Abstract
Purpose
Screening is a major contributor to colorectal cancer (CRC) mortality reductions in the U.S., but is underutilized. We estimated the fraction of CRC deaths attributable to nonuse of screening to demonstrate the potential benefits from targeted interventions.
Methods
The established MISCAN-colon microsimulation model was used to estimate the population attributable fraction (PAF) in people aged ≥50 years. The model incorporates long-term patterns and effects of screening by age and type of screening test. PAF for 2010 was estimated using currently available data on screening uptake; PAF was also projected assuming constant future screening rates to incorporate lagged effects from past increases in screening uptake. We also computed PAF using Levin's formula to gauge how this simpler approach differs from the model-based approach.
Results
There were an estimated 51,500 CRC deaths in 2010, about 63% (N∼32,200) of which were attributable to non-screening. The PAF decreases slightly to 58% in 2020. Levin's approach yielded a considerably more conservative PAF of 46% (N∼23,600) for 2010.
Conclusions
The majority of current U.S. CRC deaths are attributable to non-screening. This underscores the potential benefits of increasing screening uptake in the population. Traditional methods of estimating PAF underestimated screening effects compared with model-based approaches.
Keywords: Adenomas, Adenomatous Polyps, Colorectal Neoplasms, Epidemiology, Secondary Prevention, Screening and Early detection, Computer Simulation
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the U.S., and is estimated to cause 50,310 deaths in 2014.(1) Both the absolute number of cases as well as the incidence and mortality rates have declined over the last three decades despite a high prevalence of risk factors, in contrast to trends observed in some other countries.(2) Evidence indicates that the increasing use of CRC screening has been the major contributor to the declining incidence and mortality rates in the U.S. from this disease.(3, 4) However, screening remains underutilized, suggesting that a substantial proportion of current CRC deaths in the U.S. are avoidable. This has galvanized public action on increasing the uptake of screening;(5) however, lack of clarity persists regarding the proportion of current CRC deaths occurring as a result of nonuse of screening, and thus the potential public health benefits from increasing screening uptake.
The population attributable fraction (PAF) proposed by Morton Levin in 1953 has been widely used to assess the proportion of a disease outcome that occurs as a result of exposure to a risk factor, and thus the potential benefits from public health interventions to eliminate that exposure.(6) This concept, which is a function of the level of exposure to the risk factor and the size of the effect of exposure on the disease outcome, has been previously applied to assess the impact of underutilization of CRC screening on disease mortality.(7) Using this approach, Stock and colleagues reported that about 28 – 44% of deaths from CRC in the U.S. in 2005 may be attributable to nonuse of colonoscopy. However, this study used somewhat conservative estimates for the effect of colonoscopy screening that may not be applicable for the U.S.(8-10) Also, the study did not consider specific features of CRC epidemiology that are important for valid estimation of PAF. First, apart from colonoscopy, flexible sigmoidoscopy or fecal occult blood tests are also used for screening in the U.S., and therefore need to be considered in estimating PAF. Second, CRC is a heterogeneous disease characterized by a long latency between risk factor exposure and outcome. Mortality benefits from screening are derived not only from cancer detection, but also from the detection and treatment of precursor or early more curable invasive lesions. Thus, valid estimates of PAF require the consideration of benefits of screening that are realized over long time periods after the test date. Finally, patterns of exposure to CRC screening have evolved since the 1980s. According to data from the National Health Interview Survey (NHIS), the proportion of the U.S. population recently exposed to CRC screening tests increased from about 39% in 2000 to 58% in 2010.(11, 12)
In the present study, we used microsimulation modeling to estimate the PAF of U.S. CRC deaths from non-screening. We compared these PAFs with an estimate of PAF using Levin's formula to gauge how this simpler more accessible approach may differ from the microsimulation approach.
