Abstract
Chimeric antigen receptor (CAR) T-cells have shown remarkable results in patients with B-cell leukemia and lymphoma. However, while CAR T-cells have shown complete responses in a majority of patients with acute lymphoblastic leukemia (ALL), lymphomas are more difficult to treat. Different CAR designs and conditioning protocols seem to affect the persistence of patient responses. However, factors that determine if patients receiving the same CARs will respond or not remain obscure. In Sweden, a phase I/IIa trial using third-generation CAR T-cells is ongoing in which we intend to compare tumor biology and immunology, in each patient, to treatment response. CAR T-cell therapy is a powerful tool to add to the treatment options for this patient group but we need to perform the necessary basic research on the multifactorial mechanisms of action to give patients the best possible option of survival. Such studies are also crucial to expand the success of CAR T-cells beyond CD19+ B-cell malignancy. This review will focus on possible barriers of treating lymphoma to define factors that need to be investigated to develop the next generation of CAR T-cell therapy.
Introduction
Chimeric antigen receptor (CAR) T-cells are T-cells genetically engineered to express a tumor-targeting receptor. The receptor is a chimera of a signaling domain of the T-cell receptor (TcR) complex and an antigen-recognizing domain, such as a single chain fragment (scFv) of an antibody.1 Hence, independently of the native TcR, CAR T-cells can recognize tumor cells via the CAR receptor. In contrast to TcR-mediated recognition of target cells via protein peptides displayed on major histocompatibility complex (MHC) molecules, the CAR is not dependent on MHC. The CAR molecule will recognize any target on the tumor cell surface and it is not limited to be a protein since antibodies can bind also carbohydrates and lipids. As for all targeted cancer therapeutics, the target needs to be specific for the cancer cells to avoid damage of healthy tissues. In many ways B-cell malignancy is the ideal indication for targeted therapy such as CAR T-cell therapy. B-cells are easily targeted via specific and selective markers such as CD19, CD20, and the Ig kappa or light chains. Considering that persisting problems with infectious disease because of B-cell deficiency can be handled with immunoglobulin replacement therapy, eradication also of the healthy B-cell population along with the malignant B-cells is manageable. Moreover, new B-cells will develop from the hematopoietic stem cells since these cells lack aforementioned B-cell markers and are, hence, not killed by CAR T-cells.
B-cell malignancy is a heterogeneous indication with both solid lesions and circulating cells in blood and bone marrow. Treatment of B-cell malignancy using CAR T-cells presents a unique opportunity to learn mechanisms of action of different CAR designs, to define on and off target toxicity, as well as to understand the limitations of CAR T-cells in terms of sensitivity to immune escape mechanisms and physical barriers of solid tumors.
B-cell Malignancy
B-cell malignancy encompasses a heterogeneous group of cancers derived from B-cells of different differentiation stages. For example, pre-B acute lymphoblastic leukemia (pre-B-ALL) derives from progenitor cells at the pre-B-cell developmental phase in the bone marrow, while diffuse large B-cell lymphoma (DLBCL) derives from B-cells present in the germinal centers of lymphoid tissues.2 Further, chronic lymphocytic leukemia (CLL) has a mature B-cell phenotype and tumor cells are present in blood, bone marrow, and lymphoid tissues. Nevertheless, they all have in common that they are derived from B-cells and share a few common B-cell linage markers that can be used for targeted therapy. For example, CD20 is expressed on mature B-cells and the CD20-targeting antibody rituximab is currently used together with chemotherapy regimens for CD20+ malignancies. Another linage marker on B-cells is CD19. CD19 is expressed already from the progenitor B-cells to mature B-cells, and to some extent on healthy, but unfortunately not on malignant, plasma cells. Clinical trials using CD19-targeting CAR T-cells have demonstrated remarkable results, mostly in ALL patients but lately also in lymphomas.3–5 Another B-cell target is the membrane-bound antibody, and CAR T-cells are being developed that target either the Ig kappa or the lambda chain.6
