Abstract
Skin is an easily accessible organ, and therapeutic gene transfer to skin remains an attractive alternative for the treatment of skin diseases. Although we have previously documented potent lentiviral gene delivery to human skin, vectors based on adeno-associated virus (AAV) rank among the most promising gene delivery tools for in vivo purposes. Thus, we compared the potential usefulness of various serotypes of recombinant AAV vectors and lentiviral vectors for gene transfer to human skin in a xenotransplanted mouse model. Vector constructs encoding firefly luciferase were packaged in AAV capsids of serotype 1, 2, 5, 6, 8, and 9 and separately administered by intradermal injection in human skin transplants. For all serotypes, live bioimaging demonstrated low levels of transgene expression in the human skin graft, and firefly luciferase expression was observed primarily in neighboring tissue outside of the graft. In contrast, gene delivery by intradermally injected lentiviral vectors was efficient and led to extensive and persistent firefly luciferase expression within the human skin graft only. The study demonstrates the limited capacity of single-stranded AAV vectors of six commonly used serotypes for gene delivery to human skin in vivo.
Introduction
Gene vectors derived from adeno-associated virus (AAV) have been widely explored as carriers of genetic material for therapeutic gene transfer, and numerous ongoing clinical gene therapy trials in humans are exploiting the gene transfer capacity of such vectors (see National Institutes of Health, ClinicalTrials.gov). Successes include conversion of severe hemophilia B to mild or moderate disease in patients by intravenous infusion of AAV vectors expressing factor IX (FIX)1 and achievement of improved vision in patients suffering from Leber's congenital amaurosis by intraocular injection of AAV vectors expressing the RPE65 gene.2,3
As the most prevalent strain in the human population, AAV serotype 2 (AAV2) is the best characterized AAV serotype, and until recently AAV2-derived capsids have been used for most applications including clinical trials. However, several other AAV serotypes have now been characterized, and unique properties of these serotypes are currently being explored, including differential tropisms that reflect differences in the AAV capsid structure. The typical vector design includes an expression cassette, consisting of a promoter of interest and a downstream transgene, flanked by the AAV2 inverted terminal repeats (ITRs). The recombinant AAV2 (rAAV2) vector encoding the transgene can then be directed toward a specific cell type or tissue by cross-packaging into the capsid of any of the AAV serotypes.4 For several tissues, appropriate rAAV serotypes have already been identified. For gene transfer to the heart, AAV1,5 AAV6,6 and AAV9 capsids7 are appropriate, whereas AAV8 is efficient for gene transfer to the liver.1 AAV1,8 AAV6,9 and AAV8 capsids10 are suitable for gene transfer to skeletal muscle, whereas AAV1,11 AAV5,12 and AAV913 are efficient for targeting various regions of the brain. AAV1,14 AAV6,15 AAV8,16 and AAV917 have been used to target lung tissue, whereas AAV2,2 AAV5,18 and AAV819 have been successfully used for gene transfer to the eye.
We and others have previously demonstrated efficient lentiviral gene transfer to xenotransplanted human skin.20,21 We used such potency for treatment of an inflammatory skin disease model by small hairpin RNA (shRNA)-directed targeting of tumor necrosis factor-α.22 In continuation of such observations, we wanted to explore the applicability of AAV-derived vectors for gene transfer to human skin. AAV-mediated gene transfer into murine skin in vivo has been described,23,24 but primary human keratinocytes seem refractory to transduction in vitro by AAV-based vectors.25–28 Reports on using rAAV for gene transfer to human skin in vivo are not currently available. Therefore, we compared the gene transfer capability of a selection of frequently used AAV serotypes (AAV1, 2, 5, 6, 8, or 9) in xenotransplanted human skin by intradermally administering AAV vectors expressing a cytomegalovirus (CMV)-driven firefly luciferase gene. Notably, we demonstrate for all tested rAAV serotypes a lack of capacity to transduce human skin cells after intradermal injection, whereas neighboring murine tissues were effectively transduced. Vesicular stomatitis virus envelope glycoprotein (VSV-G)-pseudotyped lentiviral vectors, in contrast, successfully transduced human skin cells and did not leak to surrounding tissues. Our data support the use of lentiviral vectors, and not rAAV, for viral gene transfer to human skin.
Materials and Methods
Plasmid construction
The lentiviral vector encoding firefly luciferase (pLV/PGK-FLuc) was generated as previously described.20 In brief, the firefly luciferase gene was amplified by PCR from the commercially available pGL3-Control vector (Promega, Madison, WI) and cloned into the lentiviral vector pLV/PGK-puro,22 using the BamHI/XhoI sites, thereby replacing the puromycin resistance gene. To generate the pLV/CMV-FLuc vector, the CMV promoter sequence was amplified by PCR from the recombinant adeno-associated viral vector, rAAV-FLuc, purchased from the Gene Core Facility of the University of North Carolina (Chapel Hill, NC) and cloned into the pLV/PGK-FLuc vector, using the ClaI/BamHI sites, thereby replacing the phosphoglycerate kinase (PGK) promoter fragment. Thus, LV and AAV vectors expressing FLuc from the CMV promoter contained identical expression cassettes. All constructs were verified by restriction analysis and sequencing. Primer sequences are available on request.
Cell culturing
HEK293 cells and normal human dermal fibroblasts (NHDFs) were cultured under standard conditions at 37°C in 5% (v/v) CO2 and maintained in Dulbecco's modified Eagle's medium or RPMI (Lonza, Verviers, Belgium), respectively, supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (0.1 mg/ml), and l-glutamine (265 mg/liter). Human primary keratinocytes were cultured as previously described29 under standard conditions at 37°C in 5% (v/v) CO2 and maintained in Gibco keratinocyte-SFM (serum-free medium) containing 0.09 mM CaCl2 (Invitrogen, Taastrup, Denmark) supplemented with gentamicin (Invitrogen).
