Mice were euthanized on day 333, 11 months after the start of the study at 20 months of age. Cohort 2: Animals were treated acutely for 1-month (n = 3–4 per group), to determine the acute effects of treatment on the survival of new neurons labeled in the DG. At the end of each study, animals were deeply anesthetized by isoflurane inhalation and perfused with phosphate buffered saline. Animals were decapitated and brains were immediately removed. The right hemisphere was placed in 4 % paraformaldehyde for 48 h, followed by equilibration in 30 % sucrose. Tissue was sectioned coronally (40 µm) on a freezing microtome (Thermo-Fisher) and stored at −20 °C in cryoprotectant solution. The left hemisphere was dissected and frozen on dry ice for biochemical analysis of BDNF. All animal procedures were done in accordance and were approved by the National Institute of Health Animal Care and Use Committee.
2.2 Administration of Fluoxetine in Drinking Water and Measurement of Running Distance
Fluoxetine was dissolved in drinking water and replaced every 7 days. A pilot study established that running mice drink comparable amounts of water as sedentary controls. Fluoxetine is soluble in water up to a concentration of 4 mg/ml; in this study, we dissolved fluoxetine at 0.12 mg/ml such that oral dosing was 18 mg/kg/day (based on pilot data on water consumption).
All of the mice were individually housed (n = 10 per group) at the start of the experiment with two groups assigned to running wheel cages. The average distance run per day was 2.2 ± 0.6 km for runners and 1.1 ± 0.5 km for runners that were additionally treated with fluoxetine (see Table 1), but this was not statistically significant.
Table 1.
Total distance range and average for Flu and FluxRun groups
| Groups | Days | Range: Total distance (km) | Average distance (km) | Stdev. (km) |
|---|---|---|---|---|
| Run | 1–28 | 5.4–123.0 | 54.2 | 51.4 |
| Fluxrun | 1–28 | 2.1–120.4 | 40.3 | 44.3 |
| Run | 1–333 | 231–1367 | 738 | 461 |
| Fluxrun | 1–333 | 28–1188 | 367 | 420 |
Data collected from the running wheels reflects that both Run and FluxRun groups had a high degree of variation. While exercise significantly increased the distance ran FluxRun animals showed a trend for running less distance on average than the Run cohort alone, however the means were not significantly different (Student’s 2-tailed t-test, 28 days p = 0.61, 333 days p = 0.09)
2.3 Behavior: Morris Water Maze
Mice were trained in the Morris water maze (Morris 1984), to find a platform hidden 5 mm below the surface of a pool (1.40-m dia.) that was filled with water made opaque with white nontoxic paint. Starting points were changed daily for each trial. A trial lasted either until the mouse had found the platform or for a maximum of 60 s. Mice rested on the platform for 10 s after each trial. Mice were trained with four trials per day over 6 days. Upon completion of training, the platform was removed for 60-s probe trials; probe trial were held 4 h and 24 h after the last training session. Latency to reach the platform was recorded during the first 6 days and the time in each quadrant was measured during the probe trials (Anymaze, Stoelting Co., USA).
2.4 Behavior: Rotarod Performance
The rotarod test was used to assess sensorimotor coordination and motor performance at 10 and 20 months of age. The total number of falls were measured during three trials of 5 min each using a program with constant acceleration to 25 rpm (Med Associates, VT).
2.5 Bromo-deoxy-uridine Immunohistochemistry and Cell Counts
In order to analyze newborn cells, Bromo-deoxy-uridine (BrdU) (50 mg/kg) was injected i.p. for the first 10 days for each cohort of animals. A one-in-six series of free-floating sections (40 µm) was washed in PBS and pre-incubated with 0.6 % H2O2 for 30 min. After rinsing, the sections were incubated in 2 N HCl at 37 °C for 30 min to denature DNA and then neutralized in 0.1 M borate buffer at room temperature (RT). After thorough washing, the sections were blocked with PBS ++ (3 % donkey serum-0.05 M PBS, 0.5 % Triton-X 100) for 30 min at RT and incubated with rat anti-BrdU (1:200 in PBS, Accurate Chemical Westbury NY) overnight at 4 °C. Thereafter, the sections were washed and immersed in biotin- SP-conjugated donkey anti-rat IgG (1:250, Jackson Immuno Research, West Grove, PA) followed by 2 h in ABC reagent (1:800, Vestastain Elite; Vector Laboratories, Burlingame, CA). The sections were then incubated with the substrate 3, 3′-Diaminobenzidine (D4418, Sigma, St. Louis, MO) for 5 min to visualize the cells that had incorporated BrdU. BrdU-positive cells were counted in a one-in-six series of sections (240 µm apart) through a 20X objective (Olympus, BX51) throughout six hippocampal sections per animal starting at approximately Bregma −1.46.
The volume of the dentate gyrus (DG) for each group of animals was determined by DAPI staining a 1:6 series of sections and outlining the granular cell layer (GCL) and subgranular zone (SGZ) on a microscope equipped with Stereo investigator software (Microbrightfield, Burlington, VT). Seven sections were outlined making boundary contour tracings to determine the area of the DG at each level. Area values were analyzed with the Cavalieri method to determine hippocampal volume.
2.6 Double Immunofluorescence for Cell Fate Analysis
Free-floating sections (1:6 series) were simultaneously incubated with primary antibodies against BrdU (1:100 Accurate Chemical Westbury NY) and the neuronal marker NeuN (1:100 Millipore, Billerica, MA) after the denaturation, neutralization, washing, and blocking steps described above. Antibodies were diluted in PBS ++ and then sections were incubated for 48 h at 4 °C. After rinses with PBS and blocking in PBS ++, sections were co-incubated with donkey anti-rat Alexa Fluor 488 (1:250, Molecular Probes, Carlsbad, CA) and donkey anti-mouse Alexa Fluor 568 dyes (1:250, Jackson Immuno Research, West Grove, PA) for 2 h at RT. A laser-scanning microscope was used to identify cells positive for both BrdU and NeuN markers. Fluorescent signals were imaged with a Zeiss LSM 510 confocal laser-scanning microscope and confocal and z-stacked images were used in coordination to determine the percentage of BrdU-positive cells with a neuronal phenotype by co-labeling expression of BrdU with NeuN.
2.7 Ab Immunohistochemistry and Densitometry
The extent of Aβ immunoreactivity was determined by using a technique documented previously that utilizes formic acid (FA)-based antigen retrieval (Christensen et al. 2009). This procedure was modified to accommodate floating sections cut at 40 µm sections. These sections were washed and mounted on Superfrost slides. Antigen retrieval was achieved by boiling sections in 0.01 M citrate buffer (pH 6.0) followed by a 3-min incubation with 88 % FA. The primary Ab was directed against N-terminal Aβ peptides (1:250 IBL Japan #18584). Sections were developed using biotinylated secondary Ab, Goat anti-Rabbit 1:200, and Vector Labs ABC (Vector Labs, Burlingame, CA USA). Images, taken at total 100x magnification, were collected on an Olympus BX-51 microscope equipped with a DP-50 camera (Olympus, Hamburg, Germany). Images were converted into 8-bit grayscale images with NIH Image J (v. 1.61) and then converted into binary positive/negative images by using a threshold limit held constant for all images in a given brain region. Percent area fraction was determined through the use of a macro in Image J.
2.8 Tau Immunohistochemistry and Densitometry
As protein tau is aberrantly hyperphosphorylated at serine and threonine residues in AD, we used the monoclonal Ab AT8 that recognizes tau protein phosphorylated at both serine 202 and threonine 205 (Goedert et al. 1995). Tau accumulations can occur in the absence of Aβ and serve as hallmarks for neurodegenerative tauopathies (Lee et al. 2001; Goedert et al. 1992).
AT8, a mouse monoclonal Ab, was blocked with goat anti-mouse FAb fragments to bind endogenous mouse antigens (1:200 goat anti-mouse FAb fragments diluted in 2 % NGS, 0.4 % triton, 0.1 M PBS). After subsequent thorough washing (6 × 10 min in PBS), sections were incubated with 1:1000 AT8 for 1 h at RT and 4 °C overnight. The following day sections were washed and incubated with biotinylated sheep anti-mouse (1:200). Avidin–Biotin complex was applied at the lab-tested concentration (1:800) and sections were developed with diaminobenzidine (DAB) according to standard procedures.
Densitometry was used to measure Aβ deposition as labeled by immunohistochemistry with anti-Aβ antibody (IBL #18584) recognizing the N-terminus of Aβ present in both intraneuronal and extracellular Aβ. Densitometry employed a thresholding technique that captures intraneuronal and extracellular Aβ deposition information indiscriminately and simultaneously. For each brain subregion, available images were saved as an image sequence and a threshold was determined that identified specific staining while omitting background staining in the sections. The image sequence was then evaluated for percent area fraction using three threshold values, namely the originally determined threshold value in addition to one higher and one lower. Under no circumstances did changing the threshold value, used to determine the percent area fraction, change the outcome of the statistical comparison between groups.