B-cell leukemia and lymphoma respond differently to treatment.7 ALL has rapid progression and can be cured by chemotherapy but patients that relapse or are refractory to chemotherapy have dismal prognosis. For refractory ALL, allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative option, but relapse after HSCT has so far been uncurable.8 CLL is a slowly progressing chronic disease with varying clinical course and varying response to chemotherapy. For patients with refractory CLL, there are now a new set of signaling inhibitors that target the PI3Kδ and the Bruton's tyrosine kinase (BTK) that inhibits the B-cell receptor-driven proliferation in CLL.9 DLBCL is an aggressive lymphoma and is initially treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). DLBCL commonly responds well, and about 60% of the patients can be cured with R-CHOP. Relapsing patients show increased resistance but may still respond to high-dose chemotherapy and autologous HSCT.10
Immunotherapy has shown great effect in cancer patients and for B-cell malignancy, and genetically engineered T-cells expressing a CD19-targeting CAR receptor have shown spectacular results during the past few years.3–5 T-cells are excellent serial killers that under the right conditions can expand, survive, and kill tumor cells. Furthermore, they can maintain responses if they survive in vivo as effector memory cells in contrast to, for example, antibody-based targeted therapies such as rituximab that do not induce tumor immunity. Further, one single antibody will only have the potential to bind to one tumor cell and induce antibody-mediated cytotoxicity to kill the tumor cell, while CAR T-cells proliferate in vivo and will go from tumor to tumor with sustained cytotoxic activity. Nevertheless, some trials have shown complete responses and other transient partial responses.11,12 There are many discrepancies that make it difficult to directly compare the published results such as the use of CAR T-cells with different designs, various preconditioning strategies, and the selection of patients (e.g., leukemia versus lymphoma).
Car T-cell Design
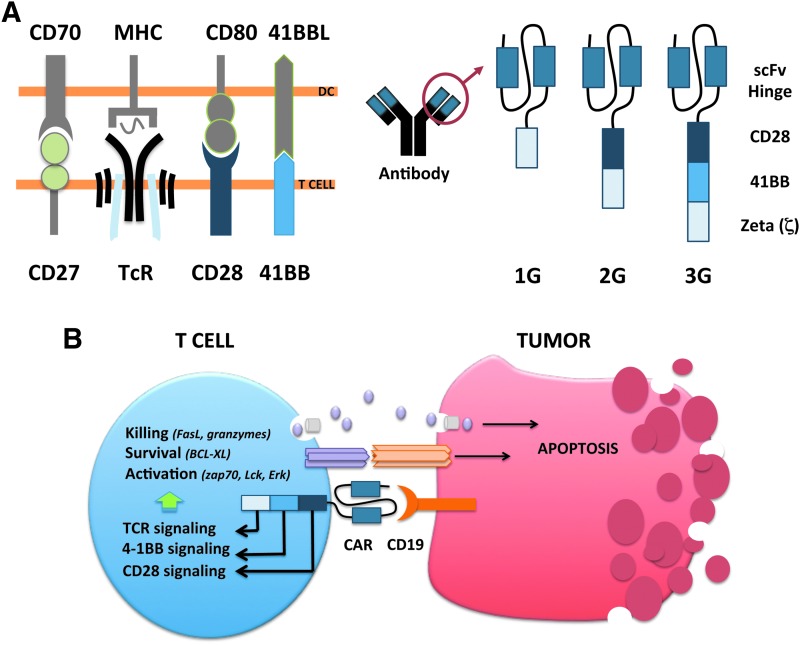
The CAR molecule consists of an antigen-recognizing extracellular domain and an intracellular signaling domain. The extracellular portion is typically an antibody single-chain fragment (scFv) directed against a cell surface antigen, while the intracellular domain consists of merged signaling domains from the TcR complex and costimulatory proteins (Fig. 1). T-cell activation is controlled by multiple signaling cascades induced by antigen-presenting cells. For full activation, T-cells need signals from antigen recognition through TcR stimulation in combination with costimulation via a range of proteins such as CD28, CD27, and 4-1BB. The first-generation CAR T-cells mimicked only TcR stimulation by combining a tumor-targeting scFv to the zeta (ζ) chain of the TcR CD3 complex, which allows T-cells to recognize and kill tumor cells in vitro but in vivo persistence was lacking.1 The second-generation CAR includes a signaling domain from a costimulatory molecule. Costimulatory signaling provides the T-cell with, for example, a better proliferative capacity or increased cytokine production depending on which costimulator that is fused to the CAR. Second-generation CAR T-cells with CD28 as a costimulator were less sensitive to Tregs and their suppressive molecules IL10 and TGFβ.13 Such CARs have shown effective in ALL but in vivo persistence can be improved for lymphoma.5,13 The CAR T-cells developed by the University of Pennsylvania included the 4-1BB molecule as a costimulator instead of CD28. This has shown to give an important survival and expansion signal to the CAR T-cells and may explain the persistent responses in their patients.3 In subsequent trials, initial complete responses were seen in about 90% of the ALL patients and the majority had sustained responses.11 CAR T-cells have also shown to be effective in different lymphomas but only if the patients are treated with aggressive doses of chemotherapy aiming to reduce regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs) before infusion. These are immunosuppressive cells that otherwise hamper the function of CAR T-cells. In a study performed by NIH using CD28-containing second-generation CAR T-cells, it was recently shown that the high-dose conditioning required for complete responses resulted in serious toxicity of the lymphoma patients.5 It is not understood why ALL patients respond much better than lymphoma patients and if other types of CAR T-cell designs may be more favorable for lymphomas.
