Abstract
Toxoplasma gondii is an obligate intracellular protozoan that has a major importance in public health, in addition to veterinary medicine. Therefore, the development of an effective vaccine for controlling toxoplasmosis is an important goal. Excretory/secretory antigens (ESA), were previously identified as potential vaccine candidates, proved to play important roles in the pathogenesis and immune escape of the parasite. In addition, autoclaved Toxoplasma vaccine (ATV) is a special type of killed vaccine, recently characterized. The aim of the present work was, to compare between excretory/secretory and ATV against RH strain of T. gondii in mice based on; parasitological and histopathological levels. Tachyzoites were harvested from peritoneal exudates of infected mice and were used for challenge infection and vaccine preparation. BCG was used as an adjuvant. Mice were allocated equally into five groups; they were vaccinated intradermally over the sternum. The results of this study showed that the survival time after challenge, extended up to 16 days in ESA vaccinated group and up to 15 days in autoclaved Toxoplasma vaccinated group. ESA vaccinated group exhibited a profound decrease in parasite load following parasite challenge with a higher percentage of reduction in parasite count in all examined organs than the autoclaved Toxoplasma vaccinated group. The histopathological picture of the liver in both immunized groups, revealed marked reduction in the pathological changes observed as compared to controls, especially in ESA vaccinated group. It was concluded that vaccination with ESA showed more promising results versus ATV, as demonstrated by the survival rate of vaccinated mice, tachyzoites count and histopathological examination.
Keywords: Toxoplasma gondii, Excretory/secretory antigens, Autoclaved Toxoplasma vaccine, RH strain
Introduction
Toxoplasma gondii is an obligate intracellular coccidian protozoan which disseminates throughout the body causing toxoplasmosis (Dimier-Poisson et al. 2003). It is considered an important food-borne parasitic disease, transmitted primarily through meat consumption, mainly pork and lamb. It is also disseminated into the environment through food contaminated with oocysts shed in the stool of cats (Costa-Silva et al. 2008).
In humans, toxoplasmosis is generally benign in immunocompetent hosts usually resulting in mild, influenza-like symptoms. However, there are frequent reports of eye disease following acquired infection (Innes 2010) and primary infection during pregnancy can result in abortion or severe neonatal malformations (Liu et al. 2009). Furthermore, under conditions of immunosuppression, such as those with AIDS, neoplastic disease and transplant recipients, the infection may be reactivated resulting in fatal encephalitis (Yuan et al. 2007).
So far, there is no drug available that has the ability to eliminate the parasite, some drugs can help limit the multiplication of the parasite during the active stage of replication. However, once the parasite encysts in the tissues, the drug loses its effectiveness (Coombs and Muller 2002; Innes 2010). Therefore, an obvious long-term goal of many scientists is to develop of an effective vaccine, which would be of great medical and veterinary value (Tan et al. 2011).
The parasite causes a spectrum of different diseases and clinical symptoms within the intermediate hosts and following infection most hosts develop adaptive humoral and cell-mediated immune responses. The development of protective immunity to T. gondii following natural infection in many host species has led researchers to look at vaccination as a strategy to control disease, parasite multiplication and establishment in hosts (Innes et al. 2009).
Many efforts have been made to develop vaccines against T. gondii to reduce oocyst shedding in cats and tissue cyst formation in mammals over the last 20 years, but only a live-attenuated vaccine based on the S48 strain has been licensed for veterinary use (Verma and Khanna 2012; Zhang et al. 2013). To date, there have been numerous attempts at developing successful vaccines against human parasites, despite these substantial efforts by many laboratories, there is currently no licensed vaccine available for human against parasitic diseases (Eissa et al. 2012).
Several T. gondii antigens, such as the major immunodominant surface antigen SAG-1 (Bullow and Boothroyd 1991; Khan et al. 1991; Darcy et al. 1992). Excretory/secretory antigens (ESA), have been identified as potential vaccine candidates, they are also thought to play an important role in the pathogenesis and immune escape of the parasite (Yamamoto et al. 1998). The ESA can stimulate a better cell-mediated immune response as compared to soluble or cyst antigens, therefore, ESA antigen is an excellent candidate for research as immunizing agents against T. gondii infection (Rahman and Anuar 1992). Daryani et al. (2003) compared between, Total lysate antigen and ESA-fractions and they suggested that ESA-F2 could be used as a good candidate for the development of new immunization strategy against toxoplasmosis.
