Abstract
Objectives
Little is known about the effects of commonly used lubricants on detection of biomarkers of semen exposure. We investigated the in vitro effect of Gynol®, K-Y Jelly®, Replens®, Astroglide®, Carbopol, and Silicorel on quantitative detection of prostate specific antigen (PSA).
Study Design
A predetermined concentration of each of the gels was added to serially diluted semen samples. Additionally, serial dilutions of each of the gels were added to three different semen dilutions (high, medium, or low). The resulting samples were tested for PSA on the Abbott ARCHITECT System.
Results
When using the Abbott ARCHITECT system, the only products that inhibited PSA detection were Gynol® and Replens®. The inhibition caused by Gynol® was dose-dependent, but that of Replens was dose-independent. K-Y Jelly®-spiked samples had higher PSA values than controls.
Conclusions
Caution is warranted when using the Abbott quantitative assay for PSA detection as a biomarker of semen exposure in settings where Gynol®, Replens® or K-Y Jelly® might also have been used. Neither Astroglide® nor Silicorel inhibited PSA detection. Additional studies evaluating other vaginal products, including microbicides, and their effects on other assays, are needed. In vivo studies will be especially important to optimize PSA detection from clinical samples.
Implications
Researchers should consider the potential for specific lubricants or any vaginal products to affect the particular assay used for semen biomarker detection. The Abbott ARCHITECT’s total PSA assay should not be used with the product Replens. Caution is warranted when using the assay in settings where Gynol or K-Y jelly may have been used.
Keywords: Semen biomarkers, Lubricants, Vaginal products, Spermicide, Prostate-specific antigen
1. Introduction
Biomarkers of semen exposure such as prostate specific antigen (PSA) can be useful in HIV/STI prevention research in several ways. They can be used as an indicator of semen exposure in cervical barrier and condom effectiveness trials [1–4]. Researchers have used PSA as an indicator of recent semen exposure in order to assess the effectiveness of condoms [1], and experts in the field have called for more studies to use biomarkers of semen in order to better evaluate the effectiveness of physical barriers such as diaphragms [2–4]. Early clinical trials evaluating the effectiveness of physical barriers of semen often enroll women at very low risk for HIV and other sexually transmitted infections and advise them to engage in intercourse with only the barrier in place. Semen biomarkers can help determine whether sex took place (the biomarker should be present in the condom or on the vaginal side of the diaphragm) and whether the barrier was effective (the biomarker should not be found in the vagina when a condom was used, and not on the cervical side of a diaphragm). Semen biomarkers can also be used as a qualitative adjunct in early evaluations of microbicides in which women are advised to refrain from sex while using a particular product in order to assess product-specific irritation or the immune response in the female genital tract [2,5]. If there is biomarker evidence that the woman recently engaged in intercourse, then irritation or other effects may not be attributable to the product [4,5]. In later microbicide trials, intercourse protected by condoms may be permitted. If there is biomarker evidence that women had unprotected sex, this may inform the study investigators that the participants were not adherent to the protocol and may help with the interpretation of effectiveness [2].
Vaginal lubricants and spermicides are routinely used before or during intercourse, and microbicides could become readily available for the prevention of HIV/STIs. However, little is known about whether such vaginal products (lubricants, spermicides, or microbicides) inhibit detection of PSA with available assays. There are several such assays and particular products may have different effects, depending on the individual characteristics of each product and assay. For example, nonoxynol 9 (N9) interferes with the ELISA PSA assay [6], and has also been shown to interfere with Seratec’s semi-quantitative PSA assay from specimens collected from the inside of spermicidal condoms [7]. Interference and false-positive results were reported with N9 when using Biofilm’s PSA membrane test [8], but not when using the Abacus ABAcard for PSA detection [9]. We have previously reported that N9 in saline did not interfere with the ABAcard’s PSA detection, but Gynol (N9 in propylene glycol) did interfere, and at high concentrations resulted in false positive results [10]. Other substances such as male urine and caustic soda also appear to interfere with PSA detection [11,12].
The Abbott ARCHITECT PSA assay is used in reproductive health research because it is a chemiluminescent immunoassay which yields quantitative results [13] with a readable range of 0–100 ng/mL [14]. Since there is no information as to whether vaginal products interfere with PSA detection using the Abbott ARCHITECT PSA assay, we undertook a series of laboratory experiments to investigate the in vitro effects of several commonly used vaginal products on PSA detection using the Abbott ARCHITECT Total PSA assay.