Figure 1.
(A) T-cells interact with antigen-presenting cells such as dendritic cells (DCs) to become activated. The first signal of activation is transmitted via the T-cell receptor (TcR) that binds to major histocompatibility complex (MHC) molecules presenting antigen peptides to the T-cell. The second signal is delivered in terms of multiple interactions with co-stimulatory molecules such as CD80, 41BBL, and CD70 presented to the T-cell by mature DCs. A chimeric antigen receptor (CAR) receptor consists of an antigen-binding region such as a single chain fragment (scFv) from a tumor-targeting antibody and an intracellular signaling region. The signaling region of the first-generation (1G) CAR mimicked TcR signaling via fusing the antigen-binding region to the CD3-ζ chain. The second-generation (2G) CAR mimicked both TcR and costimulatory signaling by adding, for example, CD28 or 41BB domains to the intracellular region, while the third-generation (3G) CAR has two costimulatory domains fused with the TcR CD3-ζ chain. (B) The CAR gene is inserted to T-cells and expressed to produce protein CAR, which is transported to the plasma membrane. A CD19-targeting CAR interacts with CD19+ malignant B-cells to receive activation signaling leading to FasL and perforin/granzyme B-mediated cytotoxicity.
Experimental studies have shown that T-cells stimulated via both CD28 and 4-1BB have greater antitumor activity and longer in vivo persistence than T-cells stimulated by either moiety alone, suggesting that these two molecules have synergistic effects.14,15 At Uppsala University in Sweden we are currently investigating the clinical capacity of such third-generation CAR T-cells (Fig. 1). We have seen responses in both ALL and lymphoma but the latter is more resistant to therapy, and we are currently investigating biopsies from lymphoma lesions to elucidate mechanisms of resistance that can aid future CAR design or define necessary combination drugs. There are three reasonable explanations to why CAR T-cell therapy is not as effective for lymphoma as for leukemia that need further investigation. First, lymphomas are solid tumors that can provide physical barriers for the CAR T-cell to come in close contact with the tumor cells. Second, regulatory immune cells and inhibitory proteins are concentrated in the vicinity of the tumor. Hence, a solid tumor may have a higher level of immunosuppression at the tumor site to shield the tumor from CAR T-cells. Third, CAR T-cells may lack homing receptors to enter solid tumors. Activated effector T-cells are prone to migrate to the circulation rather than returning to lymphoid tissues, which may further complicate clearance of tumor cells present in lymph nodes.