Autoclaved vaccine is a special type of killed vaccine that contains all the antigens of the parasite resulting in better stimulation of the immune system, resembling what occurs in the natural course of infection (Modabber 1995). Eissa et al. (2012) reported that Toxoplasma autoclaved vaccine (ATV) resulted in better stimulation of the immune system when compared to total lysate antigen. Thus, the aim of this study was to compare ESA and ATV as vaccine candidates based on parasitological and histopathological levels.
Materials and methods
Parasite
Tachyzoites of the highly virulent RH strain of T. gondii were maintained in our laboratory by intraperitoneal passages into a laboratory bred Swiss albino mice every three days. Tachyzoites were harvested from peritoneal exudates of infected mice and were used for challenge infection and vaccine preparation. Peritoneal exudates were passed 10 times through a 27-gauge needle to release the intracellular tachyzoites and were harvested in RPMI-1640 medium (Gibco, Invitrogen, USA) and washed twice in the same medium containing 100 IU/ml penicillin and 100 μg/ml streptomycin. The concentration of tachyzoites was determined after adequate dilution in RPMI-1640 medium by enumeration in a Neubauer counting chamber at 400× magnification (Araujo and Remington 1984).
Vaccines preparation
ATV preparation
The peritoneal exudates of several mice previously infected with T. gondii for three days were collected. They were centrifuged at 500×g for 5 min to sediment heavier particles and leukocytes. Then, the supernatant was re-centrifuged at 2,000×g for 5 min. The sediment was washed three times with PBS (pH 7.4) by centrifugal sedimentation at 2,000×g for 5 min. The pellet was resuspended in saline to give the required concentration of 8 × 106 in a volume of 0.1 PBS. The vaccine was dispensed into autoclaved screw capped three ml vials, (1 ml/vial) that were subjected to autoclaving for 15 min, at 120 °C, under pressure of 15 lb and kept at −20 °C until used. Vaccination dose was 0.1 ml of the vaccine containing 8 × 106 T. gondii tachyzoites (Eissa et al. 2012).
ESA preparation
For preparing ESA in cell-free incubation media, each 1.5 × 108 RH strain tachyzoites per milliliter were aliquoted into 10 tubes and incubated at 37 °C for 3 h under mild agitation. Tubes were centrifuged at 1,000×g for 10 min and their supernatants were filtered by passing through 0.22 μm Millipore membrane filter (Millipore Corp., Bedford, MA, USA), and stored at –20 °C until use (Zenner et al.1999).
Adjuvant
Bacille Calmette–Guérin (BCG) (Japan BCG laboratory) was used as an adjuvant in a dose of 5 × 105 CFU/0.1 ml injection. Animals received the adjuvant intradermally over the sternum.
Experimental animals, infection and vaccination schedule
Experiments were carried out on 75 Swiss albino mice, 6–8 weeks old. They were purchased from the animal house of the Medical Research Center, Faculty of Medicine, Ain Shams University. They were allocated equally into five groups (15 mice each), group 1 = ATV+BCG, group 2 = ESA+BCG, group 3 = BCG, group 4 = infected control and group 5 = non infected control. Groups 1 and 2 mice were vaccinated intradermally over the sternum by ATV and ESA, respectively; in three doses of 0.1 ml. BCG was included in equal volumes in all inoculants (Krahenbuhl et al. 1972). The first vaccination dose was given, followed 10 days later by the second dose and the third dose was given 20 days after the second dose. Mice of groups 1 and 2 were challenged four weeks after the last vaccination dose by 2,500 viable tachyzoites of RH strain injected intraperitoneally, the same dose of viable tachyzoites was used for infecting mice of group 3 and 4 (Araujo et al. 1992).
Assessment of vaccine efficacy
Parasitological study
The survival periods
The survival periods were recorded daily until all mice died; survival was recorded up to 16 days after challenge. Then cumulated frequency of mortality and survival percent were determined using the Kaplan–Meier curve (Yap et al. 1998).
Estimation of parasite count
Impression smears were made from liver, brain, spleen and lung, stained with Giemsa stain. Counting of T. gondii tachyzoites in different tissue smears were carried out using oil immersion objectives (100×) lens. The mean of ten different fields from each organ of each mouse and the mean of each group of mice were calculated (El-Temsahy et al. 2002).