Methods
Population attributable fraction
The population attributable fraction (PAF) for CRC is defined as the proportion of CRC deaths in adults who are age 50 years or older that is due to non-receipt of screening as recommended by national guidelines. Analogous to the first definition discussed by Rockhill and colleagues, a short treatise on the most common definitions used for PAF, this is expressed algebraically as:(13)
| (1) |
where RT is the observed CRC mortality risk within the population per year, R0 is the risk in those screened (unexposed) per year, and RRT/0 is the ratio. We used a Microsimulation Screening Analysis (MISCAN) model to generate the entries RT and R0 in definition (1). To compare the model approach and simple approach, the risk in the absence of screening, R1, was also assessed. Since the use of screening, disease incidence and mortality, and risk of death from competing causes change over a person's lifetime, we derived PAF according to three age strata (age 50 – 64, 65 – 74, 75 and older). It was first derived for calendar year 2010 based on observed patterns of exposure to non-screening from national survey data up to 2010, and then extended to 2030, assuming a constant rate of exposure to screening after 2010 to explore the lagged effects from recent increases in screening uptake. See the supplementary appendix for more precise definitions of PAF according to stratum and calendar year.
This study was conducted within the National Cancer Institute's (NCI) Cancer Research Network (CRN) and as part of the NCI-funded Population-Based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium. The aim of PROSPR is to conduct multi-site, coordinated, trans-disciplinary research to evaluate and improve cancer screening processes.
MISCAN-colon microsimulation population
The MISCAN-colon microsimulation model was used to stochastically generate a virtual population similar to the U.S. population in terms of the life expectancy and the natural history and occurrence of CRC. This model was defined for the 1980 – 2030 period, to cover both historical and possible future patterns of screening use and the corresponding CRC mortality effects. U.S. birth and all-cause mortality for the model were based on U.S. Census Bureau population estimates from 2000(14) and generational U.S. Berkeley Mortality tables,(15) respectively. Cancers were assumed to develop along the adenoma-carcinoma sequence, i.e. originate from small adenomatous lesions (≤5mm) which first slowly grow to become medium (6-9mm in diameter) or large adenomas (≥10mm) before turning malignant.(16) The size-specific prevalence of adenomas by age was based on autopsy and colonoscopy data from before the era of CRC screening.(17-20) The stage- and location-specific incidences of CRC by age were based on SEER program data from the pre-screening era.(21) The model was developed by the Department of Public Health at the Erasmus Medical Center in Rotterdam, the Netherlands, as part of the NCI-funded Cancer Intervention and Surveillance Modeling Network (CISNET), and has been described more extensively elsewhere.(22, 23)
Exposure to non-screening
To derive PAF, we simulated two scenarios on the uptake of screening in the U.S. First, we closely replicated age- and test-specific screening patterns for the U.S. as observed in 8 waves of NHIS from 1987 – 2010 (Figure 1). The NHIS is a cross-sectional survey with a complex design on a nationally representative sample of the U.S. population.(24) Questions regarding the use of CRC screening tests were asked during the following survey years: 1987, 1992, 1998, 2000, 2003, 2005, 2008, and 2010. The estimated overall screening rate in 2010 (ages 50-100 years) was 59%. We assumed screening rates levelled off at ∼60% (i.e. a 40% non-screening rate) after 2010. Screening as measured in the NHIS is comprised of home-based fecal occult blood testing, and endoscopy (particularly flexible sigmoidoscopy, or optical colonoscopy).
Figure 1.
Colorectal cancer screening trends* in National Health Interview Survey (NHIS) data and MISCAN.
* The red line plots the proportion of U.S. population which had a home FOBT in the previous year, the blue and green lines plot the proportions which had an endoscopy in the previous 5 or 10 years, respectively.
In the second scenario to assess the mortality risk from CRC that persisted despite complete screening of the population, after 1980 everyone was assumed to be fully compliant with using a single test (screening colonoscopy) at ages 50, 60 and 70 in accordance with U.S. guideline recommendations.(25)
In the first scenario above patients screened with a positive fecal test or sigmoidoscopy were invited for a diagnostic colonoscopy. The assumed adherence rate was 80%. In both of the above scenarios patients in whom precancerous adenomas were detected during colonoscopy were invited for surveillance colonoscopy at 3 – 5 yearly intervals in accordance with U.S. guideline recommendations for polyp size, number and histology.(26) The adherence rate for surveillance colonoscopy was 80% and 100%, respectively, for the two scenarios.