Physical Barriers of Car T-cell Infiltration
It is known that T-cells are present in most tumors and that the number of tumor-infiltrating T-cells is correlated to a positive overall survival.16 However, when investigating the localization of the T-cells within a tumor lesion, it is evident that most T-cells remain in the tumor stroma and only few cells, or none, have infiltrated into the parenchyma. Several factors may be restricting infiltration into the parenchyma. For example, blood vessels are dysfunctional in tumor lesions and may not express the necessary receptors for T-cell attachment, rolling, and diapedesis such as ICAM-1, VCAM-1, and P/E-selectins. It may also be the T-cells that lack their counterpart receptors such as the integrins α4β1 (VLA-4) and LFA-1, but also PSGL-1 and CD43 to migrate into the parenchyma.17 Endothelial cells can be activated via CD40 to upregulate receptors for attachment, which may aid T-cell transmigration.18 A combination of CD40-targeted therapies may therefore be of interest for CAR T-cell therapy of lymphoma. Moreover, CD40 stimulation on dendritic cells (DCs) provides Th1-mediated immunity, which would further complement CAR T-cell therapy.19 In fact, Curran et al. recently published CAR T-cells that express recombinant CD40 ligand (CD40L) aiming to support the T-cell survival in the tumor milieu.20
Expression of CD40L may also enhance T-cell infiltration into the tumor if it activates the endothelial cells. However, in a mouse model, constitutive CD40L expression in lymphoid cells led to a lymphoproliferative disorder and the in vivo use of CAR T-cells expressing CD40L needs to be evaluated with care.21 CAR T-cells may also be engineered to express chemokine receptors to facilitate homing to the tumor. For example, the tumor often releases CCL2 and CXCL5, which aid recruitment of macrophages, MDSCs, and neutrophils to the tumor.22 By overexpressing the CCR2 or CCR4 on CAR T-cells, they may better home to tumor lesions. Nevertheless, lymphoma situated in lymph nodes may be targeted by other means. Naïve T-cells in blood have access to lymph nodes via specialized high endothelial venules (HEVs) but for effector cells this process is far less efficient. Instead, effector and memory cells enter lymph nodes via the lymphatics.23 T-cells enter the lymphatics via HEVs and binding to selectins is crucial. The chemokine CCR7 is important to home T-cells to the lymph nodes and CCR7 is expressed on naïve and memory T-cell populations, while effector cells usually lack CCR7.24 Hence, protocols that expand CAR T-cells with an effector/memory phenotype with CCR7 expression will preserve their capacity to migrate into lymph nodes.25 Nevertheless, the T-cells can lose CCR7 expression in vivo and CCR7 expressed in trans with CAR may be needed to maintain access to lymph nodes.
Tumor cells and tumor stroma, such as M2 macrophages, often express VEGF to stimulate angiogenesis26 and this holds true also for lymphoma.27 It has been demonstrated that VEGF prevents T-cell infiltration into tumors and VEGF blockade by the tyrosine kinase inhibitor (TKI) sunitinib upregulated chemokines, which was followed by an increased T-cell infiltration.28 Sunitinib is also known to inhibit MDSCs in renal cell carcinoma,29 which would further potentiate CAR T-cell therapy if used in combination settings. However, the effect of sunitinib on T-cells needs further analysis since many signaling pathways downstream of the TcR complex and costimulatory molecules may depend on tyrosine kinases.
Physical barriers could also entail the extracellular matrix in solid tumors that may impede T-cell penetration, especially in tumors with a dense nature, for example, because of collagen-producing fibroblasts.30 Interestingly, the expression levels of genes associated with remodeling of extracellular matrix and inflammatory responses were higher in DLBCL patients who were later cured by chemotherapy compared with the expression levels in nonresponders. For example, the matrix metalloproteinase-12 produced by macrophages was increased in the patients who were later cured and these patients also had a higher number of infiltrating T-cells.31 In an elegant study by Caruana et al., CAR T-cells engineered to constitutively express heparanase showed better capacity to infiltrate tumors and an improved overall survival in a mouse model. Collectively, the data demonstrated that physical barriers may be circumvented by appropriate CAR T-cell design.32
The Hostile Tumor Microenvironment
Immunosuppression as a means to escape antitumor immune responses is the most difficult obstacle for effective immunotherapy. The tumor can release inhibitory substances like TGFβ and IL10 that directly hamper T-cell proliferation and cytotoxic function. These substances can also inhibit antigen-presenting cells, leading to hampered activation of tumor-reactive T-cells. Further, TGFβ plays a crucial role to drive differentiation of naïve T-cells into Tregs and IL10 promotes differentiation of M2 macrophages. Tregs will then contribute with more TGFβ, IL10, and also other suppressive agents like IL35 and adenosine.33 M2 macrophages are anti-inflammatory, proangiogenic, and protumorigenic.
