Abstract
Retinitis pigmentosa (RP) is a group of diseases in which a mutation in one of the large variety of genes causes death of rod photoreceptors. After rods die, cone photoreceptors gradually die resulting in constriction of visual fields and eventual blindness in many patients. Studies in animal models of RP have demonstrated that oxidative damage is a major contributor to cone cell death. In this study, we extended those findings to patients with RP, because compared to control patients, those with RP showed significant reduction in the reduced to oxidized glutathione (GSH/GSSG) ratio in aqueous humor and a significant increase in aqueous protein carbonyl content. In contrast, there was no significant decrease in the serum GSH/GSSG ratio or increase in carbonyl content of serum proteins. These data indicate that patients with RP have ocular oxidative stress and damage in the absence of manifestations of systemic oxidative stress and/or damage indicating that demonstrations of oxidative damage-induced cone cell death in animal models of RP may translate to human RP. These observations lead to the hypothesis that potent antioxidants will promote cone survival and function in patients with RP and that the aqueous GSH/GSSG ratio and carbonyl content on proteins may provide useful biomarkers. Antioxid. Redox Signal. 23, 643–648.
Introduction
Retinitis pigmentosa (RP) is a group of diseases in which a mutation in one of the large variety of genes causes death of rod photoreceptors. The death of rod photoreceptors causes night blindness, the earliest symptom of RP. While difficulty seeing in dim illumination can be inconvenient, patients can continue to read, drive, and function normally at this stage of the disease. However, after rods die, there is gradual degeneration of cones starting in the midperiphery of the retina often resulting in a ring scotoma. Cone cell death progresses on each side of the scotoma posteriorly and anteriorly until the entire peripheral field is extinguished leaving a central island of vision. Cones are eliminated at the margin of the island causing gradual reduction in the size of the remaining central field, which may be eventually extinguished.
Studies in rat and mouse models of RP have provided insights into the mechanism of cone cell death. In humans, rods constitute 95% of the cells in the outer nuclear layer with cone photoreceptors accounting for the other 5%, while in rodents, the ratio is 98 to 2. As rods die, oxygen utilization is markedly reduced, but the choroidal circulation is not regulated and continues to provide the same blood flow and oxygen delivery resulting in markedly elevated oxygen levels in the outer retina (9). The high levels of oxygen increase production of superoxide radicals in cones, both in mitochondria and also in the cytoplasm due to activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (8). The excess superoxide radicals generate other reactive oxygen species, including some that are more damaging such as peroxynitrite, which attack lipids, proteins, and deoxyribonucleic acid (DNA) leaving signatures of oxidative damage that increase in cones over time as cone cell death is occurring (5). These signatures include increased carbonyl content on proteins and lipid hydroperoxides such as malondialdehyde (MDA). Antioxidants reduce oxidative damage and cone cell death in models of RP indicating that oxidative damage is a major contributor to cone cell death (2, 3).
Innovation.
There are extensive data suggesting that oxidative damage contributes to cone photoreceptor cell death in animal models of retinitis pigmentosa (RP). This study demonstrates oxidative damage and ongoing oxidative stress in the eyes of patients with RP, suggesting that the mechanistic data in animal models may translate to patients. This supports proceeding with a clinical trial to investigate the therapeutic effects of potent antioxidants in patients with RP and suggests that aqueous protein carbonyl content and GSH/GSSG ratio should be tested as potential biomarkers.
While the occurrence of oxidative damage in the retinas of rodents or pigs with RP suggests that it is likely to be present in patients with RP, it is difficult to directly test this proposition. In this study, we sought to indirectly test for oxidative stress and oxidative damage in the retinas of patients with RP by using aqueous humor as a surrogate. Since oxidative damage is indiscriminant in its targets, it should affect soluble macromolecules that may exit the retina and enter other ocular compartments as well as those that are integral to the retinal structure and remain in place. Glutathione (GSH) is a thiol that is a critical part of the intracellular antioxidant defense system that neutralizes reactive oxygen species through which it is converted to oxidized glutathione (GSSG). Reduction of the GSH/GSSG ratio is a measure of ongoing oxidative stress in a tissue. GSH is present in the aqueous humor and vitreous, and we hypothesized that it serves as a reservoir of reducing capacity for surrounding tissues, including the retina, and that severe oxidative stress in the retina could result in a reduction in the GSH/GSSG ratio in the aqueous. We sought to determine if compared to control patients, those with RP have a reduction in the GSH/GSSG ratio and an increase in markers of oxidative damage in the aqueous humor and thus providing a biomarker for oxidative damage in the retina.