Screening and treatment effects
The effects of screening follow from the test performance assumptions in Table 1. We defined for each test the sensitivity and specificity for adenomas and adenocarcinomas, and in the case of endoscopic procedures, the extent of the colon evaluated by the exam. For detected incident adenomas, we assumed a 100% efficacy of treatment; for detected cancers, stage-specific survival was based on SEER mortality data for people with CRC diagnosed between 2000 – 2003. A model including these test characteristics was previously validated to data from trials on the effectiveness of sigmoidoscopy(27) and of fecal occult blood tests.(28-30) The latter also included validation of the effect of colonoscopy after a positive test.
Table 1. Test performance assumptions in MISCAN.
| Performance characteristic | Colonoscopy 1 | Sigmoidoscopy 2 | FOBT 3 |
|---|---|---|---|
| Sensitivity: | |||
| - Adenomas ≤ 5 mm | 0.75 | 0.75 | - |
| - Adenomas 6 - 9 mm | 0.85 | 0.85 | 0.013 |
| - Adenomas ≥ 10 mm | 0.95 | 0.95 | 0.065 |
| - Stage I adenocarcinoma | 0.95 | 0.95 | 0.182 / 0.508 |
| - Stage II adenocarcinoma | 0.95 | 0.95 | 0.182 / 0.508 |
| - Stage III adenocarcinoma | 0.95 | 0.95 | 0.182 / 0.508 |
| - Stage IV adenocarcinoma | 0.95 | 0.95 | 0.182 / 0.508 |
| Specificity: | NA | NA | 0.02 |
| Reach endoscope: | Cecum | Splenic Flexure | NA |
| Completeness rate:4 | 0.98 | 0.80 | NA |
FOBT = Fecal Occult Blood Testing; iFOBT = immunochemical FOBT; NA = Not Applicable
Colonoscopy sensitivity for each adenoma, and completeness of colonoscopy were based on a systematic review of adenoma miss rates in tandem colonoscopy studies by Van Rijn and colleagues(42).
Sensitivity of sigmoidoscopy was also based on van Rijn and colleagues (42).
We assumed that fecal occult blood testing is more sensitive in preclinical cancers that are close time-wise to becoming symptomatic. This assumption showed good concordance with Fecal Occult Blood Test trial results (48).
This is the proportion of endoscopies visualizing the maximum point of reach of the endoscope.
Absolute CRC mortality risks
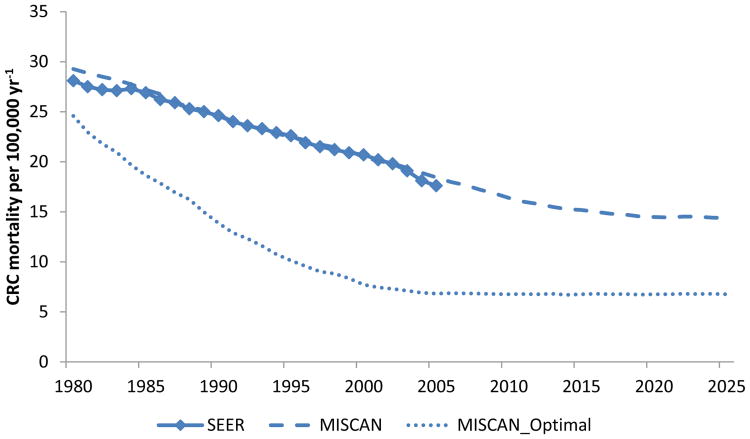
The CRC mortality risk was determined by the model assumptions for the risk of CRC, levels of screening uptake, and the effects of screening and treatment. The 2010 (baseline) mortality rate over all ages was aligned with the SEER mortality database by scaling the CRC incidence rate in the model (Figure 2). (31) Absolute mortality numbers for 2010 were derived by multiplying the mortality rates with 2010 population estimates from the U.S. Census bureau.(32)
Figure 2. U.S. age-standardized* colorectal cancer (CRC) mortality rates by calendar year in Surveillance Epidemiology and End Results program (SEER) data and MISCAN.