Besides the production of IL10 and TGFβ, M2 macrophages produce CCL22, which attracts CCR4+ Tregs. Further, they express PDL1 and can inhibit activated PD1+ T-cells.34 The tumor also produces prostaglandin E2, which drives expansion of MDSCs. MDSCs are immature myeloid cells at different differentiation stages and these cells also have suppressive capacity.35 They have many inhibitory mechanisms; for example, they release arginase-1 and upregulate nitric oxide synthase 2, both involved in the metabolism of L-arginine. T-cells deprived of L-arginine reduce CD3ζ and lose their proliferative capacity.36 Tregs, MDSCs, and M2 macrophages can together create a very hostile milieu for T-cells, which is concentrated to the tumor lesions but affect the whole patient.33 In CLL, immunosuppression occurs early in disease with malfunction of T-cells, natural killer (NK) cells, and monocytes.37 Lymphoma lesions are infiltrated with CD163+ M2 macrophages and their presence is associated with worse prognosis.38,39 The presence of MDSCs is correlated to a poor overall survival.40 However, the role of Tregs in B-cell malignancy is contradictive and has even been associated to a better prognosis.41
Tregs may have a dual role in tumors derived from immune cells since their main function is to suppress such cells. Hence, while suppressing antitumor immune responses, Tregs may as well suppress the tumor. In previous work, we demonstrated that Tregs in patients with B-cell leukemia or lymphoma had increased levels of Tregs and that their Tregs expressed cytolytic markers. In vitro, these Tregs could kill B-cell tumors.42 Nevertheless, the net outcome of all suppressive cells and cytokines in B-cell lymphoma is an immunosuppressive environment that will hamper T-cell efficacy. In a pilot study we have demonstrated that Tregs are elevated in CLL and DLBCL, while the pediatric ALL patients had similar Treg levels as age-matched controls.42 However, a high level of TGFβ has been associated to high-risk ALL.43 Nevertheless, these data support a mechanism that explains why this patient group seems to be the best responder to CAR T-cell therapy.
Conditioning of Patients Receiving Car T-cell Therapy
CAR T-cell therapy has limited effect if the patients do not receive preconditioning therapy. Preconditioning chemotherapy is often given to patients receiving immunotherapy to decrease Tregs and MDSCs that may otherwise hinder the intended immune activation. Further, chemotherapy-induced lymphocyte or myeloid cell depletion may induce bone marrow cytokine production that restores the immune cell populations and favors the activation of antitumor responses. The most commonly used protocol was developed at NIH when using fludarabine and cyclophosphamide before infusion of in vitro–expanded melanoma-specific T-cells.44 This protocol is also used before infusion of CAR T-cells5 but because of the toxicity, other regimens have been used as well depending on the indication.4,5 Metronomic cyclophosphamide has been given to patients undergoing immunotherapy in an attempt to control suppressive immune cells over time.45 Such supportive chemotherapy protocols may be of great value if they do not hamper the desired antitumor responses. One such supportive chemotherapy of interest may be gemcitabine. Gemcitabine is a nucleoside analog that replaces cytidine during DNA replication, which leads to growth arrest and apoptosis. Gemcitabine also targets ribonucleotide reductase, thereby blocking the function of this enzyme. Several studies have shown that patients treated with gemcitabine had significantly lower levels of the immunosuppressive molecule TGFβ, Tregs, and MDSCs but an increased number of DCs, monocytes, and activated T-cells.46,47
CLL is commonly treated with fludarabine, bendamustine, and lenalidomide.48 While both fludarabine and bendamustine are immunosuppressive per se, lenalidomide enhances the degradation of Ikaros 1 and 3. Since Ikaros 1 represses production of IL2, its inhibition will release IL2 production and enhance the function of both T and NK cells.49 Interestingly, lenalidomide inhibits Tregs.50 Hence, lenalidomide may also be of interest as combination treatment with CAR T-cells. There are also other interesting possibilities with the new generation of cancer therapeutics such as the BTK inhibitor ibrutinib. Ibrutinib inhibits ITK that drives the development of Th2-type CD4+ T-cells. By inhibiting ITK, Th1 T-cells that are essential in the antitumor responses were promoted.51 Signaling pathway inhibitors such as BTK and PI3K inhibitors as well as lenalidomide are evaluated also in DLBCL in phase II–III trials.10 Thus, combining standard-of-care treatments with CAR T-cell therapy may be an interesting and accessible option to enhance efficacy in both CLL and non-Hodgkin's lymphoma.