Viral vector production
Lentiviral vectors encoding firefly luciferase (designated LV/CMV-FLuc and LV/PGK-FLuc) and pseudotyped with VSV-G were produced in 293T cells that were seeded in 15-cm dishes (7 × 106 cells per dish). Twenty-four hours later, cells were transfected by CaPO4 treatment with 7.26 μg of pRSV-Rev, 9.07 μg of pMD.2G, 31.5 μg of pMDGP-Lg/RRE, and 31.5 μg of pLV/PGK-FLuc. Forty-eight hours after transfection the viral supernatant was harvested and filtered through 0.45-μm filters to remove cellular debris (Sarstedt, Nümbrecht, Germany). The lentiviral vectors were ultracentrifuged for 2 hr (4°C at 25,000 rpm) in an SW28 rotor (Beckman Coulter, Fullerton, CA). Virus pellets were allowed to resuspend overnight in PBS−/− at 4°C at 1/250th the original volume. The lentiviral vector yield was determined by measuring the p24 Gag protein concentration, using an HIV-1 p24 antigen ELISA kit (ZeptoMetrix, Buffalo, NY) according to the manufacturer's protocol. FLuc-encoding recombinant adeno-associated viral vectors of various serotypes (rAAV2/1-CMV-FLuc, rAAV2/2-CMV-FLuc, rAAV2/5-CMV-FLuc, rAAV2/6-CMV-FLuc, rAAV2/8-CMV-FLuc, and rAAV2/9-CMV-FLuc) were all purchased from the Gene Core Facility at the University of North Carolina. The vectors are designated according to the backbone and the serotype, for example, a recombinant AAV vector of serotype 1 with an AAV2 backbone is designated rAAV2/1.
In vitro transduction experiments
Human primary keratinocytes and NHDF cells were seeded at 2.5 × 104 cells per well in 24-well dishes. The next day rAAV-FLuc vectors (multiplicity of infection [MOI], ∼1 × 105 vector genomes [VG]/cell) or LV-FLuc vectors (MOI, ∼30 transducing units/cell; lentiviral titer determined by transduction of HEK293 cells) were added to the cells in a volume of 250 μl of the appropriate medium. The keratinocytes were allowed to incubate with the viral vectors for 3 hr at 37°C before another 250 μl of medium was added to the cells and then incubated until the medium was changed on the next day. On days 3 and 7 after transduction, firefly luciferase activity was analyzed with the Dual-Luciferase reporter assay system (Promega) according to the manufacturer's instructions. Reactions were performed in 96-well arrays and reading was performed in a multisample plate-reading luminometer (Berthold, Bad Wildbad, Germany).
The xenograft transplantation model
Normal skin was kindly provided by healthy individuals undergoing corrective surgery. Informed consent was obtained. The study was approved by the Central Ethics Committee and conducted according to the Declaration of Helsinki protocols. All animal studies were carried out with permission from the Danish Experimental Animal Inspectorate. Mice xenotransplanted with human skin were achieved as follows: skin biopsies, containing both epidermis and dermis (split skin), were divided into several grafts (each 1.5 × 1.5 × 0.05 cm) and transplanted onto C.B-17 SCID mice (C.B-Igh-1b/IcrTac-Prkdcscid), 6–8 weeks old (Taconic Europe, Lille Skensved, Denmark) as described.30 Briefly, the mice were anesthetized before surgery by a subcutaneous injection of ketamine (Ketaminol vet., 100 mg/kg; Intervet, Skovlunde, Denmark) and xylazine (Narcoxyl vet., 10 mg/kg; Intervet). The back of each mouse was shaved and part of the exposed skin removed. The human skin grafts were sutured with absorbable 6-0 suture (Caprosyn; Tyco, Copenhagen, Denmark) and covered with Xeroform dressings (Sherwood Medical, Markham, ON, Canada) for 1 week. The mice were kept under pathogen-free conditions throughout the study. The grafts healed for at least 28 days before viral vector administration.
In vivo administration of AAV-derived and lentiviral vectors
The administration of viral vectors to xenotransplanted human skin was performed essentially as previously described.20,22 Briefly, xenotransplanted C.B-17 SCID mice were anesthetized by a subcutaneous injection of ketamine–xylazine or with 3.75% isoflurane (Forene; Abbott Scandinavia, Solna, Sweden) (2% isoflurane for anesthesia maintenance) and then injected intradermally (into the xenograft) with a total volume of 150 μl of viral vector suspension. rAAVs were injected at a dose of 5 × 1010 VG/mouse, whereas LV vectors were injected at a dose of 2 μg of p24 units per mouse. Saline was injected as negative control. To test rAAV transduction efficiency at a higher dose, mice (n = 5) were injected with rAAV2/6-FLuc at a dose of 2.5 × 1011 VG/mouse whereas the LV/CMV-FLuc dose (n = 4) was unchanged relative to previous experiments. For analysis of gene delivery to muscle, 8- to 10-week-old BALB/cJBomTac female mice (Taconic Europe) were injected intramuscularly via the quadriceps femoris muscle in the right hind leg. A total volume of 40 μl of rAAV suspension, corresponding to a dose of 5 × 1010 VG/mouse, was injected into four separate sites in the same muscle. Each group consisted of two to five mice that received an aliquot from the same viral vector batch. The mice were monitored by in vivo imaging up to 28 days postinjection.
In vivo bioluminescence imaging
Analysis of mice for bioluminescence on days 3, 7, 14, 21, and 28 after viral vector administration was carried out as previously described.20,22 Briefly, mice were injected intraperitoneally with the substrate d-luciferin potassium salt (Synchem OHG, Felsberg-Altenburg, Germany) at a dose of 150 μg/g of body weight and subsequently anesthetized with 3.75% isoflurane (Forene; Abbott Scandinavia). Anesthesia was maintained at 2% isoflurane during the bioluminescence scan in the IVIS Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA). Data were analyzed using the software Living Image 4.0. Up to 10 images were acquired at an interval of 2 min and peak-intensity images were selected for subsequent analysis. For quantification of bioluminescence from each skin graft or muscle, a standardized “region of interest” (ROI) was defined and the radiance intensity was measured (photons/sec/cm2/sr). An equivalent ROI was used for all human xenografts at each time point and likewise for analysis of the bioluminescence signal from the hind limb quadriceps muscles. Before scanning the excised xenografts, the mice were injected with d-luciferin and anesthetized and scanned as described previously. Immediately after reaching peak intensity the mice were killed and the grafts were excised and subsequently scanned. Data from each group in the two skin experiments were pooled and used for data analysis. For grafts injected with a high dose (HD) of the rAAV2/6 vector as well as grafts injected with LV/CMV-FLuc, 4-mm punch biopsies were excised from the centrum of the graft and scanned separately. Finally, mice were scanned to reveal any bioluminescent signal remaining after graft removal.