Histopathological study of the liver, brain, spleen and lung
Specimens from the organs were fixed in 10 % formalin, dehydrated in ascending series of ethyl alcohol, cleared in xylol, and then embedded in paraffin. Serial sections, five microns thick, were cut using microtome, and processed for staining using haematoxylin and eosin stain. Sections were put in xylol, passed in descending series of ethyl alcohol, rinsed in water, stained in Harris haematoxylin, washed under running water, then counter-stained using eosin. They were dehydrated using ascending series of ethyl alcohol, and then mounted in Canada balsam (Drury and Wallington 1980).
Semiquantitative histopathological assessment of the submitted specimens from tested groups was done for tissues of the liver, spleen, lung and brain tissue. Liver sections were scored according to (Batts and Ludwig 1995). Score (0) was given for liver specimens showing an almost normal hepatic tissue or mild portal tract inflammation. Score (1) was given for liver specimens showing mild periportal inflammation, kupffer cells hyperplasia in addition to previously described changes. Score (2) was given to liver specimens showing beading of hepatic sinusoids by mononuclear inflammatory cells in addition to previously described changes. Score (3) was given for liver specimens showing focal central necroinflammatory areas±hepatocyte ballooning degeneration in addition to previously described changes. Score (4) was given to liver specimens showing multifocal necroinflammatory areas, disruption in hepatic lobular architecture in addition to previously described changes.
Statistical analysis
SPSS software package version 19.0 was used for data analysis. Quantitative data was expressed using mean and standard deviation. Differences were considered significant if P values were equal to or less than 0.05 and highly significant when the P value was <0.001.
Ethical consideration
All animal studies presented here have been approved by the local government based on national regulations for animal experimentation.
Results
Survival rate
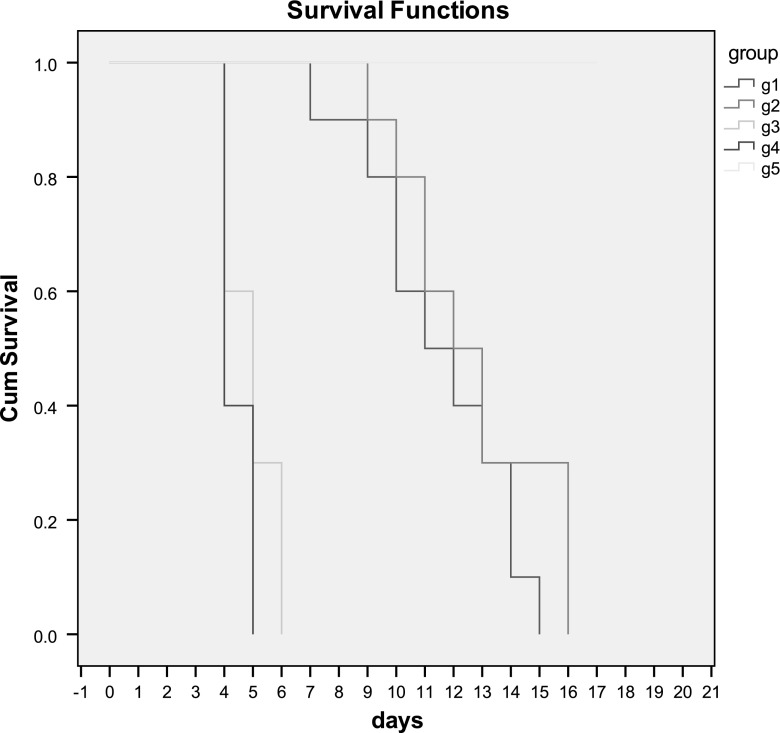
Mice of the infected control group (group 4), all died by the fifth day post infection with survival rate of 40 % on the 4th day. In the BCG infected control group (group 3) the survival rate was 60 % on the 4th day, 30 % on the 5th day, and 0 %on the 6th day. On the other hand, animals that were vaccinated with either ATV (group 1) or ESA (group 2) in combination with BCG as an adjuvant, showed significant increase in their survival rates, which were 100 % on the fifth day post infection, dropped to 90 % on the 7th day for group 1 and on the 9th day for group 2. The maximum survival time extended up to 15th and 16th days post infection in ATV (group 1) and ESA (group 2) vaccinated groups, respectively, survival in different groups of mice was shown in (Fig. 1).
Fig. 1.
Kaplan–Meier curve for survival in mice of group 1, group 2, group 3 and group 4 challenged with 2,500 RH strain tachyzoites
No significant difference was found between survival curves of groups 1 and 2, no significant difference between survival curves of groups 3 and 4, but highly significant difference was found between survival curves of groups 1 and 3; groups 1 and 4; groups 2 and 3 and groups 2 and 4. The mean of survival in group 1, group 2, group 3 and group 4 was 11.5, 12.7, 4.9 and 4.4 days respectively (Table 1).