The capacity of CAR T-cells may be enhanced by other immunotherapies. For example, the checkpoint blockade antibodies targeting the CTLA-4 and PDL1/PD1 pathways52 may prevent CAR T-cell exhaustion in lymphoma lesions. Another proposed combination is the use of oncolytic viruses and CAR T-cell therapy.53 This is of high interest since most viruses are by nature immunostimulatory and attract T-cells to the site of infection. Oncolytic viruses conferring expression of immunostimulatory proteins to the tumor area may even further promote CAR T-cell efficacy. For example, we have shown that adenoviruses expressing CD40L in the tumor enhance Th1 immunity with infiltration of T-cells at the same time reducing Tregs and MDSCs and promoting M2 to M1 switch.54–56 In human bladder cancer patients, an adenovirus expressing CD40L induced large T-cell infiltrates in the bladder wall.57
Future Considerations
There is an immediate need to solve CAR T-cell accessibility and survival in lymphoma and more research is clearly needed concerning the homing of CAR T-cells to lymphoid tissues and enhancing their capacity to migrate in the tumor extracellular matrix. Even if high-dose chemotherapy before CAR T-cell infusion can lead to complete responses, some patients will die from such harsh preconditioning and it also limits the number of patients who can receive CAR T-cell therapy. Nevertheless, there are other agents available to target tumor-induced immune inhibition. For example, the currently used lenalidomide or signaling pathway inhibitors may be used alongside with CAR T-cells. Further, combining CAR T-cells with other immunotherapeutics such as checkpoint blockade antibodies or oncolytic viruses may increase their survival in the tumor lesions and support efficacy. The combination with other immune therapeutics is very interesting to broaden the immune activity against the tumor since both checkpoint blockade antibodies and oncolytic viruses will activate not only the CAR T-cells but also the naturally occurring tumor-recognizing T-cells. This could prevent escape mutant tumor cells that are not positive for the CAR target. In the trials using CD19-targeting T-cells, CD19-negative clones have expanded and caused progressive disease.58 There are also novel agents being developed blocking IL35 that may support CAR T-cell therapy by reducing the inhibitory effect of Tregs that may be of value in the future.59 Such treatments may as well release the ongoing immune responses and not only support the CAR T-cells.
The lessons learned from the clinical use of CD19-targeting CAR T-cells may be valuable for other indications in terms of CAR design and suitable preconditioning or supportive combination treatments as discussed in this review. Suitable targets are easier to determine for cells of hematopoietic origin, especially B-cells, but trials are ongoing to target also solid malignancies with CAR T-cells targeting Her2, GD2, IL13Ra2, and mesothelin. ROR1 is also an interesting target present both on B-cells and on cancers of epithelial origin.60 A study was performed in nonhuman primates that demonstrated a good safety profile61 and a clinical trial to evaluate efficacy in CLL patients is listed at the ClinicalTrials website but not yet recruiting patients. In light of the results of CAR T-cell treatment of lymphomas, there will likely be a greater focus on the tumor micromilieu (including endothelium, stroma, as well as immune cells) in future trials to find means to increase infiltration of T-cells into the parenchyma and to change the environment to allow T-cell survival and activation. Instead of only providing preconditioning, solid malignancies will likely benefit from supportive combination treatments that affect the tumor microenvironment for weeks or months after CAR T-cell infusion.
Acknowledgments
The authors want to acknowledge Prof. Malcolm Brenner and Prof. Gianpietro Dotti at Baylor College of Medicine, Houston, for the collaborative project using CAR T-cells in Sweden. The CAR T-cell research in Uppsala is supported by AFA Insurance AB, the Swedish Cancer Society, the Swedish Childhood Cancer Society, Lions Cancer Fund at Uppsala University Hospital, and the Swedish State Support for Clinical Research.