Results
Increased oxidative damage to aqueous proteins in patients with RP
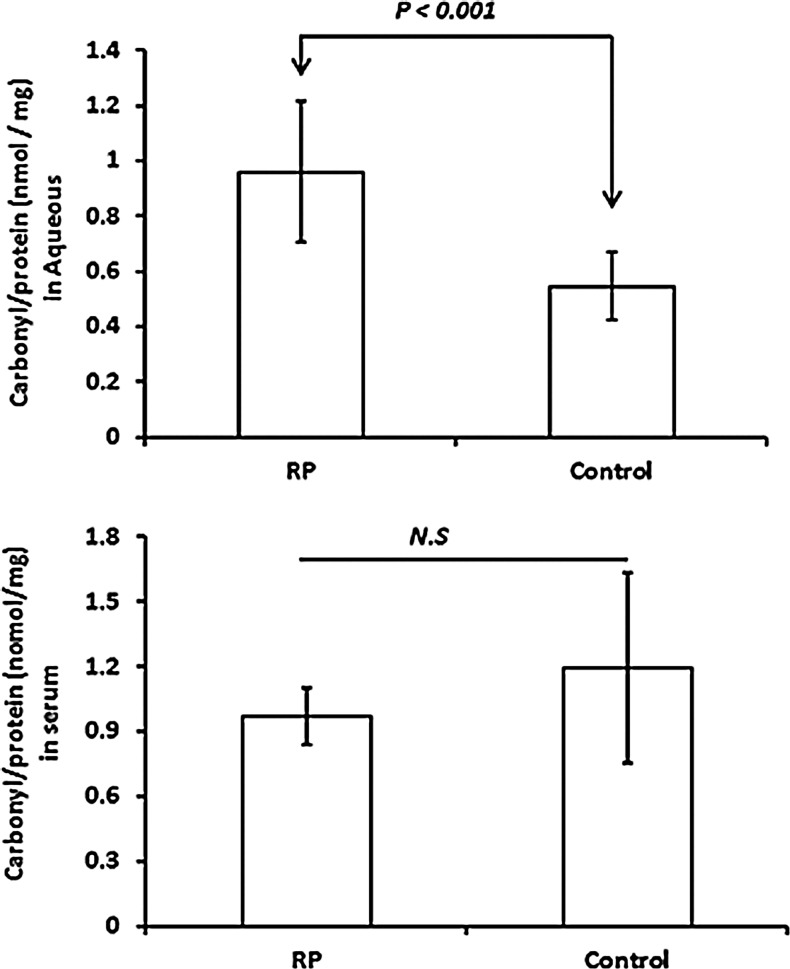
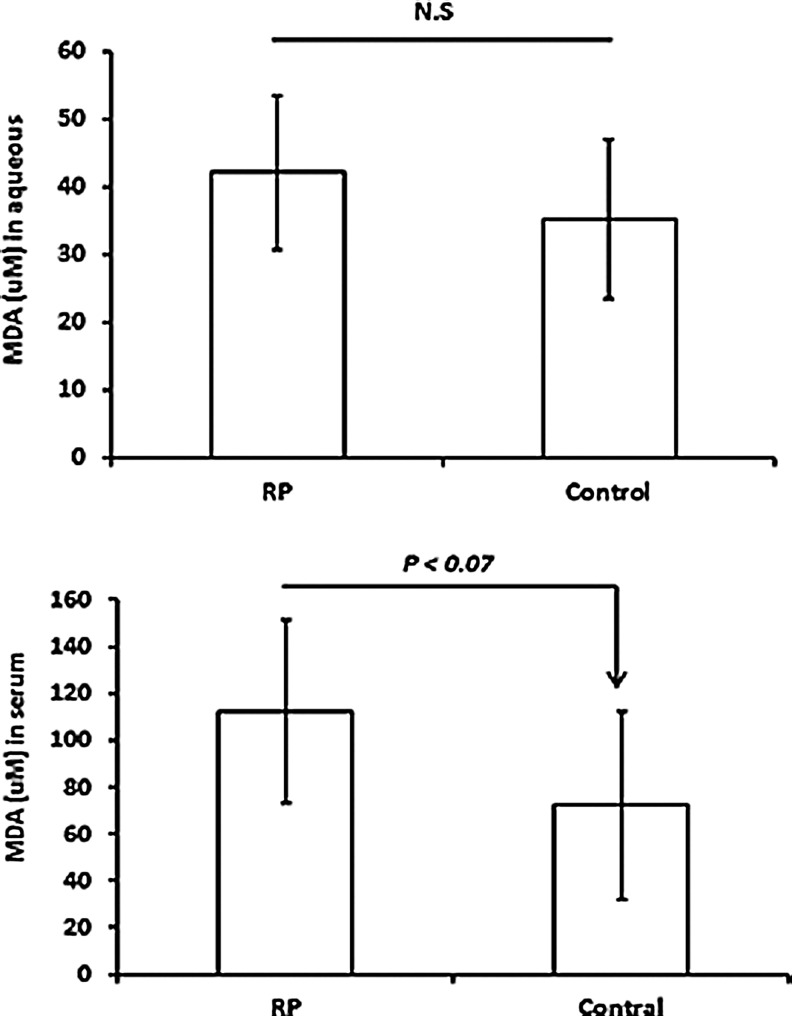
Oxidative damage to proteins causes carbonyl groups to be introduced into side chains, and enzyme-linked immunosorbent assay (ELISA) for carbonyl adducts provides a quantitative measure of oxidative damage to proteins in a tissue. Compared to aqueous samples from control patients who were undergoing vitreous surgery for macular pucker or macular hole, aqueous samples from patients with RP had a significant elevation in mean carbonyl content of proteins (p<0.001; Fig. 1). There was no significant difference in carbonyl content of serum proteins in RP patients compared with controls. Oxidative damage to lipids results in lipid hydroperoxides that break down to form 4-hydroxynonenal, MDA, and acrolein. There was no significant difference in the mean level of MDA in the aqueous or serum of patients with RP compared to those of controls (Fig. 2).
FIG. 1.
Comparison between retinitis pigmentosa (RP) patients and controls in carbonyl content of proteins in aqueous and serum. Aqueous samples from nine RP and nine control patients were assayed for