* Adjusted to the total 2000 U.S. standard population
Alternative approach to assess PAF
We also derived PAF using the formula as proposed by Morton Levin in 1953, to help gauge the difference of this simpler more accessible approach with the model-based estimate.(6) Similar to the second definition in Rockhill and colleagues, this can be expressed algebraically as:(13)
| (2) |
Here P1 is the population proportion exposed to nonuse of screening, and the RR1/0 is the ratio of the CRC mortality risks or rates in the non-screened versus the adequately screened population. This approximation is based on the assumption that the risk in the total population can be derived by linear interpolation of the risks in the non- and adequately screened groups (RT∼P1 R1 + (1 – P1) R0), which is valid only under stringent conditions such as no confounding.(13) In this study, the parameters for equation (2) were derived from the same NHIS data used to inform the model on screening uptake in the U.S., and a large prospective cohort study for the effect of colonoscopy use.(33) Since the formula allows for a single parameter on screening uptake, we used the most recent (2010) NHIS wave to estimate the proportion exposed to non-screening (Table S1). As risk ratio we used the age-adjusted hazard rate for colonoscopy use of 0.32 (95% CI: [0.24, 0.45]) derived by Nishihara and colleagues.(33) Again, more precise definitions according to age stratum and calendar year are provided in the supplementary appendix.
Results
In 2010, the overall estimated number of CRC deaths in the U.S. was 51,500 (Table 2a). From this total, an estimated 12,700 occurred within the age stratum 50 – 64, 12,300 occurred within the age stratum 65 – 74, and 26,500 occurred within age stratum 75 and older.
Table 2a. U.S. colorectal cancer deaths in 2010 attributable to nonuse of screening according to MISCAN.
| Variable | Population subgroup by age | |||
|---|---|---|---|---|
|
|
||||
| 50-64 | 65-74 | 75-100 | All | |
| Total population (million) 1 | 59.1 | 21.9 | 18.6 | 99.6 |
| Estimated number of CRC deaths without screening (MISCAN) | 19,800 | 23,000 | 51,800 | 93,400 |
| Actual number of CRC deaths in the population2 | 12,700 | 12,300 | 26,500 | 51,500 |
| Estimated number of CRC deaths with full uptake of screening (MISCAN)3 | 7,100 | 4,200 | 7,300 | 19,300 |
| CRC deaths prevented by current screening (deaths if theoretical no screening – actual deaths) | 7,100 | 10,800 | 25,200 | 41,900 |
| CRC deaths attributable to residual non-screening (actual deaths – deaths if 100% screening) | 5,600 | 8,000 | 19,200 | 32,200 |
| Attributable fractions: | ||||
| Fraction of CRC deaths attributable to non-screening if theoretical no screening, % | 64% | 82% | 86% | 79%4 |
| Fraction of actual CRC deaths attributable to non-screening, % | 44% | 65% | 72% | 63% |
Abbreviations: CRC = Colorectal cancer
Population estimates were based on U.S. Census Bureau population estimates(32). The overall population size in MISCAN was scaled to this number.
CRC mortality numbers were derived by multiplying CRC mortality rates from 2010 SEER data with the population estimates from the U.S. Census Bureau(31, 32).
This was defined as having screening colonoscopy at ages 50, 60 and 70 and lifetime surveillance follow-up of patients with adenomas detected in screening
Thus, the estimated overall relative risk for colonoscopy screening according to guideline recommendations was 0.21.
In an ideal scenario of 100% uptake of screening (i.e. 100% uptake of 10-yearly colonoscopy screening), the microsimulation model estimated the expected number of CRC deaths to be 19,300 (Table 2a). This means that 32,200 CRC deaths out of the actual total of 51,500 in 2010 were attributable to nonuse of screening, which equates to a PAF of 63%. In analyses stratified according to age, the PAF was 44% for persons 50 – 64 years of age, but was 65% for those aged 65-74. On the assumption that screening rates remained at the 2010 level of ∼60% into future years, the fraction of CRC deaths attributable to nonuse of screening decreased slightly over time to 58% in 2020 (Figure 4).