2.9 BDNF Western Blot
To measure mature BDNF peptide levels, hippocampal tissue was homogenized in 400 µl of the 1X RIPA buffer containing protease inhibitors (Complete Mini, Roche Diagnostics) using pestles and microtubes (ISC BioExpress) and then sonicated with four pulses of 10 s at scale 4 (Ultrasonic Processor, Model GE70) at RT. The lyzed samples were centrifuged at RT for 10 min and the supernatants were transferred to fresh tubes. The lysates were reduced with 100 mM DTT at 70 °C for 1 h to break the strong disulfide bonds of BDNF. The protein concentrations were measured using Bradford method (Bio-Rad). The samples were diluted to final concentration of 3 µg/µl with the lysis buffer and 4X LDS Nu-PAGE sample buffer (Invitrogen). Before electrophoresis, the samples were heated at 90 °C for 5 min, rapidly cooled on ice for 1 min and then equilibrated to RT for 10 min. Equal amounts of 15 µg of the proteins were loaded onto 4–12 % gradient NuPAGE neutral polyacrylamide gel. The electrophoresis was carried out in 1X MES buffer and the proteins in the gel were transferred to Immobilon-FL membrane (Millipore) using NuPAGE transfer buffer according to the manufacture’s protocol (Invitrogen). The polyclonal rabbit BDNF antibody (Santa Cruz Biotechnology, Inc.) and an infrared-labeled goat against rabbit secondary antibody (Li-Cor Biosciences) were used for immunostaining according to Li-Cor’s protocol. The specificity of BDNF antibody staining was confirmed by co-migration with the reduced human recombinant BDNF (0.1 mg) monomer (Neuromics).
2.10 Statistical Analysis
All statistical analyses were carried out using GraphPad Prism. For Morris water maze latency, one-way analysis of variance (ANOVA) with repeated measures was performed followed by Bonferroni posthoc tests for individual days. For the time in quadrants, a one-way ANOVA was performed on each group followed by Bonferroni post hoc tests. For comparisons of two groups, Student’s t test was used to determine if means were significantly different and assign statistical significance.
3 Results
3.1 Motor Impairment in Fluoxetine-Treated Animals
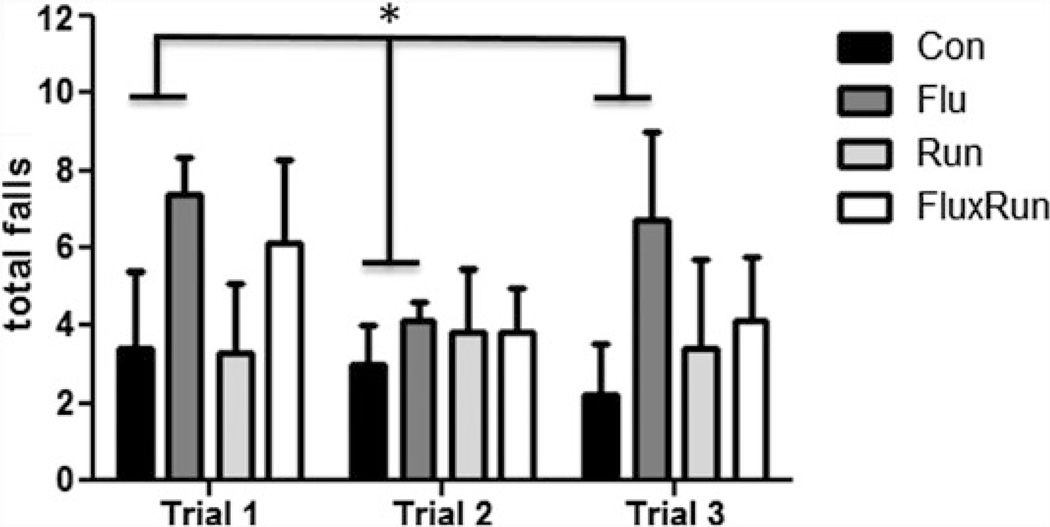
Running distances in the Run and FluxRun cohort were collected during the study. Surprisingly, a high degree of variability was present in the middle-aged mice for running distance (Table 1). When assessing motor coordination and performance using the rotarod, motor impairment was evident at 20 months of age in the 3xTg mice treated with fluoxetine compared to all our groups. Mice treated with fluoxetine displayed a significantly higher frequency of falls particularly in trials 1 and 3 (Fig. 1, One-way ANOVA p < 0.05).
Fig. 1.
Total falls in the rotarod test at 20 months of age. During a program of constant acceleration on the rotarod rod, 3xTg mice given fluoxetine showed a higher total number of falls when measured across trials. All three trials were conducted in succession on the same day with approximately 1 h between trials (repeated measures ANOVA F(3,54) = 5.82, p < 0.05)
3.2 Acute and Chronic Running Stimulate BrdU+ Cell Survival and Adult Neurogenesis
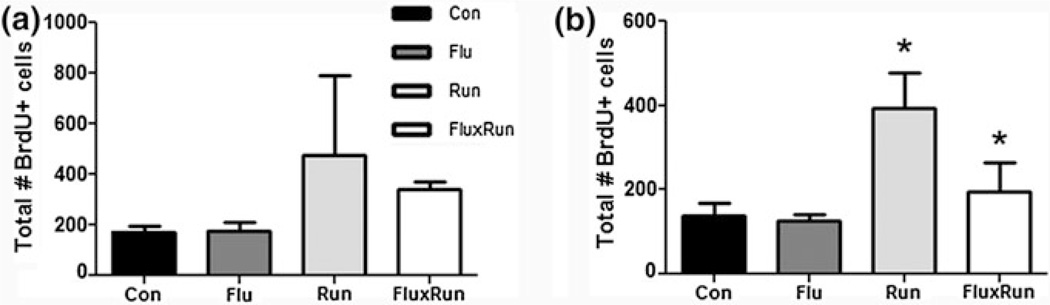
To label dividing neural progenitor cells, the thymidine analog BrdU was injected for the first 10 days. To measure the short-term effects of running and fluoxetine, a separate cohort of animals was used to quantify BrdU+ cell survival 1 month after injection when 3xTg mice were 10 months of age. BrdU-labeled cells were counted in the SGZ and granule cell layer. At this time, there was a high amount of variability between groups and mean cell survival was not significantly different between groups (Fig. 2a).
Fig. 2.
BrdU + cell survival 1 and 11 months after beginning treatment. a 1 month after treatment with fluoxetine (Flu), exposure to running (Run), or combined therapy (FluxRun), none of the treatment groups showed significant elevations in BrdU+ cell survival compared to controls (Con) (One-way ANOVA F(3, 9) = 0.97, p = 0.44). b 11 months after starting treatment, significant increases in BrdU+ cell survival were seen for both Run and FluxRun groups compared to nonrunning controls. (One-way ANOVA F(3,23) = 5.27, p < 0.01)
To measure long-term effects of running, fluoxetine, and synergistic treatment, BrdU+ cell survival was also measured after 10 months of treatment when the animals were 19 months of age. A significant difference was found between treatment groups, specifically that running and combined fluoxetine treatment had elevated the number of surviving BrdU+ cells, albeit significantly less so in the FluxRUN as compared to the Run group (Fig. 2b). However, neither group showed a significant correlation between running distances and BrdU+ survival (data not shown: groups combined, Pearson r = 0.23, p = 0.2125; Run only, Pearson r = 0.14, p = 0.38; FluxRun, Pearson r = 0.28, p = 0.27).