protein carbonyl content and total protein. Control patients had macular pucker or macular hole, but no other eye diseases. Serum samples from eight RP and seven control patients were also assayed. Each bar represents the mean (±standard deviation) carbonyl content per mg total protein and statistical comparisons were made with Student's unpaired t-test. NS, not significant.
FIG. 2.
Comparison between RP and control patients in malondialdehyde (MDA) level in aqueous and serum. Aqueous samples from seven RP and six control patients and serum samples from eight RP and seven controls were assayed for MDA. The bars represent the mean (±standard deviation). Statistical comparisons by Student's unpaired t-test showed no statistically significant differences.
Depletion of GSH in aqueous of RP patients
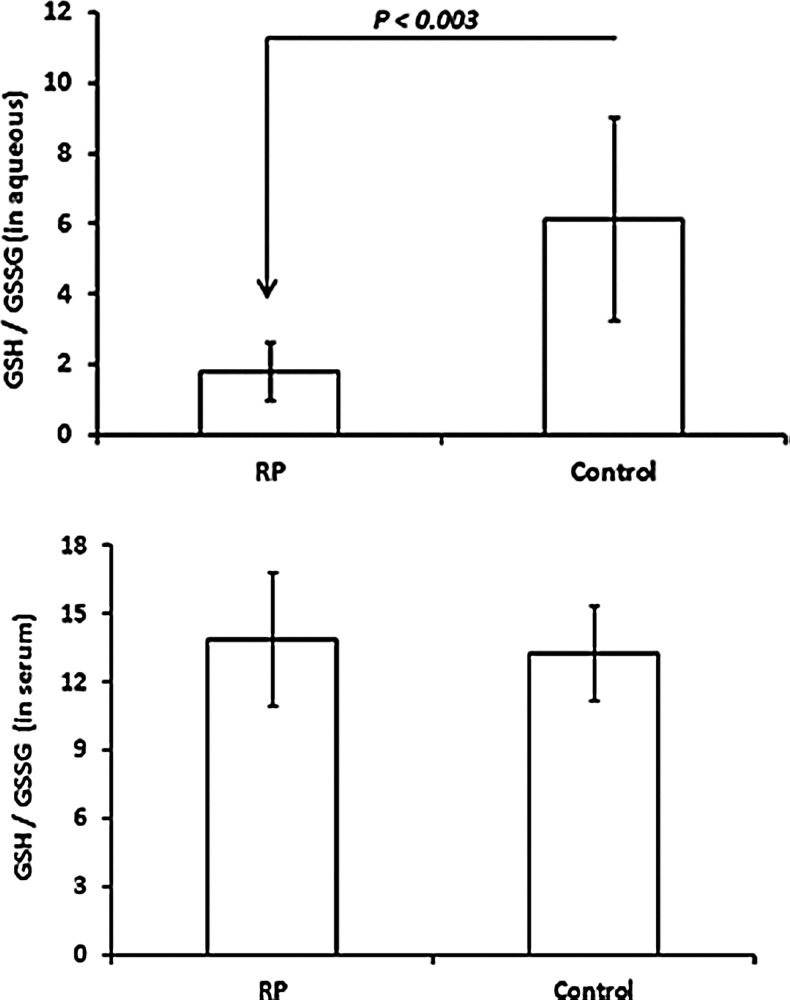
Under oxidative conditions, GSH is reversibly oxidized to glutathione disulfide (GSSG) and under reducing conditions, GSH is regenerated. Thus, the GSH/GSSG ratio provides a measure of ongoing oxidative stress. The mean aqueous GSH/GSSG ratio was significantly reduced in patients with RP compared to controls (p<0.003), but there was no significant difference in the mean serum GSH/GSSG ratio (Fig. 3).