Ex vivo bioluminescence detection
After excision the human skin grafts were snap-frozen in liquid nitrogen and stored at −80°C until further analysis. Lysis of the skin grafts was achieved by mincing the tissue and by adding passive lysis buffer (Promega) before homogenization with an Omni tissue homogenizer (Omni International, Kennesaw, GA). To ensure complete lysis, the samples were incubated at room temperature for 30 min. The samples were centrifuged to remove any remaining nonlysed tissue, and supernatant containing the lysate was analyzed for bioluminescence in the form of luciferase activity and protein concentration. Luciferase activity was analyzed with luciferase assay reagent II (Promega) in 96-well arrays and detected with a multisample plate-reading luminometer (Berthold). Protein concentration was analyzed by the Bradford protein assay (Bio-Rad, Hercules, CA) according to the manufacturer's protocol and detected with a Shimadzu UV-160A spectrophotometer. The average luciferase activity was hereafter normalized to the amount of total protein in the respective lysate.
Statistical analysis
All p values were calculated by a two-tailed Student t test to test the null hypothesis of no difference between the compared groups. The assumption of equal variances was tested by the F test. p < 0.05 was considered significant.
Results
Verification of in vivo transduction capacity of an AAV vector panel in mouse muscle
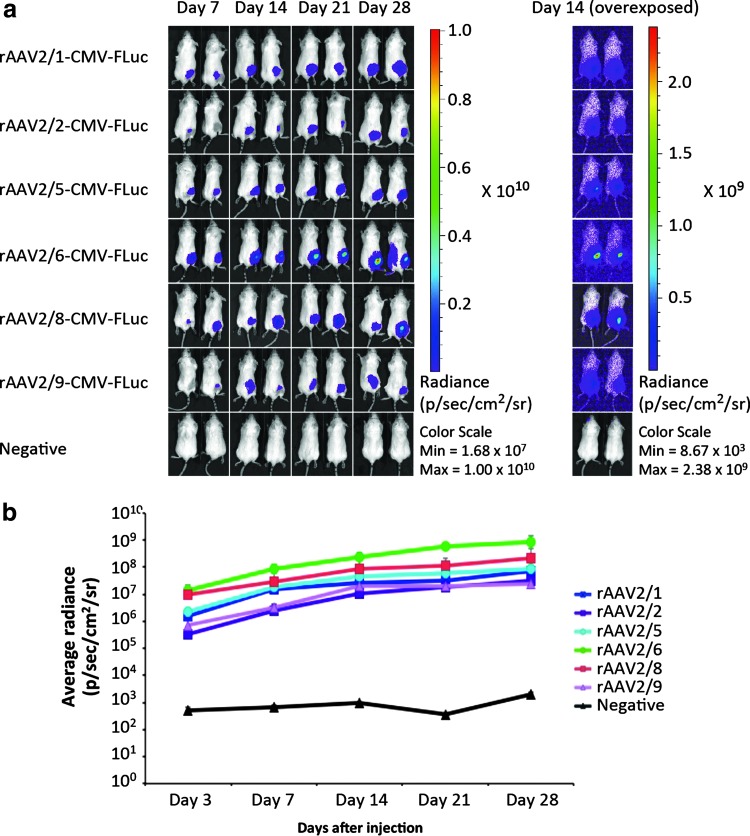
To investigate AAV serotype tropism in human skin and to identify an AAV serotype that was suited for transduction of human skin in vivo, we designed a comparative study of six commonly used AAV serotypes, including AAV serotypes 1, 2, 5, 6, 8, and 9. An AAV2 vector backbone containing the firefly luciferase (FLuc) gene driven by a human cytomegalovirus (CMV) promoter was packaged into the various AAV capsids. To confirm functionality and the capacity of the set of rAAV vectors to transduce tissues in vivo, we initially injected the vectors into the quadriceps femoris muscle of BALB/cJ mice (n = 2, dose of 5 × 1010 VG/mouse) and collected data by in vivo bioluminescence imaging on days 7, 14, 21, and 28 after viral vector administration (Fig. 1a). Irrespective of serotype, FLuc expression in the left hind leg, corresponding to the quadriceps femoris muscle in which the viral vectors were administered, was evident as early as day 3 after viral vector administration (Fig. 1b). In addition, for all AAV serotypes we observed robust FLuc gene expression for the duration of the experiment, resulting in levels of average radiance ranging from 107 to 109 photons/sec/cm2/sr with AAV6 as the most potent serotype and AAV2 and AAV9 as the least effective serotypes in muscle (Fig. 1b).
Figure 1.
In vivo intramuscular transduction efficacy of rAAV2 serotypes 1, 2, 5, 6, 8, and 9. (a) Representative images from days 7, 14, 21, and 28 of BALB/cJ mice injected intramuscularly with rAAV2/1-CMV-FLuc, rAAV2/2-CMV-FLuc, rAAV2/5-CMV-FLuc, rAAV2/6-CMV-FLuc, rAAV2/8-CMV-FLuc, rAAV2/9-CMV-FLuc, or negative controls. Right: Overexposed images from day 14. (b) Bioluminescence from injected murine hind limb muscle was recorded on days 3, 7, 14, 21, and 28, and the results are based on the bioluminescence light signal intensity computed from a standardized region of interest (depicted as the average radiance + SD) (each group, n = 2). LV, lentiviral vector; rAAV, recombinant adeno-associated virus vector. p/sec/cm2/sr, photons per second per centimeter squared per steradian.