Author Disclosure
Prof. Loskog is the CEO and board member of Lokon Pharma AB, chairman of Vivolux AB and RePos Pharma AB, and scientific advisor at NEXTTOBE AB, and has a royalty agreement with Lokon Pharma AB and Alligator Bioscience AB. Prof. Enbland and Dr. Karlsson have no conflicts of interest.
References
- 1.Dotti G, Gottschalk S, Savoldo B, et al. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 2014;257:107–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott DW, Gascoyne RD. The tumor microenvironment in B cell lymphomas. Nat Rev Cancer 2014;14:517–534 [DOI] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;19:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectvely treated with autologous T cells expressing and anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vera J, Savoldo B, Vigouroux S, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006;108:3890–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabhan C, Rosen ST. Chronic lymphocytic leukemia: A clinical review. JAMA 2014;312:2265. [DOI] [PubMed] [Google Scholar]
- 8.Dinner S, Lee D, Liedtke M. Current therapy and novel agents for relapsed or refractory acute lymphoblastic leukemia. Leuk Lymphoma 2014;55:1715–1724 [DOI] [PubMed] [Google Scholar]
- 9.Byrd JC, Jones JJ, Woyach JA, et al. Entering the era of targeted therapy for chronic lymphocytic leukemia: Impact on the practicing clinician. J Clin Oncol 2014;32:3039–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: Optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015;125:22–32 [DOI] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loskog A, Giandomenico V, Rossig C, et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia 2006;177:353–358 [DOI] [PubMed] [Google Scholar]
- 14.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA 2009;106:3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther 2007;18:712–725 [DOI] [PubMed] [Google Scholar]
- 16.Ascierto PA, Capone M, Urba WJ, et al. The additional facet of immunoscore: Immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med 2013;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agace WW. Tissue-tropic effector T cells: Generation and targeting opportunities. Nature Rev Immunol 2006;6:682–692 [DOI] [PubMed] [Google Scholar]
- 18.Urban D, Thanabalsingam U, Stilbenz D, et al. CD40/CD40L interaction induces E-selectin dependent leukocyte adhesion to human endothelial cells and inhibits endothelial cell migration. Biochem Biophys Res Commun 2011;404:448–452 [DOI] [PubMed] [Google Scholar]
- 19.Loskog A, Ninalaga C, Tötterman TH. Dendritic cells engineered to express CD40L continously produce IL12 and resist negative signals from Tr1/Th3 dominated tumors. Cancer Immunol Immunother 2006;55:588–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran KJ, Seinstra BA, Nikhamin Y, et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther 2015;23:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown MP, Topham DJ, Sangster MY, et al. Thymic lymphoproliferative disease after successful correction of CD40 ligand deficiency by gene transfer in mice. Nat Med 1998;4:1253–1260 [DOI] [PubMed] [Google Scholar]
- 22.Chanmee T, Ontong P, Konno K, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014;6:1670–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Förster R, Braun A, Worbs T. Lymp node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol 2012;33:271–280 [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–712 [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Zhang M, Ramos CA, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 2014;123:3750–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 2015;33:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duse AO, Ceausu RA, Mezei T, et al. Expression and possible significance of vascular endothelial growth factor in non-Hodgkin lymphoma. Arch Biol Sci Belgrade 2013;65:487–491 [Google Scholar]
- 28.Huang H, Langenkamp E, Georganaki M, et al. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NFkB induced endothelial activation. FASEB J 2015;29:227–238 [DOI] [PubMed] [Google Scholar]
- 29.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-erived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009;15:2148–2157 [DOI] [PubMed] [Google Scholar]
- 30.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014;15:1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linderoth J, Eden P, Ehinger M, et al. Genes associated with the tumor microenvironment are differentially expressed in cured versus primary chemotherapy-refractory diffuse large B-cell lymphoma. Br J Haematol 2008;141:423–432 [DOI] [PubMed] [Google Scholar]
- 32.Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nature Med 2015; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol 2015;33:101–111 [DOI] [PubMed] [Google Scholar]