Table 1.
Pair wise comparisons between survival curve of the group 1, group 2, group 3, group 4 and group 5 and mean of survival
| Group 1 | Group 2 | Group 3 | Group 4 | |||||
|---|---|---|---|---|---|---|---|---|
| χ2 | P value | χ2 | P value | χ2 | P value | χ2 | P value | |
| Group 1 | 1.557 | 0.212* | 20.169 | 0.000** | 19.793 | 0.000** | ||
| Group 2 | 1.557 | 0.212* | 20.169 | 0.000** | 19.793 | 0.000** | ||
| Group 3 | 20.169 | 0.000** | 20.169 | 0.000** | 2.580 | 0.108* | ||
| Group 4 | 19.793 | 0.000** | 19.793 | 0.000** | 2.580 | 0.108* | ||
| Group 5 | 11.789 | 0.001*** | 9.120 | 0.003*** | 19.069 | 0.000** | 19.81 | 0.000** |
| Means and (SD) of survival | 11.5 ± 2.54 (SD) | 12.7 ± 2.58 (SD) | 4.9 ± 0.87 (SD) | 4.4 ± 0.51 (SD) | ||||
SD standard of deviation
* P > 0.05 no significant difference; ** P < 0.001 highly significant difference; *** statistically significant at P < 0.05
Parasite count
Toxoplasma gondii tachyzoites were detected by Giemsa stained impression smears from spleen, liver, lung and brain of animals infected with 2,500 tachyzoites of the virulent RH-strain of T. gondii. There was a non statistically significant difference in the mean count of tachyzoites, in the same examined organ, between infected (group 4) and BCG-infected (group 3) controls.
On the other hand, mice that were vaccinated with ATV or ESA showed a statistically significant difference when compared to controls in all organs except for spleen in ESA vaccinated group showed highly significant difference when compared to infected control, with a percentage reduction of 80.8 % in liver, 60.2 % in brain, 72.7 % in spleen and 74.3 % in lung for the ATV vaccinated group and a percentage reduction of 85.1 % in liver, 68.2 % in brain, 84.6 % in spleen, and 82.3 % in lung for the ESA vaccinated group (Table 2).
Table 2.
Mean parasitic count and percentage reduction in the different organs in Toxoplasma vaccinated and control groups
| Liver | Brain | Spleen | Lung | ||
|---|---|---|---|---|---|
| Group 1 | 3.120 ± 1.4856 | 1.980 ± 0 .311 | 4.80 ± 1.304 | 3.140 ± 1.2422 | |
| Group 2 | 2.420 ± 0.8289 | 1.580 ± 0.370 | 2.720 ± 0.6760 | 2.340 ± 0.6542 | |
| Group 3 | 15.720 ± 4.92 | 4.70 ± 1.185 | 13.80 ± 4.324 | 11.960 ± 4.620 | |
| Group 4 | 16.320 ± 4.55 | 4.980 ± 1.448 | 17.60 ± 2.881 | 13.260 ± 4.448 | |
| Group 5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| P | 1–2 | 0.262* | 0.016** | 0.068* | 0.293* |
| 1–3 | 0.002** | 0.006** | 0.011** | 0.011** | |
| 1–4 | 0.001** | 0.012** | 0.001** | 0.012** | |
| 2–3 | 0.003** | 0.006** | 0.005** | 0.008** | |
| 2–4 | 0.002** | 0.006** | 0.000*** | 0.008** | |
| 3–4 | 0.850* | 0.762* | 0.268* | 0.707* | |
| Group 1 reduction % | 80.8 % | 60.2 % | 72.7 % | 74.3 % | |
| Group 2 reduction % | 85.1 % | 68.2 % | 84.6 % | 82.3 % |
Group 1 ATV+BCG, Group 2 ESA+BCG, Group 3 BCG infected control, Group 4 infected control, Group 5 normal non infected
* Not significant; ** P < 0.05 statistically significant difference; *** p < 0.001 statistically highly difference
There was a non statistically significant difference between the two vaccinated groups in liver, spleen and lung. There was statistically significant difference between the two vaccinated groups in brain with the least count of tachyzoites in the ESA vaccinated group in all examined organs.
Histopathological study
Semiquantitative histopathological assessment of the specimens from tested groups was done for tissues of the liver, spleen, lung and brain tissue.