Lentiviral gene delivery to human skin shows higher activity of the PGK promoter compared with CMV
We have previously shown efficient transduction of human skin in vivo by VSV-G-pseudotyped lentiviral vectors (LVs) encoding enhanced green fluorescent protein (eGFP)22 and FLuc.20 To directly compare the potency of gene transfer by AAV- and lentivirus-derived vectors in human skin, we produced LVs, designated LV/CMV-FLuc, carrying the exact same CMV-FLuc expression cassette as the panel of rAAV vectors described previously. As our previous studies of lentiviral gene transfer to skin were based on vectors expressing reporter genes from the human phosphoglycerate (PGK) promoter, we first compared the efficacy of transgene expression after lentiviral delivery of FLuc driven by the CMV and PGK promoter, respectively. Normal human skin grafts were xenografted onto the back of severe combined immunodeficient (SCID) mice, and the animals were injected intradermally, into the human skin graft, with a single dose of either LV/PGK-FLuc or LV/CMV-FLuc and imaged on days 3, 7, 14, 21, and 28 after vector administration (Supplementary Fig. S1a; supplementary data are available online at http://online.liebertpub.com/hgtb). Throughout the entire experiment, the PGK promoter resulted in levels of expression that were approximately 10-fold higher than the levels obtained with the CMV promoter (Supplementary Fig. S1b). These data show an increased potency of the PGK promoter in human skin and stress the importance of delivering similar gene cassettes in comparative studies of vector systems. To compare head-to-head gene delivery by lentiviral vectors and rAAV vectors, we therefore delivered the exact same CMV-FLuc expression cassette in our subsequent studies for all vector types.
Comparison of gene transfer to xenotransplanted human skin using AAV- and lentivirus-derived vectors harboring the CMV-FLuc expression cassette
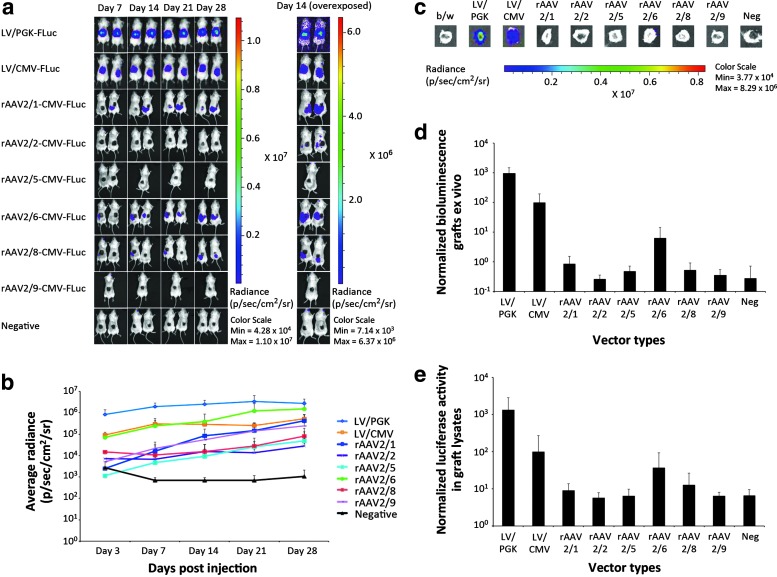
To compare the efficacy of VSV-G-pseudotyped LVs and the various AAV serotypes in human skin, we initially administered the set of vectors by intradermal injection in small groups of SCID mice (n = 2) transplanted with normal human skin. Lentiviral vectors were injected at a dose of 4 × 107 transducing units/mouse, whereas the injected dose of rAAV was 5 × 1010 VG/mouse. The mice were monitored by bioluminescence for 28 days (Fig. 2a) and were then killed, allowing subsequent isolation and analysis of the skin graft.
Figure 2.
In vivo and ex vivo detection of luciferase after intradermal injections of LV or rAAV2 serotype 1, 2, 5, 6, 8, or 9 into xenografted human skin. (a) In vivo imaging of luciferase activity after intradermal vector administration. Shown are representative images from days 7, 14, 21, and 28 of xenotransplanted mice in groups injected intradermally with LV/PGK-FLuc, LV/CMV-FLuc, rAAV2/1-CMV-FLuc, rAAV2/2-CMV-FLuc, rAAV2/5-CMV-FLuc, rAAV2/6-CMV-FLuc, rAAV2/8-CMV-FLuc, rAAV2/9-CMV-FLuc, or negative controls. (b) Bioluminescence from injected xenografted human skin was recorded on days 3, 7, 14, 21, and 28 and the results are based on the signal intensity computed from a standardized region of interest. The experiment was performed twice, and data for each group were pooled from the two experiments (LV, rAAV2/1, rAAV2/2, rAAV2/6, rAAV2/8, and negative controls, n = 4; rAAV2/5 and rAAV2/9, n = 3). (c) Ex vivo detection of luciferase activity in human skin xenografts exposed to LV or rAAV2 serotype 1, 2, 5, 6, 8, or 9. Shown are representative images of the human skin xenografts after excision of these immediately after the mice were killed on day 28. (d) Depiction of average radiance after normalization to 102 for LV/CMV-FLuc in the human skin xenografts ex vivo on day 28 (n = 3). (e) Bioluminescence intensity depicted as the average luciferase activity in lysates of the excised human skin grafts normalized to total protein concentration (n = 3). LV, lentiviral vector; rAAV, recombinant adeno-associated viral vectors; p/sec/cm2/sr, photons per second per centimeter squared per steradian. Data are presented as means + SD.
For all mice injected with rAAV vectors, we observed increasing levels of FLuc expression over time in or around the skin graft. Although the signals were weak for some of the serotypes, the light emission signals were significantly above the background level observed in uninjected mice and could be documented by overexposure of the images in all rAAV-treated groups, except in animals treated with rAAV2/5-CMV-FLuc (Fig. 2a, right). Among the panel of rAAV vectors, quantification of the emitted bioluminescence light signals showed the highest level of expression for rAAV2/6 and rAAV2/1, whereas rAAV2/2 and rAAV2/5 were the least efficient vector types (Fig. 2b). On the basis of this analysis, AAV2/6 resulted in higher levels of FLuc expression than was observed in LV/CMV-FLuc-injected animals, but lower levels of FLuc expression than was measured in LV/PGK-FLuc-injected animals. Notably, LV/PGK-FLuc again produced signals that were approximately 10-fold stronger than the signals obtained with LV/CMV-FLuc.