Figure 4. Projected CRC mortality fractions attributable to nonuse of screening *.
* The mortality rates were not standardized for age; future estimates were based on a scenario of constant screen rates of ∼60% after 2010
Levin's formula approach to estimate PAF yielded more conservative estimates of the fraction of CRC deaths attributable to underuse of screening. With this formula, 23,600 CRC deaths out of 51,500 in 2010 were attributable to underuse of CRC screening for a PAF of 46% (Table 2b, Figure 3). For the 50-64 year-old age group, the PAF was 49%, whereas for those 65-74 years old, the PAF was 41%, which was substantially lower than the result of the microsimulation approach.
Table 2b. U.S. colorectal cancer deaths in 2010 attributable to nonuse of screening according to Levin's formula.
| Variable | Population subgroup by age | ||||
|---|---|---|---|---|---|
|
|
|||||
| 50-64 | 65-74 | 75-100 | All | ||
| Total population (million) 1 | 59.1 | 21.9 | 18.6 | 99.6 | |
| Estimated number of CRC deaths without screening (not assessed) | |||||
| Actual number of CRC deaths in the population2 | 12,700 | 12,300 | 26,500 | 51,500 | |
| Estimated number of CRC deaths with full uptake of screening (Levin) | 6,400 | 7,300 | 14,100 | 27,900 | |
| CRC deaths prevented by current screening (deaths if theoretical no screening – actual deaths) | |||||
| CRC deaths attributable to residual non-screening (actual deaths – deaths if 100% screening) | 6,200 | 5,000 | 12,400 | 23,600 | |
| Attributable fraction: | |||||
| Fraction of CRC deaths attributable to non-screening if theoretical no screening, %3 | 68% | 68% | 68% | 68% | |
| Fraction of actual CRC deaths attributable to non-screening, % [Min,Max] 4 | 49% [36%, 59%] | 41% [28%, 50%] | 47% [34%, 57%] | 46% [33%, 56%] | |
Population estimates were based on U.S. Census Bureau population estimates(32). The overall population size in MISCAN was scaled to this number.
CRC mortality numbers were derived by multiplying CRC mortality rates from 2010 SEER data with the population estimates from the U.S. Census Bureau(31, 32). Likewise, numbers corresponding with the attributable fraction of CRC mortality were derived by multiplying the estimated PAF based on relative mortality rates with the observed number of deaths.
Based on the age-adjusted hazard rate for colonoscopy use derived by Nishihara and colleagues (33)
The minimum to maximum range was based on using respectively the 95% upper and lower confidence bound for the efficacy of screening reported by Nishihara and colleagues(33).
Figure 3. Proportion of U.S. colorectal cancer deaths in 2010 attributable to nonuse of screening by two approaches.
Discussion
In the present study we used a Microsimulation Screening Analysis (MISCAN) model to assess the fraction of colorectal cancer (CRC) deaths in the U.S. population among people aged 50 or older that is attributable to nonuse of screening as recommended by U.S. national guidelines. Of the estimated 51,500 CRC deaths in 2010 in the U.S., about 63% (N∼32,200) were attributable to non-screening. Under a scenario in which the screening rates attained in 2010 remained unchanged until 2030, the future population attributable fraction (PAF) attributable to nonuse of screening decreased to about 58% by 2020 due to the long-term cancer-preventive effects of adenoma removal after recent increases in screening uptake. Compared with the model-based approach, the traditional approach using the formula proposed by Levin, which utilizes static measures of screening and risk, resulted in a more conservative estimate of 46% (N∼23,600) of CRC deaths in 2010 that were attributable to non-screening.