According to the scoring criteria, examination of liver specimens revealed the following results:
Group 1 (ATV vaccinated group) scored as ‘0’ in 60 % of specimens, the remaining 40 % scored as ‘1’ where liver specimens showing mild peri-portal inflammation and kupffer cells hyperplasia (Fig. 2). All liver specimens of group 2 (ESA vaccinated group) scored as ‘0’ (100 %) displaying a near normal hepatic tissue or mild portal tract inflammation (Fig. 3). Group 3 (BCG group) revealed a score of ‘2’ in 40 % of cases, exhibiting beading of hepatic sinusoids by mononuclear inflammatory cells, whereas the rest of this group (60 %) scored as ‘3’ showing focal central necroinflammatory areas with or without hepatocyte ballooning degeneration (Fig. 4). Group 4 (infected control) were given a score of ‘3’ in all liver specimens (100 %) expressing focal central necroinflammatory areas with or without hepatocyte ballooning degeneration (Fig. 5).
Fig. 2.
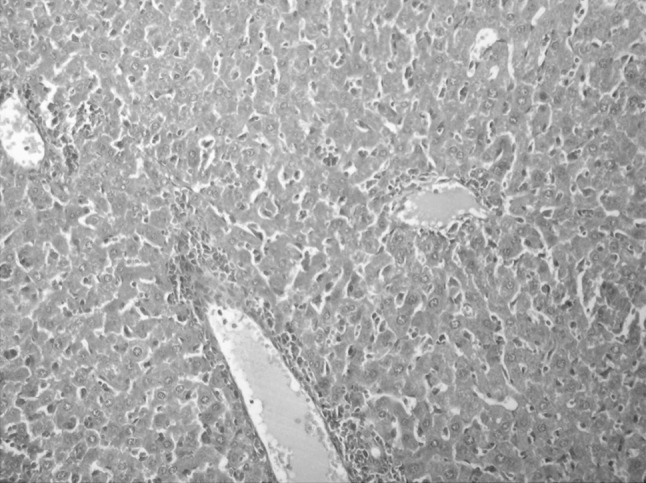
Liver specimen of group 1 showing mild inflammatory changes with preserved hepatic architecture (H&E ×200)
Fig. 3.

Liver specimen of group 2 showing mild portal inflammation and preserved architecture (H&E ×200)
Fig. 4.
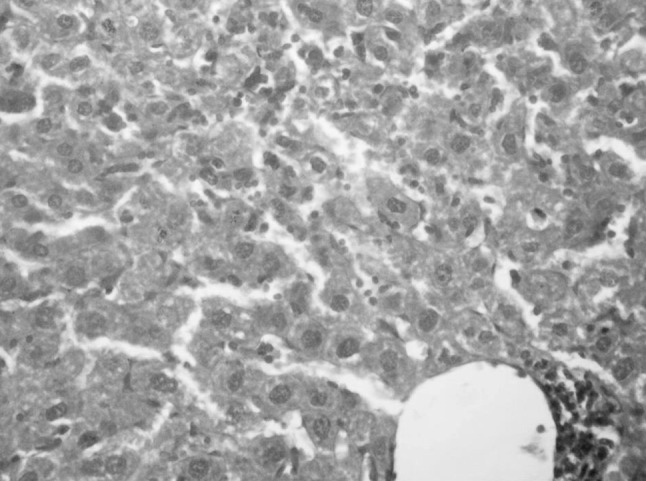
Liver specimen of group 3 central necroinflammatory areas with beading of hepatic sinusoids by mononuclear inflammatory cells (H&E ×400)
Fig. 5.

liver specimen of group 4 showing collapsing architecture with necroinflammatory areas (black arrows) and marked ballooning of hepatocytes (H&E ×200)
Brain sections of all group revealed nonspecific findings ranging from mononuclear infiltration to focal degenerative features (Fig. 6). Spleen sections revealed lack of discrimination between of both white and red pulp with geographical areas of necrosis (Fig. 7). Variations in histopathological findings among examined brain as well as splenic specimens of different groups were subtle. Lung sections revealed congestion, variable interstitial pneumonic consolidations particularly perivascular in addition to pneumocyte type 2 hyperplasia (Fig. 8). All of these changes were generally more evident in group 3 and group 4 as compared to group 1 and group 2.
Fig. 6.

Brain specimen of group 1 showing lymphoid aggregates and degenerative features (H&E ×200)
Fig. 7.