By monitoring bioluminescence signals in live animals, we were not able to conclude whether the emission signals originated from the graft or from neighboring tissues. After killing the animals on day 28 after injection, we therefore isolated the skin grafts and measured the bioluminescence signal originating from the graft only. Remarkably, strong signals were detected in grafts isolated from mice treated with LV/CMV-FLuc and LV/PGK-FLuc, whereas signals in grafts from rAAV-injected mice were markedly lower (Fig. 2c). A faint signal was detected inside the isolated rAAV2/6-CMV-FLuc-treated graft, whereas the other grafts, including the negative control, did not emit any detectable light signal. Quantification of bioluminescence signals directly in the isolated grafts (Fig. 2d) and the luciferase activity in graft lysates (Fig. 2e) confirmed these observations.
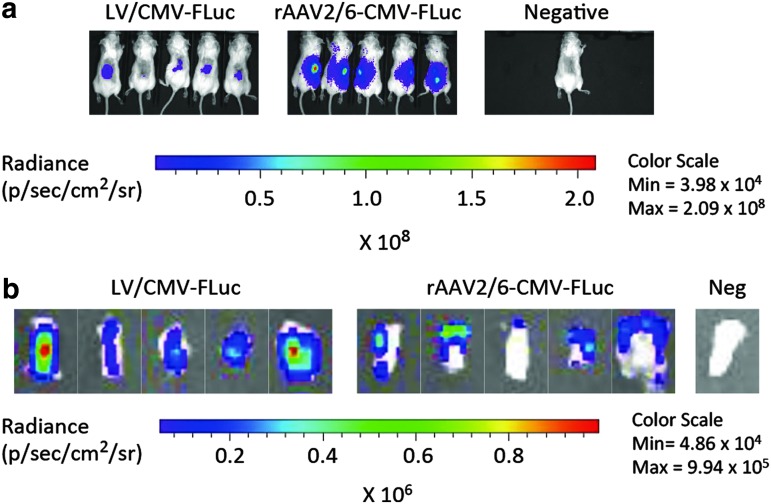
These data supported the notion that lentiviral vectors were able to transduce human skin cells within the injected graft, whereas rAAV vectors, with variable potency depending on the serotype, would transduce cells primarily outside the graft. To confirm this finding we repeated the experiment, comparing the efficacy of LV/CMV-FLuc and rAAV2/6-CMV-FLuc in larger groups of mice (n = 5). Importantly, we observed in this experiment strong bioluminescence signals in all mice treated with rAAV2/6-CMV-FLuc, whereas the signals in LV/CMV-FLuc-injected mice were less intense, but clearly limited to the graft (Fig. 3a). Next, we isolated the grafts from all 10 mice and analyzed the topology of expression within the grafts (Fig. 3b). For grafts from all five LV/CMV-FLuc-injected mice we confirmed strong gene expression centered inside the graft, whereas the remaining expression for rAAV2/6-CMV-FLuc-injected grafts was detected only in the periphery of the graft and most likely in neighboring connective tissue attached to the isolated graft.
Figure 3.
rAAV2/6 transduction of neighboring tissues but not of the skin graft after intradermal vector injection. (a) In vivo imaging of luciferase expression after intradermal vector administration. Representative images show xenotransplanted mice (day 28) in groups injected intradermally with LV/CMV-FLuc (left) or rAAV2/6-CMV-FLuc (middle), as well as a saline-injected negative control mouse (right). (b) Ex vivo detection of luciferase activity in human skin xenografts exposed to LV/CMV-FLuc or rAAV2/6-CMV-FLuc and in a graft from a negative control mouse injected with saline. Shown are representative images of the human skin xenografts excised immediately after the mice were killed on day 28. LV, lentiviral vector; rAAV, recombinant adeno-associated viral vectors; p/sec/cm2/sr, photons per second per centimeter squared per steradian. Data are presented as means + SD.
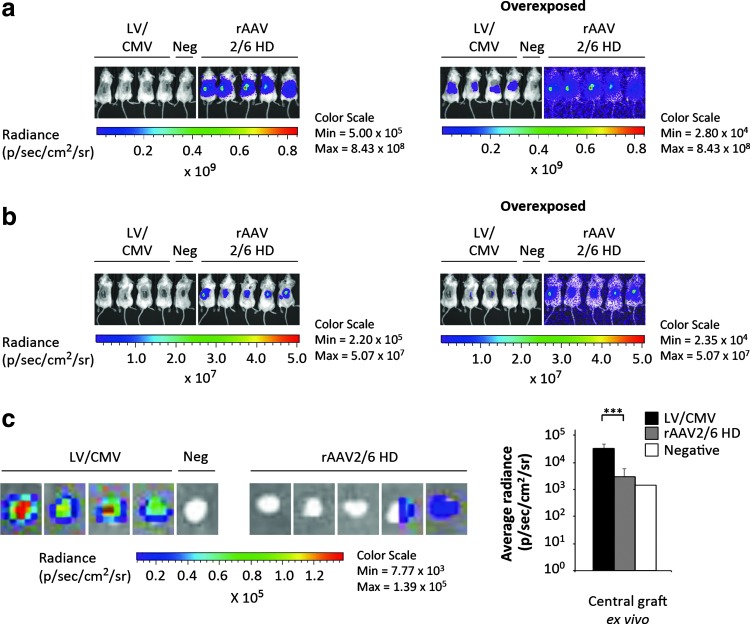
To further substantiate these findings, we repeated the experiment described previously, using higher doses of rAAV2/6-CMV-FLuc and unaltered doses of LV/CMV-FLuc. With limitations on injection volume and titers of the rAAV2/6-CMV-FLuc batch, we maximized the dose to 2.5 × 1011 VG/mouse, which corresponds to a 5-fold increase relative to our previous experiments (Figs. 2 and 3). As expected, bioluminescence imaging of the high-dose (HD) rAAV2/6 group for 4 weeks revealed a profound signal in all mice that increased over time (Supplementary Fig. S2b). Quantification of bioluminescence signals confirmed this notion, and demonstrated much stronger activity of the luciferase reporter in the HD group as compared with the lower dose (Supplementary Fig. S3). Transgene expression in the HD group increased initially after injection and seemed to plateau at about 14 days after treatment (Supplementary Fig. S3). Twenty-eight days posttreatment a head-to-head comparison of animals injected with rAAV2/6-CMV-FLuc HD and LV/CMV-FLuc clearly showed a much stronger signal in the rAAV2/6 HD group, whereas longer exposure was required to visualize luciferase activity in the LV/CMV-FLuc group (Fig. 4a). To pinpoint the location of the bioluminescence signal and to distinguish the human graft from neighboring murine tissue, we next killed the mice and removed the graft by dissection. Bioluminescence imaging without the graft revealed a high level of transgene expression in the tissue beneath the graft in all rAAV2/6-treated animals (Fig. 4b). As evident on overexposed images, and in stark contrast, we were not able to detect any transduction of murine tissue beneath or surrounding the skin graft injected with lentiviral vectors at a dose of 2 μg of p24 per mouse (Fig. 4b). As the excised graft inevitably contained murine tissue at the rim of the patch, we punched out 4-mm-wide biopsies in the center of the excised xenograft in order to separate human from neighboring murine tissue. Notably, bioluminescence analysis of the punch biopsies demonstrated highly restricted activity of the reporter gene in the central part of the graft after rAAV2/6-based delivery, whereas strong transgene