The PAF is an informative concept in providing public health policy makers with a ceiling for potential risk reductions achievable through interventions targeting the elimination of risk factors.(13) In this study we found that considerable reductions in CRC mortality of up to 63% are possible if the screening uptake in the U.S. is maximized (100% uptake). Unfortunately, the likelihood of this outcome occurring in the foreseeable future seems small. Healthcare accessibility is still a serious problem for roughly one quarter of the U.S. population,(34) and screening is underutilized particularly by populations with lower socioeconomic status, including the uninsured.(35) Even if recent health insurance reforms fulfill their promise of minimizing financial barriers to access for underserved populations,(36) the question remains whether uptake will get beyond a level of 80%. Integrated health care delivery systems have been successful in achieving compliance rates of around 80%, such as in the Kaiser Permanente Northern and Southern California member populations,(37) where all eligible adults not up-to-date with screening by endoscopic methods receive a fecal hemoglobin test over the mail (out-reach), and are reminded during primary care visits (in-reach).(38) This 80% screening rate has also been declared a national goal for the U.S. by 2018;(39) the observation that it has already been achieved in some large populations suggest this goal is, at the least, feasible. Assuming linearity in the effects of screening to screening uptake (the basic assumption behind the formula by Morton Levin(6)), our results indicate that a reduction of ∼30% (half of the effect when attaining full compliance) in CRC mortality might be expected if the US succeeds in attaining the 80% screening rate.
The model-based approach resulted in a substantially higher PAF than the traditional approach using Levin's formula. This stemmed from a number of factors. First, the model incorporated long-term patterns and effects of screening in the population, while the simpler approach used a static measure for the proportion exposure to nonuse of screening. Thus the traditional approach, for example, could not incorporate in its calculation of CRC mortality for 2010, the benefits from cancer prevention from the removal of adenomas provided by screening exams received many years earlier. Given the steep increase of screening rates in recent years, this may have contributed to the underestimation of the 2010 PAF with this method. The model suggests that this underestimation may have accounted for about one-quarter to one-third of the difference between the approaches, given the narrower gap between the two approaches for 2020.
The remainder of the difference in PAF between model-based and simple approach was attributable to different assumptions for the efficacy of screening. First, while in MISCAN the risk ratio over all ages corresponding with the use of colonoscopy screening according to guidelines was approximately 0.21 (Table 2a), a ratio of 0.32 for colonoscopy use in general was used in the simple approach. This larger risk reduction for screening leveraged the model-based PAF. Assuming a lower risk ratio of 0.21 the simple approach would have generated a PAF closer to the model estimate. Further, while the model allowed for disparities in effects of screening according to age and current versus optimal screening practice, a uniform risk ratio was applied in the simple approach. Because the model settings induced stronger effects of screening in the older age strata, the PAF difference was most pronounced in higher ages. The simpler (non-model based) approach in this study did lead to an overall PAF similar to the 44% found for 2005 in the previous study by Stock and colleagues,(7) who also utilized the simpler traditional approach to derive PAF.
We used NHIS data to inform this study on the current and past exposure to screening in the U.S., which is subject to potential biases. First, NHIS is a cross-sectional survey with repeated measurements over time. With changes in the items used on the survey to reflect changes in screening patterns and potential interference from re-sampling, estimates cannot be directly compared across survey years. Further, NHIS relies on self-reported measures of screening use, which may have caused an overestimation of the true screen rates, particularly in some demographic groups.(40) Nevertheless, the survey provides one of the best estimates of the use of screening in the U.S.