3.3 Neuronal Differentiation Is Unaffected by Treatments
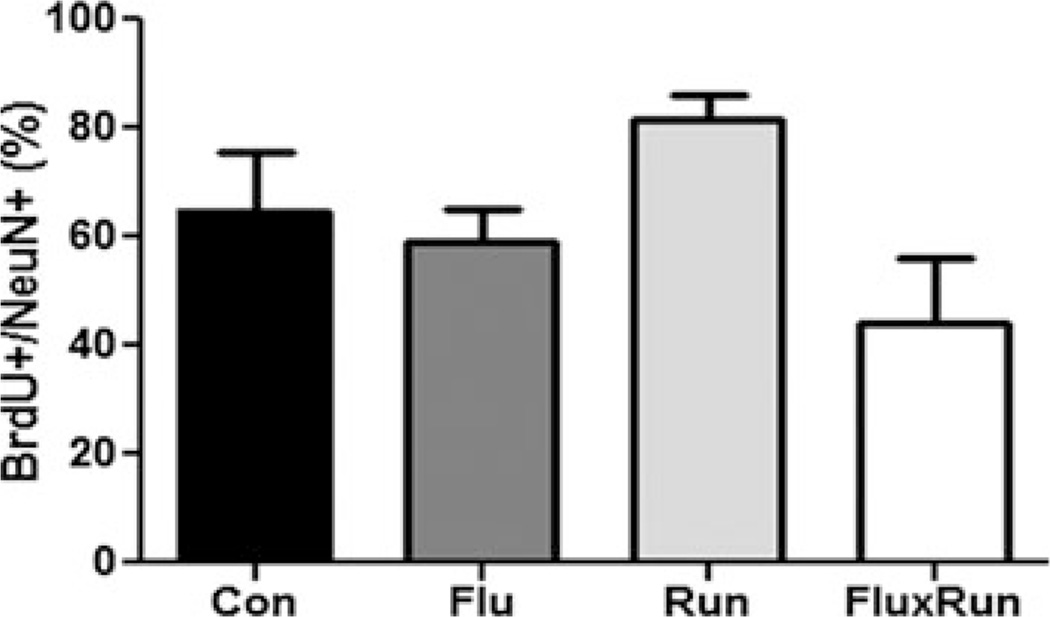
To investigate the ability of running, fluoxetine, and synergistic treatment to influence adult born cell fate and neurogenesis, confocal imaging was used to also identify BrdU +/NeuN+ cells. BrdU in this paradigm identifies cells born during the first 10 days of the experiment, while NeuN identifies mature neurons. Co-imaging of these markers across cohorts showed there were no significant differences in the percentage of cells labeled with both markers (control: 65 ± 11 %, Flu 59 ± 7 %, Run: 77 ± 7 %, and FluxRun 48 ± 12 %) (Fig. 3).
Fig. 3.
Percentage of BrdU + cells expressing the mature neuronal marker NeuN. None of the treatment groups showed significant differences in the percentage of BrdU + newborn cells expressing NeuN as marker for mature CNS neurons (One-way ANOVA F(3,19) = 2.27, p = 0.11)
The volume of the DG for each group of animals was determined by DAPI staining in a 1:6 series of sections. Area values were used by the Cavalieri method to determine hippocampal volume. No significant differences in total area were found between groups (One-way ANOVA p = 0.86, F = 0.25).
3.4 Aged 3xTg Mice Do Not Exhibit Impairments in Learning or Memory
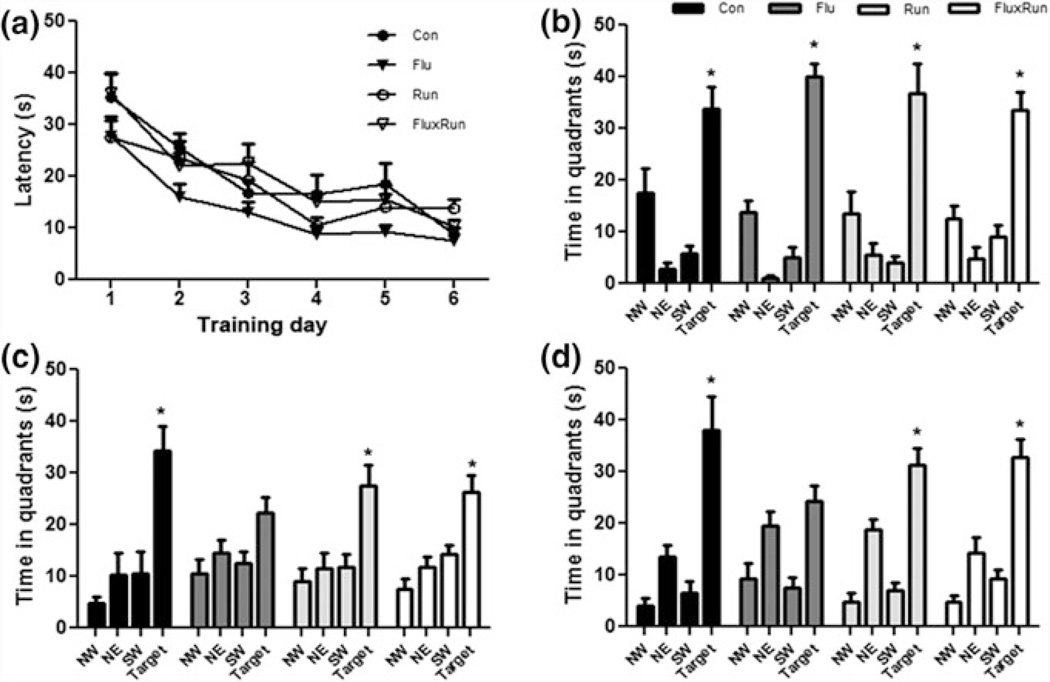
To assess spatial learning and memory, mice were tested in the Morris water maze. Surprisingly, there were no observable differences between groups after 1 month of treatment (Fig. 4a). All animals in the study learned the task to criterion within 6 days, with both groups remembering equally well the location of the platform in a probe test 24 h after the last training session. After 11 months of running, at 20 months of age, mice were trained to a different platform location. There was no difference between groups in acquisition of the task over 6 days of training or in the probe tests. During the 6- and 24-h probe tasks, mice in Con, Run, and FluxRun had a preference for the target quadrant (Fig. 4c, d. Mice treated with fluoxetine, did not show this preference. No differences in swim speed or path length were detected between groups (data not shown).
Fig. 4.
3xTg mice show no impairment in learning or memory during testing in the MWM at 10 or 20 months of age a 1 month data reflects that all groups learned the MWM to criterion (latency to platform < 20 s) (repeated measures ANOVA F(3,198) = 1.97, p = 0.16) and b all groups at the 1 month timepoint, remembered the location of the platform 24 h after their last training session (Con F(3,27) = 13.44, Flu F(3,27) = 54.91, Run F(3,24) = 11.60, FluxRun F(3,27) = 17.09, P < 0.001) Bonferroni post test p < 0.05 target versus each quandrant c 10 months after starting the experiment, 3xTg mice were re-trained and Con, Run, and FluxRun showed significant preferences for the target quadrant during 4 h probe trials (Con F(3,12) = 9.00, Run F(3,21) = 6.00, FluxRun F(3,18) = 9.34, P < 0.01) Bonferroni post-test p < 0.05 target versus each quandrant, Flu F(3,21) = 2.94, p = 0.06) d 24 h probe trials (Con F(3,12) = 13.14, Run F(3,21) = 21.50, FluxRun F(3,18) = 22.81, P < 0.01) Bonferroni post-test p < 0.05 target versus each quadrant. Flu did not show a significant preference for the target quadrant
3.5 Mature BDNF Protein Levels in the Hippocampus
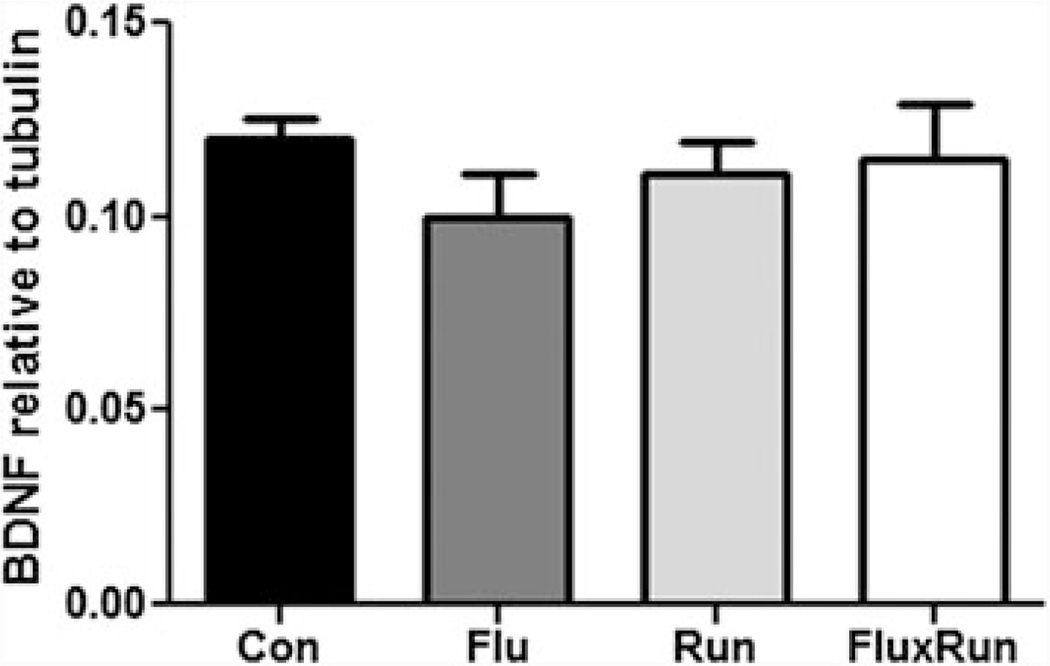
Mature BDNF was measured by Western blot; there was no significant difference in BDNF expression between the groups (Fig. 5).