expression, in contrast, was evident in lentivirally transduced grafts (Fig. 4c). The limited reporter activity from the punch biopsies for the group treated with rAAV2/6 HD reproduced the distinct pattern of expression at peripheral regions of the graft observed earlier using a lower dose (Fig. 3b). In summary, we conclude that lentiviral vectors can efficiently transduce human skin cells on intradermal injection in xenotransplanted skin, whereas AAV-derived vectors by the same administration route transduce skin cells only to a limited extent. Rather, intradermally injected rAAV vectors spread to and predominantly transduce neighboring murine tissues. Thus, our data support the use of lentiviral vectors, and not rAAV packaged in commonly used capsids, for viral gene transfer to human skin.
Figure 4.
High-dose rAAV2/6 transduction increases luciferase expression in the neighboring tissues but not in the skin graft after intradermal vector injection. (a) In vivo imaging of luciferase expression after intradermal vector administration. Representative images show xenotransplanted mice (day 28) in groups injected intradermally with LV/CMV-FLuc (left, mice 1–4; same vector dose as in previous experiments), a saline-injected negative control mouse (left, mouse 5), or high-dose (HD) rAAV2/6-CMV-FLuc (right, mice 1–5; 5-fold increased vector dose compared with previous experiments). Overexposed images are shown on the right-hand side. (b) In vivo imaging of luciferase expression in the mice shown in (a) after graft removal. After the mice were killed, the human skin xenografts were excised and new imaging was performed on the dead mice without grafts. Overexposed images are shown on the right-hand side. (c) Ex vivo detection of luciferase activity in the central part of the human skin xenografts (4-mm punch biopsy) exposed to LV/CMV-FLuc (left, grafts 1–4), saline-injected negative control (left, graft 5), or HD rAAV2/6-CMV-FLuc (right, grafts 1–5). Depiction of the average radiance in central graft tissue ex vivo from the punch biopsies are shown on the right-hand side. Data are presented as means + SD. ***p < 0.01 (p = 0.0026). LV, lentiviral vector; rAAV, recombinant adeno-associated viral vectors; HD, high dose; p/sec/cm2/sr, photons per second per centimeter squared per steradian.
Discussion
Our findings demonstrate a limited capacity of AAV-derived vectors, at least for the commonly used serotypes tested here, to transduce human skin. This is in contrast to the efficacy and extensive transduction by VSV-G-pseudotyped lentiviral vectors in human skin. This difference may be partially explained by differential diffusion of the two vector types both intradermally within the human skin graft and by diffusion away from the human skin graft due to variation in virion size. As AAV particles are only 20–25 nm in diameter,31 these may potentially diffuse more easily within the tissue than lentiviral particles with a diameter of 130–145 nm.32 As AAV binding and entry into the cells is receptor dependent, another consideration is the presence in human skin of the receptors needed. It is believed that on binding to a primary glycan receptor on the cell surface, the AAV capsid interacts with a coreceptor to facilitate internalization. For several of the AAV serotypes, the specific receptors and/or coreceptors have already been identified, helping in understanding the tropism of the serotypes. AAV1, AAV5, and AAV6 all use α2,3/α2,6 N-linked sialic acid as the primary receptor33,34 and in addition, AAV6 binds to heparan sulfate proteoglycans (HSPGs) with moderate affinity.35 As secondary receptors, the platelet-derived growth factor receptor (PDGFR) and the epidermal growth factor receptor (EGFR) are needed for internalization of AAV5 and AAV6, respectively.36,37 As both α2,3 N-linked sialic acid,38 HSPGs,39 and EGFR40 are expressed by keratinocytes in the human epidermal skin layer, AAV1 and AAV6 should be able to infect keratinocytes in the epidermis. However, as PDGFR is not expressed in the epidermis,41 this may help to explain the ineffective transduction of keratinocytes by AAV5. AAV2 requires HSPG for binding to cells42 and this serotype, among others, can use both fibroblast growth factor receptor (FGFR)43 and integrin αVβ5/α5β1,44,45 which are both present in the human epidermis,46,47 for internalization. However, primary human keratinocytes lack HSPG and, therefore, are only vaguely transduced by AAV2 vectors.48 AAV9 requires galactose for binding to the target cell,49 and both AAV8 and AAV9 need the 67-kDa laminin receptor as secondary receptor.50 Both galactose51 and the 67-kDa laminin receptor52 are found in the human epidermis, potentially rendering epidermis susceptible to transduction by AAV9 and perhaps also by AAV8, although additional receptors for this serotype have not yet been reported. In summary, on the basis of the receptors required for transduction by the various AAV serotypes, we would expect to observe transduction of keratinocytes in the human epidermis by at least some AAV serotypes.