2. Methods
Table 1 describes the vaginal products (lubricants and spermicides) that were tested. These included Replens®, Carbopol, Gynol®, K-Y Jelly®, Astroglide®, and Silicorel. Replens® is used as a long-acting vaginal moisturizer, and Carbopol is its active ingredient. Gynol® is a common vaginal spermicide (2% nonoxynol 9). Astroglide® and K-Y Jelly® are both commonly used vaginal lubricants. Silicorel is a vaginal lubricant provided in the packaging and recommended for use with the two female condoms available in the US.
Table 1.
Vaginal products tested for their effects on PSA detection by the Abbott ARCHITECT assay
| Product | Brand name (manufacturer) | Main ingredient | Type | Date tested |
|---|---|---|---|---|
| Replens | Replens (LDS consumer products) | Polycarbofil | Vaginal moisturizer | September 2009 |
| CARBOPOL | N/A (formulated at EVMS) | Polycarbofil (Acrylic Polymer) | Main ingredient for Replens | June 2009 |
| Gynol | Gynol 2 (Ortho) | 2% Nonoxynol 9 in propylene glycol | Spermicide | September 2009 |
| K-Y Jelly | K-Y Brand Jelly (Johnson & Johnson) | Hydroxyethylcellulose (HEC) | Vaginal lubricant | September 2009 |
| Astroglide | Astroglide (BioFilm) | Glycerin and propylene glycol | Vaginal lubricant | September 2009 |
| Silicorel | N/A (Formulated at EVMS) | Polydimethylsiloxane (silicone based) | Medical Grade vaginal lubricant | June 2009 |
EVMS, Eastern Virginia Medical School.
In order to investigate whether these products affected PSA detection and to determine if that is dose-dependent, we used a two-step approach.
For the first step, we varied the semen dilutions and held the gel at a constant volume. Samples were prepared from two-fold serial dilutions of pooled semen samples ranging from 1:100 to 1:6,553,600 and then combined with equal volume of vaginal product diluted 1:5 in phosphate-buffered saline (PBS), in order to achieve a final fixed gel concentration of 10% volume.
For the second step, we varied the gel concentrations and added those to three different semen dilutions (to represent a high, medium, and low semen concentrations, respectively). Semen samples were diluted to 1:200 (for high PSA), 1:3200 (for medium PSA), and 1:51,200 (for low PSA) and tested with each vaginal gel which were serially diluted two-fold from 1:10 through 1:1280.