Fig. 5.
Mature BDNF protein in the hippocampus. No significant differences were observed in the amount of soluble BDNF across cohorts. BDNF protein, as measured by Western blot, was standardized to the housekeeping gene, beta-tubulin (One-way ANOVA F(3,23) = 0.56, p = 0.64)
3.6 Aβ Expression in the 3xTg Mouse
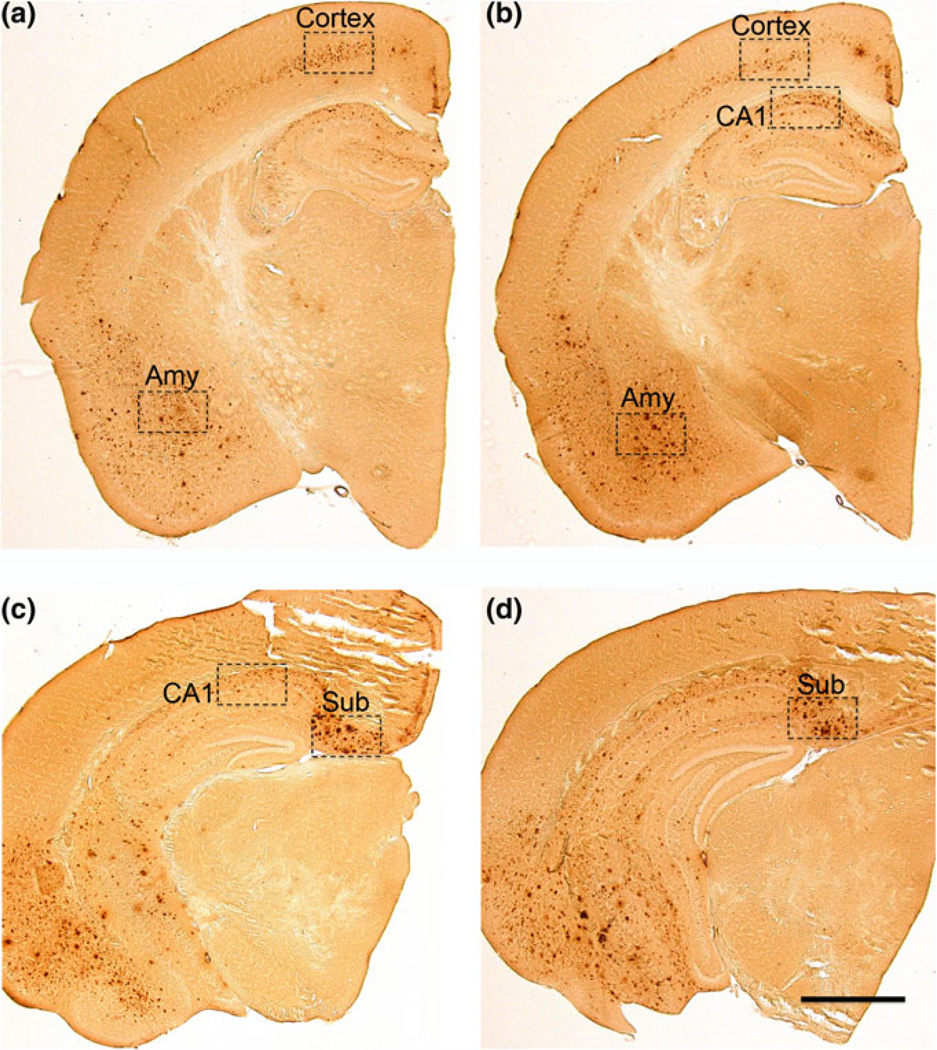
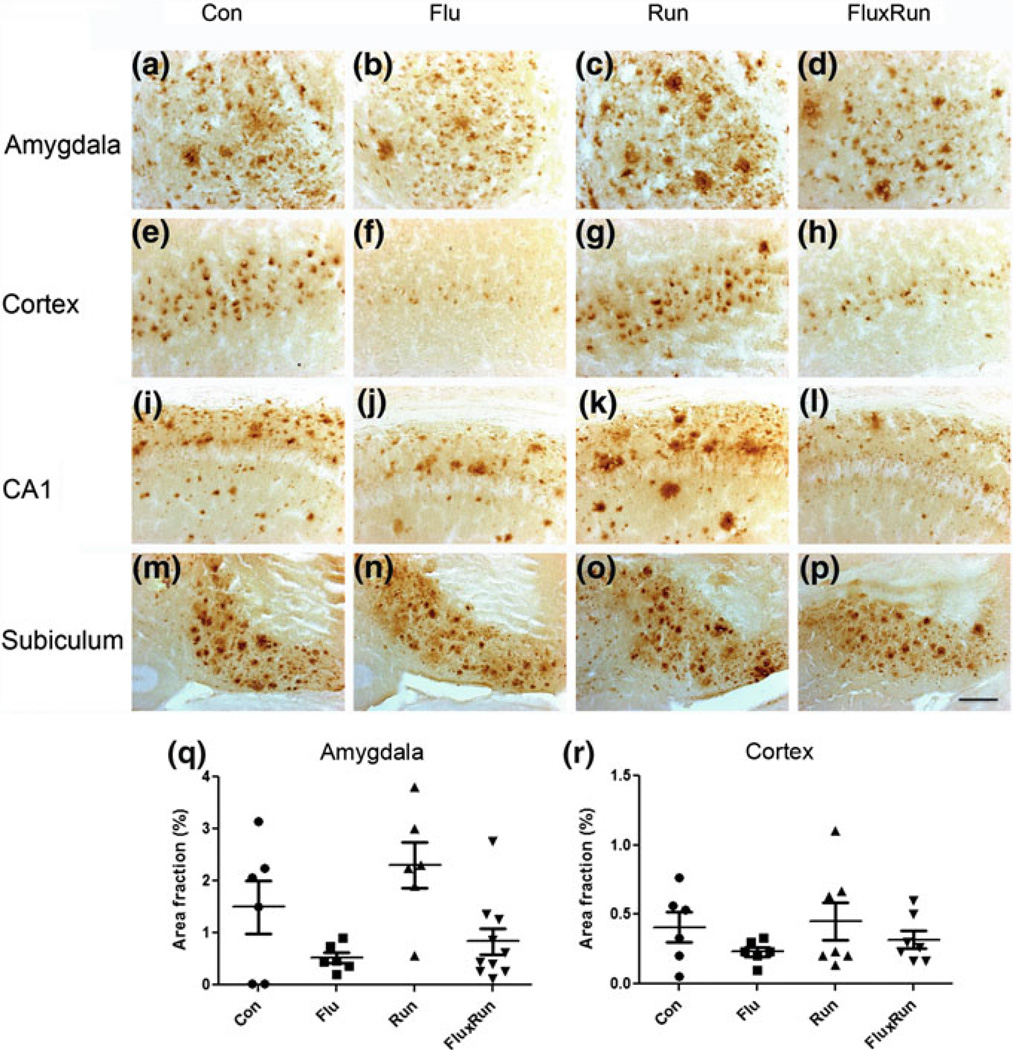
Given the important role of intraneuronal Aβ in the pathogenesis, we used immunocytochemistry to detect intraneuronal Aβ. This technique also identifies extracellular Aβ plaques, but not beta C-terminal fragments (β-CTFs). In sections of the rostral hippocampus, intraneuronal Aβ was observed primarily in the cortex and amygdala (Amy) (Figs. 6a, b, and 7a–h). In contrast to these areas, extracellular Aβ plaques were predominantly found in the CA1 region and Subiculum (Figs. 6c, d, and 7i–p). In order to make appropriate comparisons, these structures were quantified by densitometry across three separate sections that were standardized to specific rostral-caudal orientations (representative levels Fig. 6). A 1:6 series of tissue sections was used to assess levels of neuropathology, such that neighboring sections are 240 µm apart. If three consecutive sections were not available for quantification within an anatomical region, within the proper rostral-caudal orientation (amygdala and cortex −1.46 → − 2.30 mm, CA1 −1.94 → 2.78 mm, subiculum −2.46 → − 3.30 mm), the animal was not included for analysis.
Fig. 6.
Anatomical overview and regions of interest used for quantification of Aβ pathology in 20 month-old female 3xTg mice. a Cortical and amygdala Aβ pathology were quantified at approximately Bregma −1.46 mm. b Cortical, amygdala and CA1 pathology were quantified at −1.94, while quantification of the subiculum continued through −2.46, c staining of the subiculum was quantified in the caudal hippocampus, here at −2.70 mm, but as far back as −3.30 mm. scale bar = 600 µm
Fig. 7.