- 34.Ostuni R, Kratochvill F, Murray PT, et al. Macrophages and cancer: From mechanisms to therapeutic implications. Trends in Immunol 2015;36:229–239 [DOI] [PubMed] [Google Scholar]
- 35.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Rev Immunol 2009;9:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol Rev 2008;222:180–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zirlik K. MDSCs: The final frontier of the microenvironment in CLL? Blood 2014;124:666–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kridel R, Xerri L, Gelas-Dore B, et al. The prognostic impact of CD163-positive marophages in follicular lymphoma: A study from the BC Cancer Agency and LYmphoma Study Association. Clin Cancer Res 2015; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 39.Marchesi F, Cirillo M, Bianchi A, et al. High density of CD68+/CD163+ tumour-associated macrophages (M2-TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B-cell lymphoma. Hematol Oncol 2014; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 40.Tadmor T, Fell R, Polliack A, et al. Absolute monocytosis at diagnosis correlates with survival in diffuse large B-cell lymphoma-possible link with monocytic myeloid-derived suppressor cells. Hematol Oncol 2013;31:65–71 [DOI] [PubMed] [Google Scholar]
- 41.Dehghani M, Sharifpour S, Amirghofran Z, et al. Prognostic significance of T cell subsets in peripheral blood of B cell non-Hodgkin's lymphoma patients. Med Oncol 2012;29:2364–2371 [DOI] [PubMed] [Google Scholar]
- 42.Lindqvist CA, Christiansson LH, Thörn I, et al. Both CD4+ FoxP3+ and CD4+ FoxP3- T cells from patients with B-cell malignancy express cytolytic markers and kill autologous leukaemic B cells in vitro. Immunology 2011;133:296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler B, Taschik J, Haubitz I, et al. TGFβ and IL10 have an impact on risk group and prognosis in childhood ALL. Pediatr Blood Cancer 2015;62:72–79 [DOI] [PubMed] [Google Scholar]
- 44.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol 2006;3:668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerullo V, Diaconu I, Kangasniemi L, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther 2011;19:1737–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vizio B, Novarino A, Giacobino A, et al. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp Ther Med 2012;4:70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunt SK, Mohr AM, Bailey JM, et al. Rosiglitazone and gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunothera 2013;62:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghia P, Hallek M. Management of chronic lymphocytic leukemia. Haematologica 2014;99:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014;343:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother 2009;58:1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122:2539–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: What's here, what's next? Curr Opin Immunol 2015;33:23–35 [DOI] [PubMed] [Google Scholar]
- 53.Nishio N, Diaconu I, Liu H, et al. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res 2014;74:5195–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liljenfeldt L, Dieterich LC, Dimberg A, et al. CD40L gene therapy tilts the myeloid cell profile and promotes infiltration of activated lymphocytes. Cancer Gene Ther 2014;21:95–102 [DOI] [PubMed] [Google Scholar]
- 55.Loskog A, Björkland A, Brown MP, et al. Potent antitumor effects of CD154 transduced tumor cells in experimental bladder cancer. J Urol 2001;166:1093–1097 [PubMed] [Google Scholar]
- 56.Loskog A, Dzojic H, Vikman S, et al. Adenovirus CD40 ligand gene therapy counteracts immune escape mechanisms in the tumor microenvironment. J Immunol 2004;172:7200–7205 [DOI] [PubMed] [Google Scholar]
- 57.Malmström PU, Loskog AS, Lindqvist CA, et al. AdCD40L immunogene therapy for bladder carcinoma–the first Phase I/IIa trial. Clin Cancer Res 2010;16:3279–3287 [DOI] [PubMed] [Google Scholar]
- 58.Gill S, June CH. Going viral: Chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev 2015;263:68–89 [DOI] [PubMed] [Google Scholar]
- 59.Collison LW, Chaturvedi V, Henderson AL, et al. Interleukin-35-mediated induction of a novel regulatory T cell population. Nat Immunol 2010;11:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deniger DC, Yu J, Huls MH, et al. Sleeping Beauty transposition of chimeric antigen receptors targeting receptor tyrosine kinase-like orphan receptor-1 (ROR1) into diverse memory T-cell populations. PLoS One 2015;10:e0128151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berger C, Sommermeyer D, Hudecek M, et al. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol Res 2015;3:206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]