The outcomes of the model strongly depend on the test performance assumptions for colonoscopy. There are currently no trial data available to validate the effectiveness of colonoscopy in our model.(41) Thus, we used adenoma miss rates from tandem colonoscopy studies to determine its efficacy,(42, 43) and validated these estimates indirectly to outcomes of FOBT trials including colonoscopy follow-up of positive FOBT,(28-30, 44, 45) and the UK flexible sigmoidoscopy trial.(27) In our study screening alone was considered a sufficient explanation for the decrease in CRC mortality between 1980 and 2000 and beyond (Figure 2). This may have overestimated the effects of screening; a previous microsimulation analysis suggested that treatment and risk factor developments also contributed to the decrease.(4) In a sensitivity analysis with a 50% reduced sensitivity for adenomas of ≤5 mm in diameter, the PAF for 2010 was lower than our base case estimate, but remained 52%. Under these assumptions, the age-adjusted relative risk for CRC mortality corresponding with colonoscopy screening was similar to the hazard ratio of 0.32 recently reported for a prospective cohort study with 22 years of follow-up.(33)
A limitation of using the PAF as a proxy for the potential returns of public health interventions is that it requires estimates of screening rates, an unproven constant estimate of the true magnitude of the benefit from screening and an approximation of the absolute disease risk in the population, all of which may change over time. Our estimates were based on currently available knowledge for each of these factors, but may not be applicable in future years, if more interventions to increase screening rates are implemented. We used PAF over other estimations such as the prevented fraction,(46, 47) because the PAF metric can be used to provide policymakers estimates of potential future benefits of increased screening beyond current benefits of past exposure.
To conclude, a model-based approach estimated that more than half of the current CRC mortality risk in the U.S. is attributable to nonuse of screening. This underscores the need to increase screening uptake in the U.S. population. A model-based approach provided a higher estimate of screening benefit than the traditional, simpler approach to assess PAF. Valid estimation of the effects of screening requires the consideration of variable screening patterns over time, which may require more complex models than traditionally used to assess PAF.
Supplementary Material
Acknowledgments
Financial Support: This project was supported by the United States National Cancer Institute (grant numbers # U54CA163262, U01CA151736, U01 CA152959). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
List of Abbreviations
- CRC
Colorectal Cancer
- NHIS
National Health Interview Survey
- PAF
Population Attributable Fraction
- P1
Population proportion unscreened (100% exposed to non-screening)
- R1
Annual CRC mortality risk in the unscreened
- RT
Annual CRC mortality risk in the total population, given current screening utilization
- R0
Annual CRC mortality risk in the screened (0% exposed to non-screening)
- RRT/0
Ratio of CRC mortality risks in the total population versus the screened
- RR1/0
Ratio of CRC mortality risks in the unscreened versus the screened
- SEER
Surveillance Epidemiology and End Results program
Footnotes
Conflicts of Interest: None of the authors report any conflicts of interest
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014 Mar-Apr;64(2):104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010 Aug;19(8):1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014 Jun 3; doi: 10.1002/cncr.28794. [DOI] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centres for Disease Control and Prevention. [cited 2014 May, 30];March Is National Colorectal Cancer Awareness Month. 2014 Available from: http://www.cdc.gov/cancer/dcpc/resources/features/colorectalawareness/
- 6.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9(3):531–41. [PubMed] [Google Scholar]
- 7.Stock C, Knudsen AB, Lansdorp-Vogelaar I, Haug U, Brenner H. Colorectal cancer mortality prevented by use and attributable to nonuse of colonoscopy. Gastrointest Endosc. 2011 Mar;73(3):435–43 e5. doi: 10.1016/j.gie.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Hilsden RJ. Quantifying the benefit of screening colonoscopy. Gastrointest Endosc. 2011 Mar;73(3):444–6. doi: 10.1016/j.gie.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Muller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. 1995 Sep 11;155(16):1741–8. doi: 10.1001/archinte.1995.00430160065007. [DOI] [PubMed] [Google Scholar]
- 10.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009 Jan 6;150(1):1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012 Jun;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998 Jan;88(1):15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Census Bureau. National Population Projections. 2000 Available from: http://www.census.gov/population/www/projections/downloadablefiles.html.
- 15.Berkeley Mortality Database Lifetables by year of birth 1900-2000. Available from: http://www.demog.berkeley.edu/∼bmd/states.html.