Intraneuronal Aβ and Aβ plaque load in selected subregions of the 3xTg mouse brain. a–d Examples of Aβ deposition in the amygdala of 3xTg mice, showing intraneuronal Aβ and extracellular Aβ deposits across cohorts. e–h Intraneuronal Aβ was found in the cortex of all cohorts, with little evidence of extracellular deposits. i–j In the CA1 region, the majority of detected Aβ was present in extracellular plaques, but no neuronal profiles were seen when compared to the cortex. m–p A dense staining of extracellular plaques was predominantly found in the subiculum across cohorts (scale bar = 100 µm). q Densitometry of the amygdala across cohorts: Flu and FluxRun groups had lower area fractions scores compared to Run, but not to the Con groups. (One-way ANOVA F(3,24) = 4.79, p < 0.05) Bonferroni post-test Flu versus Run p < 0.05, FluxRun versus Run p < 0.05). r In the cortex, fluoxetine appears to reduce Aβ accumulations, however, these differences were not statistically significant (One-way ANOVA F(3,22) = 1.00, p = 0.41)
In the 3xTg mice, most amyloid pathology was present in the subiculum, amygdala, and hippocampal CA1 areas, whereas the dentate gyrus, e.g., was largely devoid of amyloid.
3.7 Hyperphosphorylated Tau in the Caudal Hippocampus
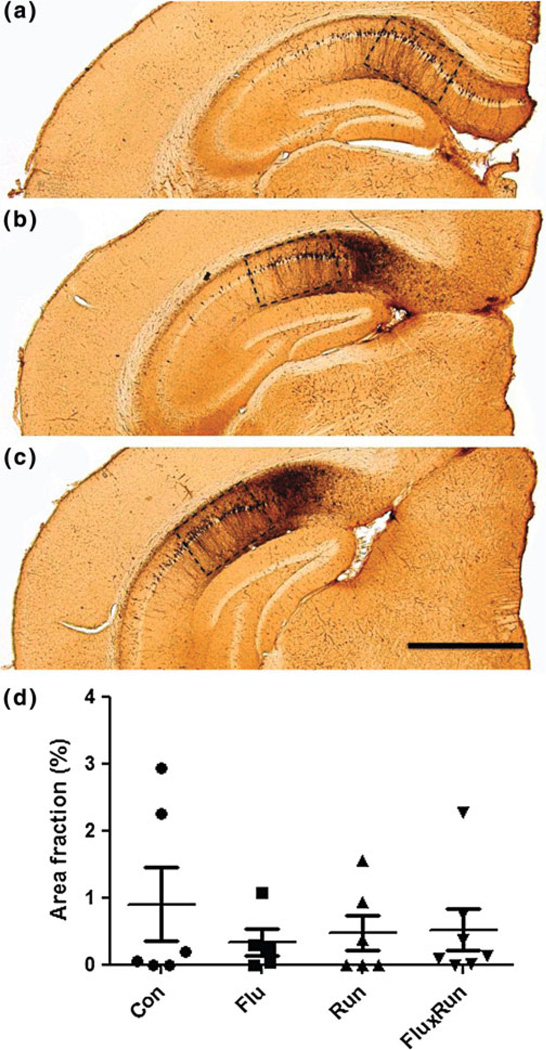
Tau pathology, known to correlate well with cognitive decline and hence an important measure for neurodegeneration in AD models, was quantified following immunocytochemistry for AT8, a well-known pathological tau epitope. AT8 expression was particularly prominent in the CA1 region in the 3xTg mice at this age, with some minor expression extending into the subiculum but hardly, if any expression in other brain regions (Fig. 8).
Fig. 8.
AT8 tau pathology in the CA1 region of the 3xTg mouse. a–c Neurons of the CA1 regions showed robust staining for hyperphorlated tau protein as identified by AT8 immunostaining. While being photographed sections were positioned to exclusively frame CA1. Quantification was carried out on sections beginning at −1.94 mm Bregma and continuing through approximately −3.16 mm Bregma, scale bar = 600 µm. d None of the interventions showed an ability to reduce pathological hyperphosphorylation (ANOVA F(3,22) = 0.58, p = 0.61)
4 Discussion
4.1 Discussion of the Present Data on the 3xTg Mice
Our current data demonstrates that running, when started in middle-age, increased survival of newborn neurons in 3xTg mice studied at old age. Cells identified by BrdU-labeling in this experiment survived for 11 months, indicating that neurons generated in middle-age are indeed maintained through old age. As we identified newborn cell survival both after 1 and 11 months of running and fluoxetine treatment, it is clear that only a minor loss of the labeled newborn neurons occurred during the 10-month period in between; in control 3xTg mice, an average of 173 labeled cells were observed after 1 month and an average of 149 surviving cells per DG after 11 months (Fig. 2a, b. Hence, the selection process for new cells appears to occur primarily during their first month of life. Further comparing the amount of neurogenesis observed here to that documented in our previous study in wildtype mice (Marlatt et al. 2012), shows that the 3xTg mice have lower neurogenesis levels per se compared to the C57Bl6 mice parental strain.
In 20-month-old mice, 11 months of running and synergistic treatment both increased survival of new cells. Interestingly, this occurred in fluoxetine treated runners despite evidence that fluoxetine impairs motor performance. Indeed, the increase in BrdU labeling was less in the FluxRun than Run animals. Data from the wheel runners indicate a trend for a reduced total distance in animals given fluoxetine in their drinking water. Previously, fluoxetine has been shown to significantly reduce running wheel activity also during acute treatment (Weber et al. 2009). While confirming evidence for reductions in running wheel activity during short time periods, our study further shows that long-term drug treatment impairs motor coordination in 3xTg mice. Prior to starting this experiment, there was evidence that fluoxetine could increase activity (Brocco et al. 2002; Prinssen et al. 2006), but currently it is unknown if this occurs in a dose-dependent manner, or how exactly fluoxetine influences activity of mice.
In the literature, benefits have further been reported with the use of antidepressants in 3xTg mice. While neurogenesis may be altered in human depression and can be responsive to antidepressive treatment (Lucassen et al. 2010a; Boldrini et al., 2009), the effects of several antidepressant drugs on neurogenesis depend on the age of the animal, its early-life history and the mouse strain under study (Couillard-Despres and 2009; Navailles 2008). Indeed, in the present study, similar to our findings in C57Bl/6 mice (Marlatt et al. 2010) fluoxetine did not increase neurogenesis in 3xTG mice. Amitriptyline when given for 4 months was shown to increase neurogenesis, to improve performance in the MWM, and to increase BDNF protein levels in male 3xTg mice (Chadwick et al. 2011). Paradoxically, however, this study also showed that amitriptyline increased Aβ deposition in the brain. This surprising finding highlights that while antidepressants, such as fluoxetine, do not have a known direct pharmacology for APP and the generation of Aβ peptide, these drugs can nonetheless impact its clearance and deposition and there may be secondary mechanisms capable of influencing Aβ accumulation in the brain. Earlier work had established that paroxetine, another antidepressant from the SSRI class, reduced expression of APP by binding a promoter region in the 5′UTR (Tucker et al. 2005) while this drug also showed cognitive benefits in the Morris Water Maze (Nelson et al. 2007). Together, this indicates that antidepressants can exert indirect effects on Aβ plaque deposition in the 3xTg model, each with unique mechanisms of action. Whether these drugs can have a meaningful impact on Aβ deposition in humans remains to be determined.
4.2 Behavioral Phenotype of 3xTg Mice
As a delayed behavioral phenotype had already been observed in younger backcrossed 3xTg mice, we started the experiment when our animals were 9 months of age. In a study of 12-month-old male 3xTg mice increased anxiety was reported during a 6-week trial of psychosocial stress (Rothman et al. 2012). A comparable study utilizing male 3xTg mice, starting at 14 months of age, reported behavioral deficits at 18 months of age (Chadwick et al. 2011). These reports on the backcrossed strains demonstrated a significant delay in the development of their behavioral phenotype when compared to the original strain, where cognitive deficits were documented as early as at 6 months of age in both male and female 3xTg mice (Billings et al. 2005). Hence, for future behavioral studies with the 3xTg mice, the original strain would be preferred as also discussed in more detail below.
Testing spatial learning and memory in the MWM is known to represent learning under stress and produces robust elevations in corticosterone release, the predominant glucocorticoid hormone in rodents (Schaaf et al. 1999). Occupancy of glucocorticoid receptors (GR) is known to play a critical role in determining performance in the MWM (Oitzl et al. 1998). Interestingly, female 3xTg mice show elevated corticosterone levels after 5 days of MWM training, a sexual dimorphism not present in male 3xTg mice (Clinton et al. 2007). In our present MWM experiments, corticosterone levels were not quantified but future studies with the 3xTg model should ideally measure corticosterone concentrations in both sexes. Also, additional spatial tasks, such as the Barnes maze or the object recognition test, that generally induce a smaller or no stress response, should be used in parallel to test the contribution of a stress response to spatial performance in these mice.