FIG. 3.
Comparison between RP patients and controls in reduced glutathione to oxidized glutathione ratio (GSH/GSSG) in aqueous and serum. Aqueous samples from seven RP and six control patients and serum samples from nine RP and seven controls were assayed for GSH/GSSG ratio. The bars represent the mean (±standard deviation) GSH/GSSH ratio and statistical comparisons were made by Student's unpaired t-test.
No difference in serum SOD3 levels in RP versus control patients
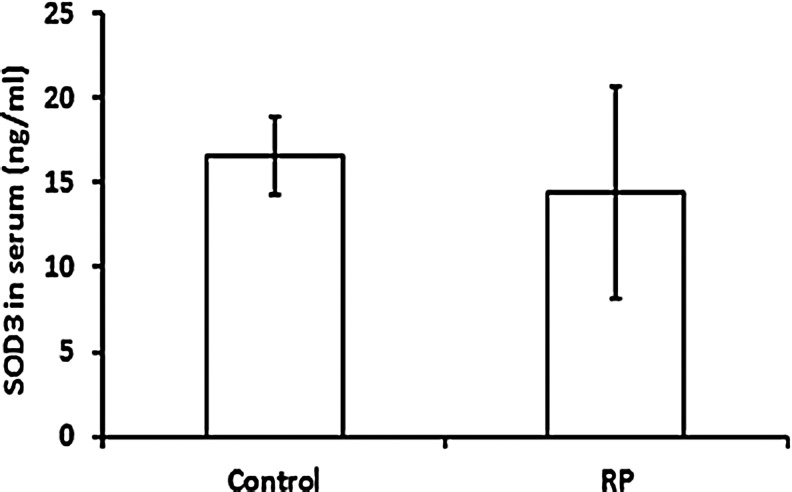
A previous study showed that compared to control patients, a group of patients with RP had a significant reduction in serum superoxide dismutase 3 (SOD3) levels (4). We therefore measured serum SOD3 levels and found that in our patient population, there was no significant difference between RP patients and controls (Fig. 4).
FIG. 4.
Comparison between RP and control patients in superoxide dismutase 3 (SOD3) level in serum. Serum samples were from eight RP and eight control patients were assayed for SOD3. Each bar represents the mean (±standard deviation) SOD3 per ml of sample. Statistical comparison by Student's unpaired t-test showed no significant difference.