- 16.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975 Dec;36(6):2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 17.DiSario JA, Foutch PG, Mai HD, Pardy K, Manne RK. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol. 1991 Aug;86(8):941–5. [PubMed] [Google Scholar]
- 18.Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, et al. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol. 1990 Aug;85(8):969–74. [PubMed] [Google Scholar]
- 19.Koretz RL. Malignant polyps: are they sheep in wolves' clothing? Ann Intern Med. 1993 Jan 1;118(1):63–8. doi: 10.7326/0003-4819-118-1-199301010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991 Aug;86(8):946–51. [PubMed] [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2013 Sub (1973-2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
- 22.Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Model Profiles. National Cancer Institute; [cited 2013 01/13]. Available from: http://cisnet.cancer.gov/colorectal/profiles.html/ [Google Scholar]
- 23.van Hees F, Habbema JD, Meester RG, Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG. Should colorectal cancer screening be considered in elderly persons without previous screening?: a cost-effectiveness analysis. Ann Intern Med. 2014 Jun 3;160(11):750–9. doi: 10.7326/M13-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. [cited 2014 March 27];National Health Interview Survey. updated March 27, 2014. Available from: http://www.cdc.gov/nchs/nhis.htm.
- 25.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov 4;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012 Sep;143(3):844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Atkin W, Edwards R, Cuzick J. Lancet. 2010. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Online. [DOI] [PubMed] [Google Scholar]
- 28.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996 Nov 30;348(9040):1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 29.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999 Mar 3;91(5):434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002 Jan;50(1):29–32. doi: 10.1136/gut.50.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cancer Institute. Age-Specific U.S. Death Rates, 2006-2010. [cited 2014 April 23];2013 updated April 2013. Available from: http://seer.cancer.gov/archive/csr/1975_2010/browse_csr.php.
- 32.U.S. Census Bureau. Population Estimates by age, sex, race and Hispanic origin. 2012 Available from: http://www.census.gov/popest/data/index.html.
- 33.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013 Sep 19;369(12):1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen C, Osborn R, Squires D, Doty MM. Access, affordability, and insurance complexity are often worse in the United States compared to ten other countries. Health Aff (Millwood) 2013 Dec;32(12):2205–15. doi: 10.1377/hlthaff.2013.0879. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Sussman DA, Doubeni CA, Anderson DS, Day L, Deshpande AR, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Cancer Society. [cited 2014 May, 30];Colorectal cancer screening - state and federal coverage laws. 2013 Available from: http://www.cancer.org/cancer/colonandrectumcancer/moreinformation/colonandrectumcancerearlydetection/colorectal-cancer-early-detection-screening-coverage-laws.
- 37.Lee JK, Levin TR, Corley DA. The road ahead: what if gastroenterologists were accountable for preventing colorectal cancer? Clin Gastroenterol Hepatol. 2013 Mar;11(3):204–7. doi: 10.1016/j.cgh.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011 Jul;33(1):101–10. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 39.American Cancer Society. The National Colorectal Cancer Roundtable. 2014 [updated July, 2013]; Available from: http://www.cancer.org/healthy/informationforhealthcareprofessionals/colonmdclinicansinformationsource/nationalcolorectalcancerroundtable/national-colorectal-cancer-roundtable.
- 40.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008 Apr;17(4):748–57. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 41.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012 Feb 23;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 42.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006 Feb;101(2):343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 43.Chung SJ, Kim D, Song JH, Kang HY, Chung GE, Choi J, et al. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut. 2014 May;63(5):785–91. doi: 10.1136/gutjnl-2013-304578. [DOI] [PubMed] [Google Scholar]
- 44.Winawer SJ, Zauber AG, O'Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993 Apr 1;328(13):901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 45.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993 Dec 30;329(27):1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 46.Gargiullo PM, Rothenberg RB, Wilson HG. Confidence intervals, hypothesis tests, and sample sizes for the prevented fraction in cross-sectional studies. Stat Med. 1995 Jan 15;14(1):51–72. doi: 10.1002/sim.4780140107. [DOI] [PubMed] [Google Scholar]
- 47.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974 May;99(5):325–32. doi: 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- 48.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, Zauber A, Habbema JD. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009 Jun 1;115(11):2410–9. doi: 10.1002/cncr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.