Wheel running in 3xTg mice has been shown to attenuate cognitive deficits. Interestingly, these were only found in the female running mice (Pietropaolo et al. 2008). Also, other behavioral changes have been observed. Chronic exercise could prevent deterioration of anxiety and startle responses in the 3xTg mice (García-Mesa et al. 2011). Outside of the traditional markers of AD neuropathology, 6 months of wheel running was further shown to reduce markers of oxidative stress in the brains of 7-month-old 3xTg mice and to protect these mice against an age-related loss of synaptic integrity (García-Mesa et al. 2011). A separate study showed that voluntary running increased neurogenesis when evaluated at 9 months of age; running, however, did not change the fate of the newborn cell phenotypes, which agrees with our current results in the same 3xTg mice (Rodriguez et al. 2011) but not with our previous study in younger C57Bl6 mice (Marlatt et al. 2010).
4.3 Backcrossed Versus Orginal 3x Tg Mice
Although the old, backcrossed 3xTg mice lack a strong behavioral phenotype, the animals used in the present experiments were systematically genotyped and did exhibit both Aβ and tau accumulations in their brains. This indicates that AD pathology can occur in absence of an appreciable behavioral phenotype. Most likely other genes from the C57 background have contributed here, that could have influenced the rate of decline, or even have overruled possible deleterious effects, if any, of the AD pathology present in these mice. An alternative option is that more abundant expression patterns might be needed before behavioral deficits can develop, while it is also possible that the presence of plaque and/or tau pathology in these key brain regions does not necessarily predict behavioral deficits and, as in other models, might follow, rather than precede, behavioral deficits in time (Oddo et al. 2003b; van Dooren et al. 2005; Götz and Ittner 2008). Identifying the exact factors responsible for these effects in the backcrossed strain of 3xTg mice and why they are different from the original strain, would require additional experiments.
One option would be to identify quantitative gene expression changes, by microarray and quantitative PCR, for each region of the brain. Identified candidates from the screening could be prioritized based on known protein structures and functions. Ideally, Western blotting data combined with immunohistochemistry could identify subcellular changes in distribution or cell type. Further analysis of soluble and insoluble fractions of Aβ peptide by ELISA might shed light on the proteolytic cleavage of APP. Further experiments would aslso be needed to identify possible changes in Aβ oligomers and determine if trafficking and/or clearance of these proteins is altered in the backcrossed strain.
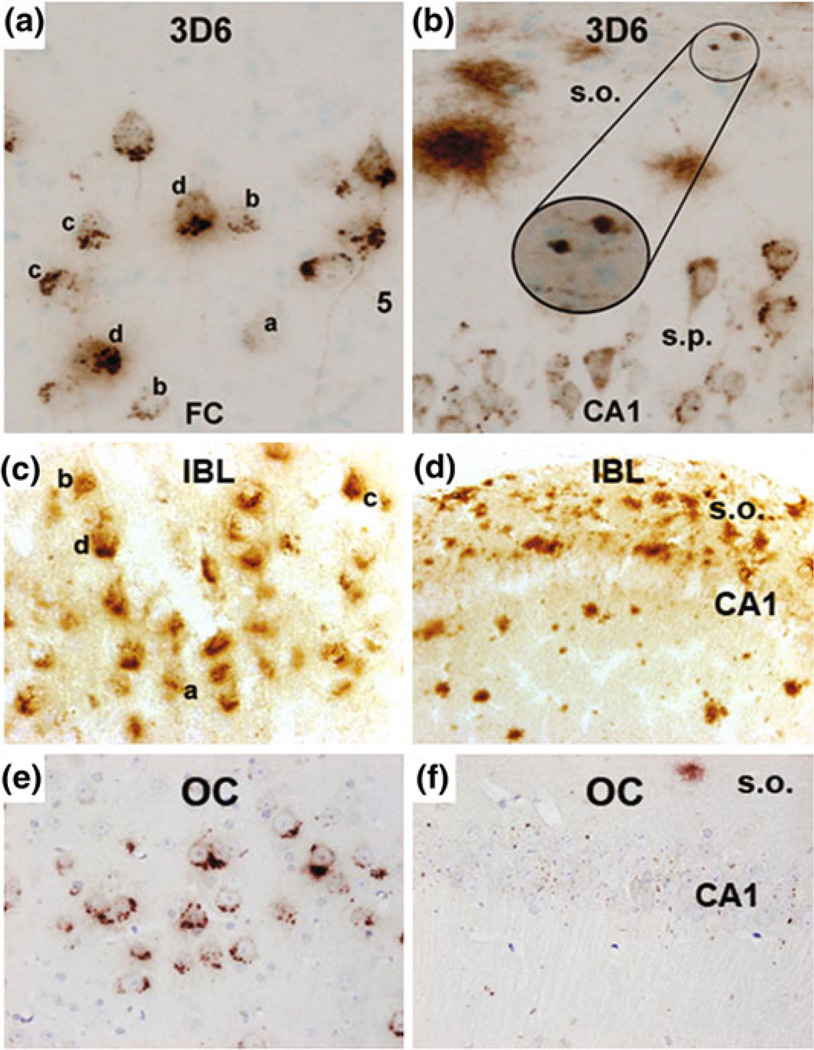
At this time we can only speculate that backcrossing the 3xTg mouse may have led to a loss of intraneuronal Aβ expression in the CA1 subregion. This is difficult to address because no mice were available from the original strain, of the same age, and stained with the same antibody. Moreover, we used an antibody directed against N-terminal Aβ (IBL #18584). Previously, in the hybrid 3xTg strain at ages of 2, 14, and 20 months, a monoclonal antibody directed at amino acids 1–5 of the N-terminal Aβ variant (3D6, Elan, South San Francisco, CA, USA) has been used to visualize Aβ deposits (Cai et al. 2012). These authors found that 3D6 labels Aβ deposits across the cortex and pyramidal layer in 2-month-old animals, where it appears as perisomatic puncta or granules. In 14-month-old animals, intraneuronal puncta labeling was also seen in the CA1. In 20-month-old transgenics, puncta are evident in the CA1, where 3D6 labels around the neuronal somata, however, this is less dense than the intraneuronal staining seen in the cortex (Cai et al. 2012) (Fig. 9, panels a, b). A conformation-specific antibody, which identifies fibrillar oligomers (Kayed et al. 2007), has also been used to identify intraneuronal CA1 staining in the 3xTg mice, aged 18 months, that were maintained on the hybrid background (Wirths et al. 2011) (Fig. 9, panels e, f). By comparing the staining patterns between hybrid and backcrossed 3xTg mice for cortical and hippocampal subregions, this limited evidence indicates that a loss of intraneuronal Aβ in the hippocampus may contribute to, or possibly, explain the loss of the present behavioral phenotype.
Fig. 9.
Comparing cortical and hippocampal Aβ accumulations in aged 3xTg mice during backcrossing with three antibodies (3D6, IBL, and OC) In an effort to identify differences due to backcrossing, this figure compares cortical and hippocampal staining in 3xTg mice. a In 20-month-old nonbackcrossed mice, intraneuronal Aβ puncta are condensed in the cortex, increasing density of puncta at the axon terminal is denoted by letters a–d. b This is also seen to a lesser extent in the CA1, where 3D6 labels neuronal somata; diffuse plaques and axonal processes are also seen in the stratum oriens (s.o.) Panels a, b reproduced from Fig. 2 of (Cai et al. 2012) c 20-month-old backcrossed mice, presented in this study, show similar intraneuronal puncta in the cortex with identifiable accumulations at the axon terminals d however, no discernable neuronal profiles are seen in the CA1 of backcrossed 3xTg mice indicating that intraneuronal Aβ is absent in these animals e 18-month-old nonbackcrossed mice are positive for intraneuronal Aβ as detected by conformation specific OC antibody in the cortex. f 3xTg mice on the nonbackcrossed background show low levels of intraneuronal Aβ in the CA1 when measured with conformation specific OC antibody. Panels e, f adapted from Fig. 4 Wirths et al. 2011
In further discussions of the 3xTg mice, others have also observed significant problems in retrieving the originally described phenotype after backcrossing (Dr. E. Hol, Amsterdam, personal communication). The 3xTg mice are now widely available from commercial vendors, namely the Jackson laboratory, and several investigators that have received mice generated from frozen embryos found that the phenotype of these animals were significantly delayed (personal communication Dr. Jorge Palop). Thus, for future studies with 3xTg mice, ideally, the original strain would be preferred.
4.4 General Discussion of Neurogenesis in Relation to Alzheimer Pathology
While synaptic plasticity is thought to be the main structural change corresponding to rapid changes in cognitive function, the addition of new neurons to an existing circuit through adult neurogenesis represents a unique form of structural plasticity for the longer term. In young rats, it has been estimated that approximately 9000 new cells per day are generated in the entire hippocampus, and a considerable proportion of these cells die within several days. While the number of new neurons incorporated into the DG may be quite low, particularly during aging, this ongoing phenomenon holds a potential for adaptation. The Neurogenic Reserve hypothesis (Kempermann 2008) states that ongoing adult neurogenesis is a special type of brain plasticity that allows for adaptation during activity, that may influence vulnerability to accumulating deleterious events and brain diseases.