All samples were generated from pooled, PBS diluted semen samples with or without the vaginal products. The experiment was replicated three different times with each replicate on different days by one female lab technician. PSA concentrations were determined by the Abbott ARCHITECT Total PSA assay (Abbott, Abbott Park, IL.)[12]. In order to determine the possible inhibitory effects of the aforementioned vaginal products, experimental samples (with vaginal gels) were compared to controls with no gels. All controls were tested on the same day as the product/experimental samples.
3. Results
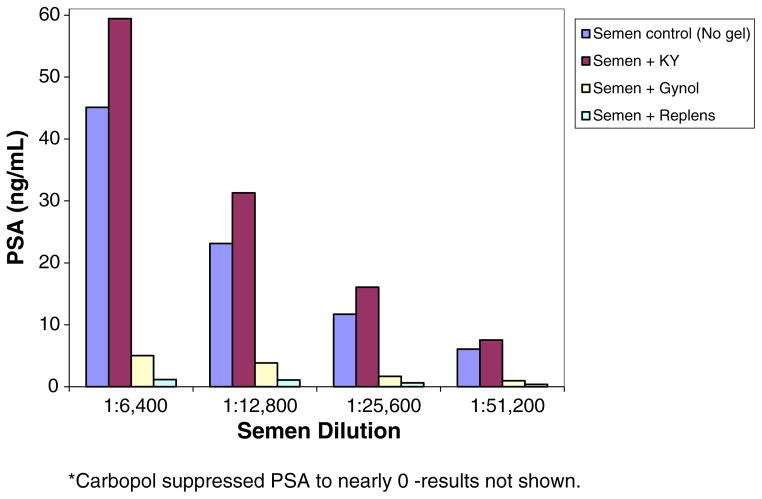
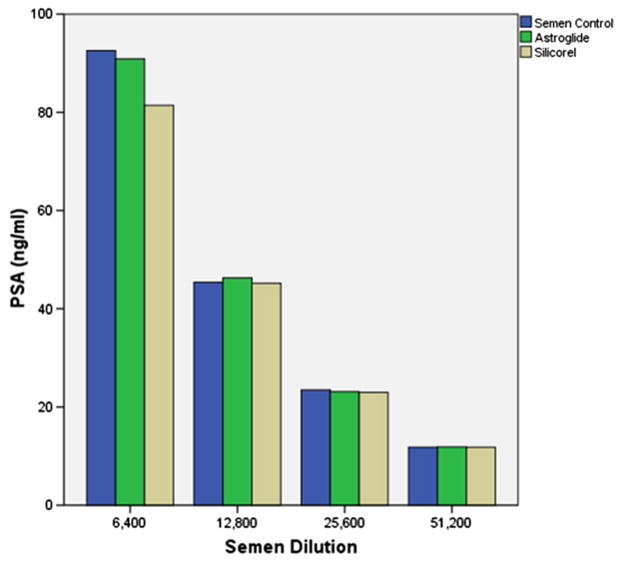
Gynol, Replens, Carbopol, and K-Y jelly affected PSA detection using the Abbott ARCHITECT Total PSA assay. PSA detection was inhibited by the products Gynol®, Replens®, and Carbopol (the active ingredient in Replens®) (Fig. 1). On the contrary, PSA values were 15–60% higher than expected when K-Y jelly® was present at the semen dilution range of 1:6400–1:51,200 (Fig. 1). Neither Astroglide® nor Silicorel affected PSA detection using the Abbott ARCHITECT Total PSA Assay (Fig. 2).
Fig. 1.
Effect of K-Y, Gynol®, Replens® and Carbopol* on PSA detection. PSA concentrations in semen samples spiked with K-Y, Gynol, Replens and Carbopol.
Fig. 2.
Effect of Astroglide® and Silicorel on PSA detection.
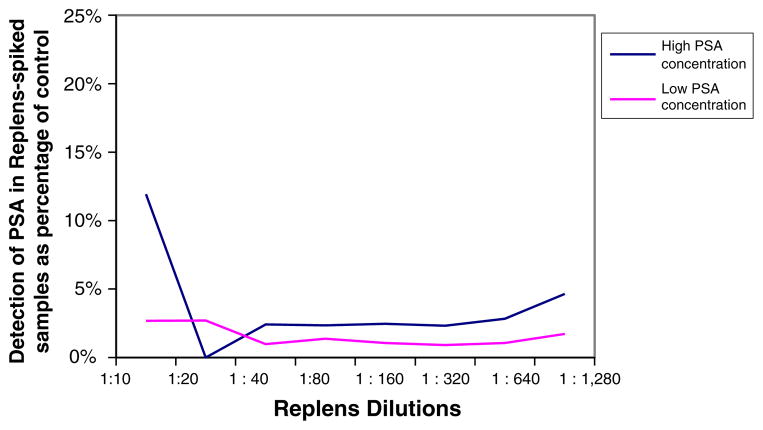
The effects of Gynol® on PSA detection were dose-dependent (Fig. 3). The higher the dose of Gynol®, the more inhibition that occurred to PSA detection. On the other hand, the effects of Replens® on PSA detection were dose-independent (Fig. 4), as Replens® inhibited PSA similarly at all amounts of the product tested. Since there was little to no variation between replicates of the same experiment, the data presented are from one replicate all completed on the same day. Although serial dilutions of pooled semen samples ranged from 1:100 to 1:6,553,600, the data presented in Figs. 1–2 are from semen dilutions that were within the readable range of the assay (within the limits of detection 0–100 ng/ml) of 1:6400 to 1:51,200.
Fig. 3.
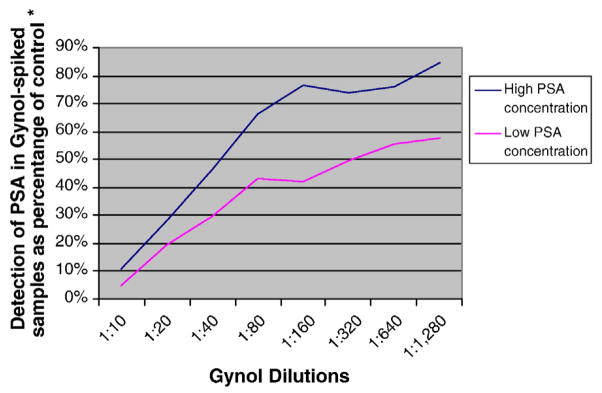
Dose-dependent inhibition of Gynol® on PSA detection.
Fig. 4.
Dose-independent inhibition of Replens® on PSA detection.