Discussion and Future Directions
In mouse and pig models of RP, after rod photoreceptors die, there is progressive oxidative damage first to cones and then to cells of the inner retina (2, 5). In this study, we have demonstrated that human patients with RP also show evidence of ocular oxidative damage, because compared to controls they have increased levels of proteins that have been subject to oxidative damage in the aqueous humor. They also show evidence of ongoing oxidative stress because compared to controls, there is significant depletion of GSH in aqueous. Our data indicate that aqueous MDA levels are not altered in patients with RP. This was worth testing, but the result is not surprising because most lipids are too hydrophobic to enter the aqueous. The oxidative stress in the eyes of patients with RP cannot be explained by a systemic problem that also affects the eyes, because there was no increase in oxidative damage to serum proteins nor was there a reduction in the serum GSH/GSSG ratio. These data indicate ongoing oxidative stress and oxidative damage in the eyes of patients with RP and suggest that the extensive evidence in mouse and pig RP models implicating oxidative damage as a mechanism of cone cell death (2, 3, 5–8) may be applicable to patients with RP.
A previous study demonstrated reduced total antioxidant capacity (TAC) in the aqueous of patients with RP compared to controls (4). Since GSH likely contributes to TAC, our observation of reduced GSH/GSSG ratio in patients with RP is consistent with the previously observed reduction in TAC. However, there may be something unusual about the population of RP patients studied in the previous report, because compared to controls, they had significant elevation of thiobarbituric acid reactive substances in serum suggesting an increase in oxidative damage to lipids throughout the body. The RP patients enrolled in our study had no difference in serum MDA levels compared to controls, suggesting that they did not have an increase in lipid peroxidation in the periphery. Compared to controls, our patients with RP also showed no significant increase in serum protein carbonyl content, suggesting against generalized oxidative damage to proteins throughout the body and they had no significant decrease in serum GSH/GSSG indicating against systemic oxidative stress. In the previous study (4), patients with RP had a significant reduction in serum SOD3 compared to controls, whereas this was not the case in the RP patients whom we studied. Therefore, some of the RP patients included in the previous study may have had a systemic problem leading to widespread oxidative stress throughout the body, which makes the findings of the study difficult to interpret.
Our data allow us to generate the hypothesis that aqueous protein carbonyl content and GSH/GSSG ratio are biomarkers for disease activity in individual patients with RP. The most definitive way to test this hypothesis is to incorporate measurement of aqueous protein carbonyl content and GSH/GSSG ratio into a clinical trial testing the effect of antioxidant therapy on functional outcomes in patients with RP. The first step in this process will be to determine if the baseline aqueous protein carbonyl content is reduced and the GSH/GSSG ratio is increased by the therapy and if so, what percentage change in these parameters correlates with functional benefit. Validation of the parameters as biomarkers for disease activity would greatly facilitate future clinical trials. Longitudinal studies in which serial measurements of aqueous protein carbonyl content and GSH/GSSG ratio are correlated with rate of reduction in visual fields and/or reduction in the length of ellipsoid zone on optical coherence tomography will also be valuable.
In summary, patients with RP show evidence of ongoing oxidative stress and oxidative damage in their eyes suggesting that previous demonstration in mouse and pig models of RP that cones die from oxidative damage may translate to humans with RP. These data support moving forward with clinical trials to test whether administration of potent antioxidants can slow loss of function and death of cones in patients with RP and as part of those trials, it will be important to determine if therapy-induced changes in aqueous protein carbonyl content and GSH/GSSG ratio occur and correlate with functional outcomes and thus validating them as biomarkers for future trials.
Notes
Subjects
The study was registered on clinicaltrials.gov (NCT01949623), the protocol was approved by the Johns Hopkins University Institutional Review Board, and all participating patients provided informed consent. The diagnosis of RP was made by a retina specialist with expertise in inherited retinal degenerations (H.P.S.) based upon eye examination, full-field electroretinography, visual field testing, and optical coherence tomography. Nine patients with RP were included in this study. An aqueous sample was obtained by administering topical anesthesia and a drop of 5% povidone–iodine into the study eye, placing a lid speculum, inserting a 30-gauge needle on a 1-ml syringe into the anterior chamber at the limbus, and gently aspirating the aqueous. Samples were frozen and stored at −80°C until assayed. A blood sample was also obtained and after clotting, the blood was centrifuged. Serum was placed in a small tube and stored at −80°C until assayed. Eleven control patients who were undergoing vitreous surgery for macular pucker or macular hole and had no other retinal or ocular disease were included. At the beginning of surgery after the eye was anesthetized and washed with povidone–iodine, a 30-gauge needle on a 1-ml syringe was inserted into the anterior chamber at the limbus and the aqueous was aspirated. Serum samples were obtained in 7 of the 11 patients; 2 patients did not have blood drawn due to poor venous access and in 2 patients there was hemolysis making the samples unusable. When aqueous samples were thawed, it was noted that the volume ranged from 50 to 150 μl and not all assays could be done with low-volume samples. The protein concentration of each sample was measured with a protein assay kit (Bio-Rad, Hercules, CA) using the manufacturer's instructions.