While the links between AD and neurogenesis are controversial, connections have been established between neurogenesis, stress, and antidepressant drug action. Of interest, high circulating levels of stress hormones form a substantial risk factor for AD. Maintenance of adult neurogenesis could stabilize synaptic density, alleviating synapse loss, promote structural plasticity, and reduce age-associated changes. Indeed, a loss of haematopoietic stem cells can result in premature aging (Nelson et al. 2012). Currently, however, no evidence has been collected regarding the relationship between loss of stem cells and specific neurologic dysfunction in humans.
So far, clear age-dependent reductions in neural progenitor cell proliferation have been shown in rodents and old monkeys, and physical activities could reduce this age-dependent decline in precursor cell proliferation. Different models of pathology have shown robust and transient increases in adult cytogenesis that are nonspecific and likely involved in gliogenesis. This nonspecific upregulation or proliferation will at least not result in acute functional recovery. A restricted view of adult neurogenesis implies that for healthy aging to occur it may be perhaps most beneficial, if activity is established in midlife to preserve adult neurogenesis prior to clinical presentation with dementia or AD. As neurons born in aged mammals are just as functional as the ones generated during developmental neurogenesis in young mammals, maintenance of stem cell proliferation, and of the local microenvironment that is responsible for proper migration and connection, is necessary to fully understand the dynamics of the niche during aging and AD.
So far, various animal studies have addressed the relation between the occurrence of Alzheimer pathology and changes in various neurogenesis markers (Verwer et al. 2007; Marlatt and Lucassen 2010). Obviously, these results depend very much on the timing of the onset of pathology, the age of animals studied, and the promotor transgene expressed.
4.5 Presenilin-1 Transgenic Mouse Models
Interest in gamma-secretase and the generation of highly fibrillogenic AB42 has led to the development of presenilin-1 (PS1) transgenic mice expressing mutant PS1. PS1 is part of the gamma-secretase complex but also participates in cellular proliferation, Notch and Wnt signaling mechanisms. PS1 signaling, are responsible for developmental maturation of glia and neurons. In Wnt signaling, PS1 is directly involved with beta-catenin turnover, a mechanism responsible for proliferation of progenitor cells in the developing brain. Normal PS1 facilitates phosphorylation of beta-catenin leading to proteasomal degradation; mutant PS1 cells have increased stability of beta-catenin leading to downstream nuclear signaling events. It is therefore not surprising that neuronal expression of mutant PS1 with a Thy1 promoter increased proliferation in the DG of 4-month-old transgenic mice. Increased cell proliferation did not result in increased neuron survival in the hippocampus of these mice (Wen et al. 2002, 2004; Wang et al. 2004).
Regarding neurogenesis, PS1/PS2 KO mice were evaluated at two ages and found to have increased proliferation and survival. A study of PS1 expressed under the NSE promoter found that proliferation was reduced by both wildtype and mutant PS1. Interestingly, the wild-type PS1 mice had increased survival of immature neurons while the mutants did not though (Wen et al. 2002). A follow-up to this study incorporated groups with environmental enrichment - expression of the wild-type protein was sufficient to increase survival of immature neurons expressing Tuj1. Enrichment in these mice increased proliferation and survival compared to the nonenriched group. This normal physiology was not preserved in mice expressing mutant PS1; enrichment increased proliferation; however, there were no changes in Tuj1 expression and less surviving BrdU+ cells (Wen et al. 2002, 2004; Wang et al. 2004; Zhang et al. 2007; Kuhn et al. 2007). A more sensitive experiment was produced by crossing mutant PS1 knockin mutants with PS1 knockouts. Mice with one mutant copy of PS1 show impaired learning in a contextual fear conditioning test. This impaired associative learning was positively correlated with impaired neurogenesis. Hence, expression of wild-type PS1 can override the mutant PS1 gene (Wen et al. 2002, 2004; Wang et al. 2004; Zhang et al. 2007; Kuhn et al. 2007).
4.6 Amyloid Precursor Protein Transgenics
Transgenic models of AD further include mice expressing mutant APP; as murine APP does not generate fibrillogenic peptides, typically bigenic mice are generated expressing mutant PS1 and human APP. As reviewed recently, most APP and APP/PS1 mouse models show reductions in cell proliferation, although exceptions exist (Marlatt and Lucassen 2010; Thompson et al. 2008; Kuhn et al. 2007). Limited information is present concerning the subsequent survival of the newborn cells. APP mice had no difference in hippocampal neurogenesis when evaluated by BrdU incorporation at young adult age. At this age the mice do not have amyloid deposits, but when the mice were evaluated at 25 months of age, APP mice exhibited significant increases in the number of BrdU and DCX-positive cells (Ermini et al. 2008) while also in affected human hippocampus and cortex, proliferative and neurogenic-like changes have been seen (Boekhoorn et al. 2006a; Verwer et al. 2007). A separate study utilizing different APP-PS1 mice at 8 months of age showed increased BrdU and NeuN-positive cells compared to controls despite finding that APP-PS1x NestinGFP mice exhibited decreases in nonproliferative Nestin-positive NPCs (Gan et al. 2008). Hence, endogenous neurogenesis appears to be elevated in response to pathology, however, the molecular mechanisms and the functionality of these new neurons are yet to be elucidated.
The relationship between Abeta and neurogenesis has also been combined in interventional studies: environmental enrichment or running was expected to lead to improvements in behavior, via reductions in Abeta plaque load and possibly increases in neurogenesis. As expected, mice provided with environmental enrichment had increases in newborn cell proliferation and survival. These changes corresponded to improved performance in a spatial memory task, but surprisingly, there was no change in plaque load. The results indicate that despite plaque burden, the neurogenic environment is preserved, which allows functional recovery (Wolf et al. 2006; Mu and Gage 2011; Li et al. 2008). Again, structural pathology appears to dissociate from functional pathology in these models (Mu and Gage 2011; Li et al. 2008; Nelson et al. 2012).
4.7 Tau Transgenic Mice
So far, only a few mouse models of tauopathy have been evaluated. A model utilizing human tau with two mutations describes induction of hyperphosphorylation and the presence of neurofibrillary tangles (NFTs) in 3–6 month-old animals in the hippocampus. Cell bodies of the DG are spared at this young age but neurites in these areas are immunopositive for AT8, indicating aberrant phosphorylation of tau, similar to what is found in AD. Compared to non-Tg mice, transgenic tau mice had 2-fold higher DCX levels and significantly higher expression of TUC-4 in the DG through 6 months (Schindowski et al. 2008). Mice with nonmutant human tau also show signs of proliferation, albeit outside the subgranular zone of the hippocampal dentate gyrus (SGZ) and the subventricular zone (SVZ) (Andorfer et al. 2005). Using a knockout–knock-in approach, it was further shown that expression of 4R tau reduces cell proliferation and increases differentiation and neuronal maturation, confirming an important role for tau in neuronal plasticity and differentiation (Sennvik et al. 2007; Fuster-Matanzo et al. 2012; Llorens-Martin et al. 2012). Also, in young mice carrying the tau-P301L mutation that is associated with frontotemporal lobe dementia, increased long-term potentiation (LTP) in the dentate gyrus was observed, notably parallel to an improved cognitive performance. As neither tau phosphorylation motor deficits nor neurogenesis could account for these changes, this demonstrated that tau plays an important role in hippocampal memory, and that it is not the tau mutations per se, but rather the ensuing hyperphosphorylation that is critical for the development of functional deficits (Boekhoorn et al. 2006b; Fuster-Matanzo et al. 2012; Nelson et al. 2012).
Ultimately, our current study reflects that despite the presence of neuropathology and the lack of behavioral deficits, newborn cell survival can still be stimulated in 3xTg mice by exercise. Notably, this occurs in absence of parallel effects on neuronal differentiation of the newborn cells, neuropathology or hippocampal BDNF levels. Furthermore, backcrossing the 3xTg mouse strain should be avoided as it seems to change its behavioral phenotype, thereby diminishing its predictive value as a model of AD.
Acknowledgments
This work was supported in part by the National Institute on Aging (NIA), Intramural Research Program. We thank Dr. Mark Mattson (NIA) for providing the 3xTg mice. MM, TAB, and PJL are supported by the EU (NEURAD consortium). MM and PJL are supported by ISAO. PJL is further supported by the Dutch Brain Foundation, by the International Parkinson Foundation IPF, and by the Netherlands Organization for Scientific Research NWO.