4. Discussion
Gynol® inhibited PSA detection by the quantitative Abbott ARCHITECT assay. However, this inhibition was dose-dependent. Higher concentrations of Gynol® (1:40–1:10 dilutions) produced approximately a 50–90% inhibition, while lower concentrations (1:640–1:80 dilutions), inhibited detection to a lesser extent (approximately 25–33%). Clinically, this degree of reduction would likely not be significant in most cases, unless very low PSA concentrations were present in the clinical specimen and/or if high concentrations of Gynol® were present. Replens® substantially inhibited PSA detection when using the quantitative Abbott ARCHITECT Total PSA assay. At the concentrations tested in this study (1:10–1:1,280 dilutions), the inhibitory effect of Replens® was dose-independent. Even small amounts of Replens® inhibited PSA detection to the same extent as higher concentrations. This inhibition appeared to be caused by Carbopol, the active ingredient in Replens® (Fig. 1), even though we do not know why or through what mechanism. Since Replens® at high concentrations only slightly inhibits the ABAcard assay [10], we suspect that its physical characteristics (i.e., the cloudy white color) may interfere with the Abbott ARCHITECT’s total PSA assay. Even though in vivo corroboration is required, these findings suggest that the assay should not be used when or if it is likely that the product Replens® is present in patient samples.
K-Y Jelly® did not inhibit PSA detection; on the contrary, samples with K-Y Jelly® gave higher PSA levels than what would be expected with the respective semen dilution alone. Clinically, this degree of increase would not be expected to substantially affect results of the quantitative assay. However, this effect should be kept in mind when setting lower cut-points for PSA detection when K-Y Jelly® is present in clinical specimens. Astroglide® and Silicorel lubricants did not interfere with PSA detection by the Abbott ARCHITECT quantitative assay.
Although a wide range of lubricant and semen dilutions were tested, the highest concentration of semen tested was 1:100, well below what may be expected in vaginal fluid after unprotected intercourse. It is unlikely that the presence of any of the vaginal lubricants/spermicides tested would prevent the qualitative detection of semen in samples collected shortly after unprotected intercourse. The interference detected in this study is more likely to occur when semen is present at much lower levels. This is informative for researchers who are interested in using the Abbott ARCHITECT PSA to identify very low concentrations of PSA exposure as might occur in early trials of barrier contraceptive methods in which small leaks are important to identify. In such trials, participants should not use Gynol, Replens, or any product with Carbopol or the researcher should use a different assay or marker.
Even though we did not test products alone without semen, we did test extremely low semen dilutions (as low as 1:6,553,600) mixed with product; PSA concentrations were lower than 0.4 ng/nL for all products tested at that semen concentration (results not shown), ruling out the possibility of false positive PSA results in the presence of the tested products. In vivo studies will be important to confirm the observed laboratory findings.
To our knowledge, this is the first study to report on how vaginal products affect PSA detection by the Abbott ARCHITECT total PSA assay. It is important to note that vaginal products may affect different assays differently. We have previously reported on the effects of K-Y Jelly®, Replens®, Gynol® and Astroglide® on semi-quantitative PSA detection by ABAcards [10]. All of these products affected detection of PSA and the performance of the ABAcard’s assay at some of the dilutions tested in that study [10]. Taken together, our findings suggest that the Abbott ARCHITECT’s quantitative total PSA assay, rather than the ABAcard’s assay, would be preferred when certain products such as Astroglide® or Silicorel may have been used [10].
Additional lubricants and microbicides, as well as alternative PSA detection assays need to be further evaluated. More studies are also needed for the clinical correlation and evaluation of the inhibitory effects of Replens® and Gynol® that were observed in this study. While we did our best to approximate the amount of product that would be potentially present intravaginally, based on typical use and/or the manufacturer’s directions for use of the products, this remains an estimate. Other factors such as vaginal inflammation, hormonal contraception use or phase of menstrual cycle may also affect some of the local environment and modify test results. Nevertheless, our in vitro study provides reassurance that several commonly used vaginal products, such as Silicorel, Astroglide®, and low concentrations of Gynol® do not affect the quantitative PSA detection by the Abbott ARCHITECT. Higher concentrations of Gynol® and all concentrations of Replens (including its active ingredient carbopol) do inhibit the PSA detection by the Abbott ARCHITECT’s quantitative Total PSA assay, and K-Y Jelly® inflated it. Particular products appear to affect specific PSA assays differently, which is important information that should be taken into account when planning reproductive health studies that will use PSA detection as a biomarker of semen exposure.