Measurement of protein carbonyl content
Protein carbonyl content was measured with an OxiSelect Protein Carbonyl ELISA Kit (Cell Biolabs, Inc., San Diego, CA) using the manufacturer's instructions. Aqueous samples from nine patients with RP and nine control patients were assayed. Serum samples were available from eight of the patients with RP and seven control patients. In brief, 50 μl of each sample (aqueous and serum) or protein carbonyl standard was added to a well of a 96-well plate and incubated at 4°C overnight. All washes described throughout the Methods section were done with phosphate-buffered saline (PBS). After washing each well, 100 μl of dinitrophenylhydrazine was added and incubated for 45 min at room temperature. After three washes, the blocking solution was added to each well and the plate was incubated for 1 h at room temperature. After three washes, a primary antibody was added to each well and incubated for 1 h at room temperature. Wells were washed thrice, a secondary antibody was added, and the plate was incubated for 1 h at room temperature. Wells were washed thrice, 100 μl of substrate was added to each well, the plate was incubated at room temperature for 25 min, and the reaction was stopped by adding 100 μl of stop solution to each well. Absorbance at 450 nm was read on a plate reader. The readings from the standards were used to generate a standard curve and the protein carbonyl content of each sample in nmol/mg protein was determined by plotting its absorbance value on the standard curve. Each bar represents the mean (±standard deviation) protein carbonyl content per mg protein and statistical comparison was made by Student's unpaired t-test.
Measurement of GSH/GSSG ratio
There was sufficient aqueous sample volume for the assays for seven RP patients and six control patients. Serum samples were available for nine RP patients and seven control patients. The GSH/GSSH ratio was determined by separate measurement of GSH and GSSG. For measurement of GSH, 50 μl of each sample (aqueous or serum), a GSH standard (ranging from 3 to 320 pmol), or a PBS blank was added to a well of a 96-well plate. After addition of 100 μl of a mixture of 2-nitrobenoic acid, NADPH, PBS, and GSH reductase, plates were incubated at room temperature for 2 min and then absorbance at 405 nm was read on a plate reader. The absorbance values of the standards were plotted to generate a standard curve, which was used to calculate the GSH level of each sample. For measurement of GSSG, 50 μl of each sample (aqueous or serum), a GSSG standard (ranging from 3 to 320 pmol), or blank PBS buffer was added to a well of a 96-well plate. A mixture of 2 μl of 2-vinylpyridine and 6 μl of triethanolamine was added to each well. After addition to each well 100 μl of a mixture of 2-nitrobenoic acid, NADPH, PBS, and GSH reductase, the plate was incubated at room temperature for 2 min, and then absorbance at 405 nm was read on a plate reader. The level of GSSG in each sample was calculated by comparison to the standard curve. The bars represent the mean (±standard deviation) GSH/GSSH ratio and statistical comparison was made by the unpaired t-test.
Measurement of MDA
There was sufficient aqueous sample volume for the assays for seven RP patients and seven control patients. Serum samples were available for eight RP patients and seven control patients. The concentration of MDA in each sample was measured as previously described (1). Malonaldehyde bisdimethyl (Sigma, Saint Louis, MO) was used to prepare samples with known MDA concentrations between 0 and 20 μM to generate a standard curve. Ten microliters of serum or 15 μl of aqueous was added to 90 or 85 μl of 100% methanol and then 200 μl of 20% trichloroacetic acid (Sigma) containing 2 μmol of ferrous sulfate (FeSO4; Sigma) and 100 μl of 0.67% thiobarbituric acid (Sigma) were added to each tube. Samples were incubated at 100°C for 30 min and after cooling at 4°C for 10 min, 200 μl of chloroform was added and samples were thoroughly mixed and centrifuged at 16,000 g for 10 min at 4°C. Supernatants were transferred to wells of a 96-well plate and 1 μl of 2% (in ethanol) butylated hydroxyanisole (Sigma) was added to each well. Absorption was measured at 532 nm for samples and standards and the MDA concentration of each sample was determined by plotting its absorption on the standard curve. The bars represent the mean (±standard deviation). Statistical comparison was made by Student's unpaired t-test.