Contributor Information
Michael W. Marlatt, Swammerdam Institute for Life Science—Center for Neuroscience, University of Amsterdam, Science Park 904 1098 XH Amsterdam, The Netherlands
Michelle C. Potter, Laboratory of Neurosciences, National Institute on Aging Intramural Research Program, Baltimore, MD, USA
Thomas A. Bayer, Division of Molecular Psychiatry, Georg-August-Universität Goettingen, Goettingen, Germany
Henriette van Praag, Laboratory of Neurosciences, National Institute on Aging Intramural Research Program, Baltimore, MD, USA.
Paul J. Lucassen, Email: p.j.lucassen@uva.nl, Swammerdam Institute for Life Science—Center for Neuroscience, University of Amsterdam, Science Park 904 1098 XH Amsterdam, The Netherlands.
References
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25(22):5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Wirths O. Intracellular accumulation of amyloid-Beta—a predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front Aging Neurosci. 2010;2:8. doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006a;24(1):1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006b;26(13):3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocco M, Dekeyne A, Veiga S, Girardon S, Millan MJ. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A pharmacological characterization of diverse classes of antidepressant agents. Pharmacol biochem behav. 2002;71(4):667–680. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, van Praag H, Winkler J, Gage FH, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Cai Y, Zhang X-M, Macklin LN, Cai H, Luo X-G, Oddo S, et al. BACE1 elevation is involved in amyloid plaque development in the triple transgenic model of Alzheimer’s disease: differential Ab antibody labeling of early-onset axon terminal pathology. Neurotox Res. 2012;21(2):160–174. doi: 10.1007/s12640-011-9256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick W, Mitchell N, Caroll J, Zhou Y, Park S-S, Wang L, et al. Amitriptyline-mediated cognitive enhancement in aged 3xTg Alzheimer’s disease mice is associated with neurogenesis and neurotrophic activity. PLoS One. 2011;6(6):e21660. doi: 10.1371/journal.pone.0021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DZ, Bayer TA, Wirths O. Formic acid is essential for immunohistochemical detection of aggregated intraneuronal Abeta peptides in mouse models of Alzheimer’s disease. Brain Res. 2009;1301:116–125. doi: 10.1016/j.brainres.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Christensen DZ, Schneider-Axmann T, Lucassen PJ, Bayer TA, Wirths O. Accumulation of intraneuronal Abeta correlates with ApoE4 genotype. Acta Neuropathol. 2010;119(5):555–566. doi: 10.1007/s00401-010-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, et al. Age-dependent sexual dimorphism in cognition and stress response in the 3×Tg-AD mice. Neurobiol Dis. 2007;28(1):76–82. doi: 10.1016/j.nbd.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, et al. Ageing abolishes the effects of fluoxetine on Neurogenesis. Mol Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, et al. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172(6):1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster-Matanzo A, Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F. Tau protein and adult hippocampal neurogenesis. Front Neurosci. 2012;6:104. doi: 10.3389/fnins.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, et al. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer’s disease like transgenic (pPDGF-APPSw, Ind) mice. Neurobiol Dis. 2008;29(1):71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mesa Y, López-Ramos JC, Giménez-Llort L, Revilla S, Guerra R, Gruart A, et al. Physical exercise protects against Alzheimer’s disease in 3×Tg-AD mice. J Alzheimers Dis. 2011;24(3):421–454. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8(1):159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci lett. 1995;189(3):167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Götz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9(7):532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, et al. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156(1):15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol. 2010;119(5):523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31(4):163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, et al. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Cooper-Kuhn CM, Boekhoorn K, Lucassen PJ. Changes in neurogenesis in dementia and Alzheimer mouse models: are they functionally relevant? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):281–289. doi: 10.1007/s00406-007-0732-4. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, Iqbal K, Grundke-Iqbal I. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67(1):78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Teixeira CM, Fuster-Matanzo A, Jurado-Arjona J, Borrell V, Soriano E, Avila J, Hernández F. Tau isoform with three microtubule binding domains is a marker of new axons generated from the subgranular zone in the hippocampal dentate gyrus: implications for Alzheimer’s disease. J Alzheimers Dis. 2012;29(4):921–930. doi: 10.3233/JAD-2012-112057. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czéh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20(1):1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Stumpel M, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010a;58:940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Lucassen PJ. Neurogenesis and dementia: biology and pathophysiology of mice and men. Curr Alzheimer’s Res. 2010;7(2):113–125. doi: 10.2174/156720510790691362. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Lucassen PJ, Van Praag H. Side by side comparison of effects of running and antidepressants on neurogenesis in mice. Brain Res. 2010;1341:93–99. doi: 10.1016/j.brainres.2010.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis and BDNF levels in female C57Bl/6 J mice. Dev Neurobiol. 2012 doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;22(6):85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, et al. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nelson RL, Guo Z, Halagappa VM, Pearson M, Gray AJ, Matsuoka Y, et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007;205(1):166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003a;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003b;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, Sutanto W, de Kloet ER. Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. Eur J neurosci. 1998;10(12):3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192(1):42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Prinssen EP, Ballard TM, Kolb Y, Nicolas LB. The effects of serotonin reuptake inhibitors on locomotor activity in gerbils. Pharmacol Biochem Behav. 2006;85(1):44–49. doi: 10.1016/j.pbb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One. 2008;3(8):e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TDD, Yeh CY, et al. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2011;8(7):707–717. doi: 10.2174/156720511797633214. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, et al. 3xTgAD mice exhibit altered behavior and elevated Ab after chronic mild social stress. Neurobiol Aging. 2012;33(4):830, e1–830, e12. doi: 10.1016/j.neurobiolaging.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101(2):305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Sibug RM, Duurland R, Fluttert MF, Oitzl MS, de Kloet ER, et al. Corticosterone effects on BDNF mRNA expression in the rat hippocampus during Morris water maze training. Stress. 1999;3(2):173–183. doi: 10.3109/10253899909001121. [DOI] [PubMed] [Google Scholar]
- Schindowski K, Belarbi K, Bretteville A, Ando K, Buee L. Neurogenesi and cell cycle-reactivated neuronal death during pathogenic tau aggregation. Genes Brain Behav. 2008;7(Suppl 1):92–100. doi: 10.1111/j.1601-183X.2007.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennvik K, Boekhoorn K, Lasrado R, Terwel D, Verhaeghe S, Korr H, et al. Tau-4R suppresses proliferation and promotes neuronal differentiation in the hippocampus of tau knockin/knockout mice. FASEB J. 2007;21(9):2149–2161. doi: 10.1096/fj.06-7735com. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thompson A, Boekhoorn K, van Dam AM, Lucassen PJ. Changes in adult neurogenesis in neurodegenerative diseases; cause or consequence? Genes Brain Behav. 2008;7:28–42. doi: 10.1111/j.1601-183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Tucker S, Ahl M, Bush A, Westaway D, Huang X, Rogers JT. Pilot study of the reducing effect on amyloidosis in vivo by three FDA pre-approved drugs via the Alzheimer’s “APP 5” untranslated region. Curr Alzheimer Res. 2005;2(2):249–254. doi: 10.2174/1567205053585855. [DOI] [PubMed] [Google Scholar]
- van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, van der Zee EA, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- van Dooren T, Dewachter I, Borghgraef P, van Leuven F. Transgenic mouse models for APP processing and Alzheimer’s disease: early and late defects. Subcell Biochem. 2005;38:45–63. doi: 10.1007/0-387-23226-5_2. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Nat Acad Sci U S A. 1999a;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RW, Sluiter AA, Balesar RA, Baayen JC, Noske DP, Dirven CMF, Wouda J, van Dam AM, et al. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain. 2007;130:3321–3335. doi: 10.1093/brain/awm264. [DOI] [PubMed] [Google Scholar]
- Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer’s disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126(2):305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Weber M, Talmon S, Schulze I, Boeddinghaus C, Gross G, Schoemaker H, et al. Running wheel activity is sensitive to acute treatment with selective inhibitors for either serotonin or norepinephrine reuptake. Psychopharmacology. 2009;203(4):753–762. doi: 10.1007/s00213-008-1420-4. [DOI] [PubMed] [Google Scholar]
- Wen PH, Shao X, Shao Z, Hof PR, Wisniewski T, Kelley K, et al. Overexpression of wild type but not an FAD mutant presenilin-1 promotes neurogenesis in the hippocampus of adult mice. Neurobiol Dis. 2002;10(1):8–19. doi: 10.1006/nbdi.2002.0490. [DOI] [PubMed] [Google Scholar]
- Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188(2):224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Bayer TA. A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide the first step of a fatal cascade. J Neurochem. 2004;91(3):513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- Wirths O, Dins A, Bayer TA. AβPP Accumulation and/or Intraneuronal Amyloid-β Accumulation? The 3×Tg-AD Mouse Model Revisited. J Alzheimers Dis. 2012;28(4):897–904. doi: 10.3233/JAD-2011-111529. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol Psychiatry. 2006;60(12):1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007;204(1):77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]