Acknowledgments
The authors would like to thank the CONRAD Clinical Working Group on Evaluation of Markers of Intercourse in Trials of Vaginal Barriers for helpful discussions. The authors also acknowledge Clair Kiernan, Laurie Howard Jones, and Edmund Gumisiriza for their expert technical assistance.
Footnotes
Conflicts of Interest and Source of Funding: For all authors none were declared.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References
- 1.Bahamondes L, Diaz J, Marchi NM, Castro S, Villarroel M, Macaluso M. Prostate-specific antigen in vaginal fluid after exposure to known amounts of semen and after condom use: comparison of self-collected and nurse collected samples. Hum Reprod. 2008;23(11):2444–51. doi: 10.1093/humrep/den283. [DOI] [PubMed] [Google Scholar]
- 2.Mauck CK, Doncel GF. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception. 2007;75(6):407–19. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz JL, Ballagh SA, Creinin MD, Rountree RW, Kilbourne-Brook M, Mauck CK, et al. SILCS diaphragm: postcoital testing of a new single-size contraceptive device. Contraception. 2008;78(3):237–44. doi: 10.1016/j.contraception.2008.04.118. [DOI] [PubMed] [Google Scholar]
- 4.Gallo MF, Steiner MJ, Hobbs MM, Warner L, Jamieson DJ, Macaluso M. Biological markers of semen exposure: tools for improving the assessment of sexual behavior in HIV/STI prevention research. AIDS & Behav. 2012 [Google Scholar]
- 5.Mauck CK, va der Straten A. Using objective markers to assess participant behavior in HIV prevention trials of vaginal microbicides. J Acquir Immune Defic Syndr. 2008;49(1):64–9. doi: 10.1097/QAI.0b013e318183a917. [DOI] [PubMed] [Google Scholar]
- 6.Sutton JG, Bosley C, Rands A. The detection by enzyme linked immunosorbent assay of P30 and 19-OH prostaglandin F1/F2, in the presence of a range of possible contaminants. Sci Justice. 1998;38(3):157–64. doi: 10.1016/S1355-0306(98)72100-8. [DOI] [PubMed] [Google Scholar]
- 7.Bitner SE. False positives observed on the Seratec(®) PSA SemiQuant Cassette Test with condom lubricants. J Forensic Sci. 2012;57(6):1545–8. doi: 10.1111/j.1556-4029.2012.02141.x. [DOI] [PubMed] [Google Scholar]
- 8.Maher J, Vintiner S, Elliot D, Melia L. Evaluation of the BioSign PSA membrane test for the identification of semen stains in forensic casework. N Z Med J. 2002;115(1147):48–9. [PubMed] [Google Scholar]
- 9.Pang BC, Cheung BK. Identification of human semenogelin in membrane strip test as an alternative method for the detection of semen. Forensic Sci Int. 2007;169(1):27–31. doi: 10.1016/j.forsciint.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Snead MC, Kourtis AP, Black CM, Mauck CK, et al. Effect of topical vaginal products on the detection of prostate-specific antigen, a biomarker of semen exposure, using ABAcards. Contraception. doi: 10.1016/j.contraception.2012.10.034. Available online 4 December 2012. ( http://www.sciencedirect.com/science/article/pii/S0010782412009663) [DOI] [PMC free article] [PubMed]
- 11.Graves H, Sensabaugh G, Blake E. Postcoital detection of a male-specific semen protein. Application to the investigation of rape. N Engl J Med. 1985;312(6):338–43. doi: 10.1056/NEJM198502073120603. [DOI] [PubMed] [Google Scholar]
- 12.Laffan A, Sawyer I, Quinones I, Daniel B. Evaluation of semen presumptive tests for use at crime scenes. Med Sci Law. 2011;51(1):11–7. doi: 10.1258/msl.2010.010040. [DOI] [PubMed] [Google Scholar]
- 13.Abbott Diagnostics, Abbott Park, IL. ARCHITECT i2000SR. [cited 2012 April 27]; Available from: http://www.abbottdiagnostics.com/Products/Instruments_by_Platform/default.cfm?system=ARCHITECT&suffix=i2000sr®=us.
- 14.Walsh T, Warner L, Macaluso M, Frezieres R, Snead MC, Wraxall B. Prostate-specific antigen as a biomarker of condom failure: comparison of three laboratory assays and self-reported condom use problems in a randomized trial of female condom performance. Contraception. 2012;86(1):55–61. doi: 10.1016/j.contraception.2011.10.018. Epub March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]