Serum SOD3
Serum SOD3 was measured by using an SOD3 ELISA Kit (Abnova, Taipei, Taiwan) according to the manufacturer's instructions. Serum samples were available from seven patients with RP and seven control patients. In brief, 100 μl of each serum sample or SOD3 standard was added to duplicate wells of a 96-well plate and incubated for 2 h at room temperature. After washing, 100 μl of working secondary antibody solution was added to each well and incubated for 1 h at room temperature. After three washes, a blocking solution was added to each well and the plate was incubated for 1 h at room temperature. After three washes, a primary antibody was added to each well and incubated for 1 h at room temperature. After three washes, 100 μl of working AV-HRP solution was added to each well and the plate was incubated for 30 min at room temperature. Wells were washed thrice, 100 μl of substrate was applied to each well, the plate was incubated at room temperature for 10 min, and the reaction was stopped by adding 100 μl of stop solution. Absorbance at 450 nm was read on a plate reader. The readings from the standards were used to generate a standard curve and the SOD3 of each sample was determined by plotting its absorbance value on the standard curve. Each bar represents the mean (±standard deviation) SOD3 per ml of sample and statistical comparison was made by Student's unpaired t-test.
Abbreviations Used
- DNA
deoxyribonucleic acid
- ELISA
enzyme-linked immunosorbent assay
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate
- PBS
phosphate-buffered saline
- RP
retinitis pigmentosa
- SOD3
superoxide dismutase 3
- TAC
total antioxidant capacity
Acknowledgments
This study was funded by Foundation Fighting Blindness, Columbia, MD and EY05951 from the National Eye Institute. P.A.C. is the George and Dolores Eccles Professor of Ophthalmology and Neuroscience. H.P.S. is supported by the Foundation Fighting Blindness Clinical Research Institute (FFB CRI) and a grant to FFB CRI by the U.S. Department of Defense USAMRMC TATRC, Fort Meade, MD (grant numbers W81-XWH-07-1-0720 and W81XWH-09-2-0189); The Shulsky Foundation (New York, NY); Ocular Albinism Research Fund (Clark Enterprises, Inc., Bethesda, MD); Unrestricted grant to the Wilmer Eye Institute from Research to Prevent Blindness; Baylor-Johns Hopkins Center for Mendelian Genetics (National Human Genome Research Institute, NHGRI/NIH; Identification number: 1U54HG006542-01). H.P.S. is the Dr. Frieda Derdeyn Bambas Professor of Ophthalmology. R.W.S. is supported by the Austrian Scientific Funds (FWF, project number # J3383-B23).
References
- 1.Esterbauer H. and Chesseman KH. Determination of aldehydic lipid peroxidation products: malondialdehyde, and 4-hydroxynonenal. Methods Enzymol 186: 407–413, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Komeima K, Rogers BS, Lu L, and Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A 103: 11300–11305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SY, Usui S, Zafar AB, Oveson BC, Jo YJ, Lu L, Masoudi S, and Campochiaro PA. N-acetylcysteine promotes long term survival of cones in a model of retinitis pigmentosa. J Cell Physiol 226: 1843–1849, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Fernandez de la Camara C, Salom D, Sequedo MD, Hervas D, Marin-Lambies C, Aller E, Jaijo T, Diaz-LLopis M, Millan JM, and Rodrigo R. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLos One 8: e74223, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen J, Yan X, Dong A, Petters RM, Peng Y-W, Wong F, and Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol 203: 457–464, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Usui S, Komeima K, Lee SY, Jo Y-J, Ueno S, Rogers BS, Wu Z, Shen J, Lu L, Oveson BC, Rabinovitch PS, and Campochiaro PA. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther 17: 778–786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usui S, Oveson BC, Iwase T, Lu L, Lee SY, Jo YJ, Wu Z, Choi EY, Samulski RJ, and Campochiaro PA. Overexpression of SOD in retina: need for increase in H2O2-detoxifying enzyme in same cellular compartment. Free Radic Biol Med 51: 1347–1354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usui S, Oveson BC, Lee SY, Jo YJ, Yoshida T, Miki A, Miki K, Iwase T, Lu L, and Campochiaro PA. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem 110: 1028–1037, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu DY, Cringle SJ, Su EN, and Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci 41: 3999–4006, 2000 [PubMed] [Google Scholar]