The lack of efficacy of rAAV transduction in intradermally injected human skin in vivo may also originate from obstacles downstream of virus attachment and internalization, involving steps such as trafficking to the nucleus, uncoating of the virus to release the genome, and conversion of the single-stranded DNA (ssDNA) to double-stranded DNA (dsDNA) serving as a template for transcription. The latter has been shown to constitute an important rate-limiting step in rAAV transduction, as ssDNA-to-dsDNA conversion relies on either host cellular replication factors for synthesis of the complementary strand or base pairing with a coinfecting complementary genome for successful transduction. Indeed, this step has been shown to reduce the transduction effectivity in many cell types,53 and it may potentially influence the low efficiency of rAAV transduction in human skin as seen in our study. Hence, by using a self-complementary rAAV vector, in which both DNA strands are packaged as a single ssDNA molecule that on uncoating refolds into dsDNA, we may be able to increase the transduction rate, as has been shown in other tissues.54
Previous studies using rAAV vectors in vitro for transduction of primary human keratinocytes have reported poor efficacy of the commonly used serotypes,28,48 although serotypes 1, 2, and 6 may be suitable for this purpose.25–27,55 Using our panel of single-stranded rAAV-CMV-FLuc vectors for in vitro transduction of primary cells of human origin, including keratinocytes, we could partially support earlier claims (Supplementary Fig. S4). Interestingly, Melo and coworkers55 reported increased transduction of keratinocytes in vitro using a novel AAV-LK19 chimera developed by DNA family shuffling technology,56 which warrants further attention for skin delivery.
During the course of our work, Sallach and coworkers reported the development of new AAV capsids with increased transduction capacity in primary human keratinocytes.48 Such capsids, selected from an AAV library depleted for mutants carrying HSPG-binding peptides, were shown to facilitate transduction of keratinocytes in vitro in human three-dimensional organotypic skin cultures and would be obvious future candidates for optimizing AAV-directed gene delivery in the skin xenotransplantation model.
Another step to further enhance rAAV transduction efficiency in the skin may be taken by using tyrosine-mutated capsids. During intracellular trafficking, the AAV capsids are at risk of being phosphorylated on surface-exposed tyrosine residues by the epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK).57 This is followed by ubiquitination and subsequent proteasomal degradation, leading to reduced transgene expression. Indeed, the ubiquitin–proteasome pathway has been demonstrated to present a barrier to transduction of human keratinocytes in vitro.25 However, this tyrosine phosphorylation can be circumvented as mutagenesis of the surface-exposed tyrosine residues into phenylalanine residues has shown a dramatic increase in transduction efficacy in various cell types and tissues. 58,59
In summary, we have tested frequently used AAV serotypes for gene transfer to human skin transplanted onto mice. As we have previously shown, VSV-G-pseudotyped lentiviral vectors transduced human skin grafts with high efficacy. In contrast, injection of rAAV vectors packaged in serotypes 1, 2, 5, 6, 8, and 9 capsids all resulted in poor, if any, transgene expression in human skin grafts, but were found to transduce neighboring tissues, indicating that the vector particles diffused out of intradermally injected human skin grafts without transducing human keratinocytes. On the basis of our findings, we recommend using VSV-G-pseudotyped lentiviral vectors for viral gene transfer to human skin.
Supplementary Material
Acknowledgments
The authors thank Birgit Holm Hansen for technical assistance. The authors also thank Daniel Miotto Dupont for help with bioluminescence and the Interdisciplinary Nanoscience Center at Aarhus University for the use of the IVIS Spectrum imaging system. Furthermore, this study was made possible through support by the Karen Elise Jensen Foundation, the Riisfort Foundation, and the Gene Therapy Initiative Aarhus (GTI-Aarhus) funded by the Lundbeck Foundation (grant no. R126-2012-12456). J.G.M. is a member of the Aarhus Research Center for Innate Immunity funded by the Aarhus University Research Foundation. M.J. and A.L.A. were funded by Ph.D. grants from the Faculty of Health, Aarhus University, Denmark.
Author Disclosure
No competing financial interests exist.
References
- 1.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009;374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinowitz JE, Rolling F, Li C, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002;76:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawase Y, Ly HQ, Prunier F, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol 2008;51:1112–1119 [DOI] [PubMed] [Google Scholar]
- 6.White JD, Thesier DM, Swain JB, et al. Myocardial gene delivery using molecular cardiac surgery with recombinant adeno-associated virus vectors in vivo. Gene Ther 2011;18:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 2006;99:e3–e9 [DOI] [PubMed] [Google Scholar]
- 8.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther 2011;22:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Storb R, Halbert CL, et al. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Mol Ther 2012;20:1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodino-Klapac LR, Montgomery CL, Bremer WG, et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther 2010;18:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutson TH, Verhaagen J, Yanez-Munoz RJ, et al. Corticospinal tract transduction: a comparison of seven adeno-associated viral vector serotypes and a non-integrating lentiviral vector. Gene Ther 2012;19:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger C, Gorbatyuk OS, Velardo MJ, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 2004;10:302–317 [DOI] [PubMed] [Google Scholar]
- 13.Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 2012;23:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flotte TR, Fischer AC, Goetzmann J, et al. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol Ther 2010;18:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human α1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther 2010;18:1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liqun Wang R, McLaughlin T, Cossette T, et al. Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of α-1-antitrypsin using invasive and noninvasive delivery. Mol Ther 2009;17:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeifer C, Aneja MK, Hasenpusch G, et al. Adeno-associated virus serotype 9-mediated pulmonary transgene expression: effect of mouse strain, animal gender and lung inflammation. Gene Ther 2011;18:1034–1042 [DOI] [PubMed] [Google Scholar]
- 18.Pang JJ, Deng WT, Dai X, et al. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PloS One 2012;7:e35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenberghe LH, Bell P, Maguire AM, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med 2011;3:88ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bak RO, Stenderup K, Rosada C, et al. Targeting of human interleukin-12B by small hairpin RNAs in xenografted psoriatic skin. BMC Dermatol 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siprashvili Z, and Khavari PA. Lentivectors for regulated and reversible cutaneous gene delivery. Mol Ther 2004;9:93–100 [DOI] [PubMed] [Google Scholar]
- 22.Jakobsen M, Stenderup K, Rosada C, et al. Amelioration of psoriasis by anti-TNF-α RNAi in the xenograft transplantation model. Mol Ther 2009;17:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jazwa A, Kucharzewska P, Leja J, et al. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther 2010;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keswani SG, Balaji S, Le L, et al. Pseudotyped adeno-associated viral vector tropism and transduction efficiencies in murine wound healing. Wound Repair Regen 2012;20:592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun-Falco M, Eisenried A, Buning H, et al. Recombinant adeno-associated virus type 2-mediated gene transfer into human keratinocytes is influenced by both the ubiquitin/proteasome pathway and epidermal growth factor receptor tyrosine kinase. Arch Dermatol Res 2005;296:528–535 [DOI] [PubMed] [Google Scholar]
- 26.Ellis BL, Hirsch ML, Barker JC, et al. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with nine natural adeno-associated virus (AAV1–9) and one engineered adeno-associated virus serotype. Virol J 2013;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petek LM, Fleckman P, and Miller DG. Efficient KRT14 targeting and functional characterization of transplanted human keratinocytes for the treatment of epidermolysis bullosa simplex. Mol Ther 2010;18:1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagnoux-Palacios L, Hervouet C, Spirito F, et al. Assessment of optimal transduction of primary human skin keratinocytes by viral vectors. J Gene Med 2005;7:1178–1186 [DOI] [PubMed] [Google Scholar]
- 29.Primo MN, Bak RO, Schibler B, et al. Regulation of pro-inflammatory cytokines TNFα and IL24 by microRNA-203 in primary keratinocytes. Cytokine 2012;60:741–748 [DOI] [PubMed] [Google Scholar]
- 30.Stenderup K, Rosada C, Worsaae A, et al. Interleukin-20 plays a critical role in maintenance and development of psoriasis in the human xenograft transplantation model. Br J Dermatol 2009;160:284–296 [DOI] [PubMed] [Google Scholar]
- 31.Chen H. Comparative observation of the recombinant adeno-associated virus 2 using transmission electron microscopy and atomic force microscopy. Microsc Microanal 2007;13:384–389 [DOI] [PubMed] [Google Scholar]
- 32.Briggs JA, Wilk T, Welker R, et al. Structural organization of authentic, mature HIV-1 virions and cores. EMBO J 2003;22:1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z, Miller E, Agbandje-McKenna M, et al. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol 2006;80:9093–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters RW, Yi SM, Keshavjee S, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem 2001;276:20610–20616 [DOI] [PubMed] [Google Scholar]
- 35.Ng R, Govindasamy L, Gurda BL, et al. Structural characterization of the dual glycan binding adeno-associated virus serotype 6. J Virol 2010;84:12945–12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Pasquale G, Davidson BL, Stein CS, et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med 2003;9:1306–1312 [DOI] [PubMed] [Google Scholar]
- 37.Weller ML, Amornphimoltham P, Schmidt M, et al. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med 2010;16:662–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holikova Z, Hrdlickova-Cela E, Plzak J, et al. Defining the glycophenotype of squamous epithelia using plant and mammalian lectins: differentiation-dependent expression of α2,6- and α2,3-linked N-acetylneuraminic acid in squamous epithelia and carcinomas, and its differential effect on binding of the endogenous lectins galectins-1 and −3. APMIS 2002;110:845–856 [DOI] [PubMed] [Google Scholar]
- 39.Tomas D, Vucic M, Situm M, et al. The expression of syndecan-1 in psoriatic epidermis. Arch Dermatol Res 2008;300:393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanney LB, McKanna JA, Stoscheck CM, et al. Visualization of epidermal growth factor receptors in human epidermis. J Invest Dermatol 1984;82:165–169 [DOI] [PubMed] [Google Scholar]
- 41.Rollman O, Jensen UB, Ostman A, et al. Platelet derived growth factor (PDGF) responsive epidermis formed from human keratinocytes transduced with the PDGF β receptor gene. J Invest Dermatol 2003;120:742–749 [DOI] [PubMed] [Google Scholar]
- 42.Summerford C, and Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998;72:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qing K, Mah C, Hansen J, et al. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med 1999;5:71–77 [DOI] [PubMed] [Google Scholar]
- 44.Summerford C, Bartlett JS, and Samulski RJ. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med 1999;5:78–82 [DOI] [PubMed] [Google Scholar]
- 45.Asokan A, Hamra JB, Govindasamy L, et al. Adeno-associated virus type 2 contains an integrin α5β1 binding domain essential for viral cell entry. J Virol 2006;80:8961–8969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takenaka H, Yasuno H, and Kishimoto S. Immunolocalization of fibroblast growth factor receptors in normal and wounded human skin. Arch Dermatol Res 2002;294:331–338 [DOI] [PubMed] [Google Scholar]
- 47.Adams JC, and Watt FM. Expression of β1, β3, β4, and β5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol 1991;115:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallach J, Di Pasquale G, Larcher F, et al. Tropism-modified AAV vectors overcome barriers to successful cutaneous therapy. Mol Ther 2014;22:929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen S, Bryant KD, Brown SM, et al. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem 2011;286:13532–13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akache B, Grimm D, Pandey K, et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol 2006;80:9831–9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgiou S, Pasmatzi E, Monastirli A, et al. Age-related alterations in the carbohydrate residue composition of the cell surface in the unexposed normal human epidermis. Gerontology 2005;51:155–160 [DOI] [PubMed] [Google Scholar]
- 52.Cavalieri S, Rotoli M, Feliciani C, et al. Expression of the high-affinity laminin receptor (67 kDa) in normal human skin and appendages. Int J Immunopathol Pharmacol 2005;18:223–231 [DOI] [PubMed] [Google Scholar]
- 53.Fisher KJ, Gao GP, Weitzman MD, et al. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol 1996;70:520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarty DM, Monahan PE, and Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 2001;8:1248–1254 [DOI] [PubMed] [Google Scholar]
- 55.Melo SP, Lisowski L, Bashkirova E, et al. Somatic correction of junctional epidermolysis bullosa by a highly recombinogenic AAV variant. Mol Ther 2014;22:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lisowski L, Dane AP, Chu K, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 2014;506:382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong L, Li B, Jayandharan G, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 2008;381:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiao C, Zhang W, Yuan Z, et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum Gene Ther 2010;21:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrs-Silva H, Dinculescu A, Li Q, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther 2009;17:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.