Splenic specimen of group 4 with lack of discrimination of red and white pulp and diffuse necrosis (H&E ×100)
Fig. 8.

Lung section of group 4 showing interstitial perivascular pneumonia (H&E ×200)
Discussion
Toxoplasma gondii is a parasite causing toxoplasmosis in healthy persons in addition to the immunocompromised individuals such as AIDS patients and in newborns during congenital infection (Choi et al. 1997). Although most infections are asymptomatic and self-limiting in immunocompetent hosts, but individuals remain chronically infected, which may result in toxoplasmosis by reactivation of the tissue-cyst (Kim et al. 2000). The importance of T. gondii infection makes the development of an effective vaccine an attractive option to control disease and to limit the spread of the parasite both within the host and in the environment. As T. gondii is an obligate intracellular parasite, protective immunity to infection is generally considered to be cell-mediated involving both the innate and adaptive immune responses, which are important in protecting the host and limiting multiplication of the parasite so, the majority of infected individuals demonstrate mild symptoms, and long-term complications are rare (Dubey 2004). The lack of CD8_T and CD4_T cells lead to increased parasite multiplication within the tissues (Casciotti et al. 2002; Gazzinelli et al. 1991). Other immune mechanisms of protection could act otherwise or in concert with such a cellular mediated protection; therefore, it would appear that cell-mediated immune responses are involved in protective immunity and recovery from a primary infection, while specific antibody may be more important in defending the host following a secondary challenge (Innes and Vermeulen 2006). It was possible to induce solid protection against infection with T. gondii virulent strains by immunization with live attenuated variants, the live attenuated S-48 strain of Toxoplasma was proven to be an effective vaccine for veterinary use; however, it is not widely accepted because of its side effects, short shelf life and high cost (Buxton 1993). Live vaccines would not be considered safe to use in people as they carry risks of infection and unexpected severe harmful mutations (Bout et al. 2002).
As Toxoplasma secretion is an important event in the production of circulating antigens during the early stages of toxoplasmosis (Hughes and Vanknapen 1982), ESA was shown to be highly immunogenic (Saadatnia et al. 2012). ESA might be one of the first targets for the immune system; the protective potentialities of ESA would suggest an alternative approach for vaccine development (Daryani et al. 2003).
The efficacy of autoclaved vaccine, against leishmaniasis (Tafaghodi et al. 2008), Schistosomiasis (Eissa et al. 2003a) and Trichinellosis (Eissa et al. 2003b) was reported. Eissa et al. (2012), showed that significant immune responses were mounted against toxoplasmosis following vaccination with ATV. In this context, this work was carried out to compare between ESA vaccine against acute toxoplasmosis in comparison to ATV. Adjuvant play an important role in the efficacy of vaccines. In addition to increasing the strength and kinetics of an immune response, they also play a role in determining both the type of immune response generated and the potential application in animals and humans (Cox and Coulter 1997; Martinez-Gómez et al. 2009). As the main protection in toxoplasmosis, is carried out by the cellular arm of the immune response (Liu et al. 2006), therefore, the use of a Th1-driving adjuvant can cause better enhancement of the vaccine efficacy via stimulating IL-12 production by macrophages (Yap et al. 2000). In this study, BCG was used as an adjuvant in an attempt to enhance the efficacy of the Toxoplasma vaccine.
As parasite count estimation could detect the density of infection in tissues, in the present work, impression smears from the different organs examined revealed; rapid dissemination of the parasite to the spleen, liver, lung and brain in the infected control (group 4). This finding was in agreement with the study done by Sibley et al. (2002) and Eissa et al. (2012). The present results showed that when BCG was given alone to experimental animals prior to infection (group 3), there was no statistically significant difference in survival rate and there was no statistically significant difference in the mean parasitic count in the different studied organs when compared to infected control which can be attributed to the fact that BCG is a non-specific stimulator of the reticuloendothelial system as previously reported by Berenhaum and Brown (1964). The absence of protection when BCG was given alone without the vaccine was in agreement with the work of Eissa et al. (2012), it can be attributed to the lack of Toxoplasma antigen that made the immune response produced by the adjuvant non-specific. The survival time extended up to 15th days (mean of survival = 11.5 days) in ATV vaccinated group and highly significant difference was found between survival curves of ATV vaccinated group and both control groups, these results agreed with the results of Eissa et al. (2012) who suggested that significant immune responses were developed following vaccination with ATV as demonstrated by the delayed death of vaccinated mice following RH challenge by nine days. The survival time extended up to 16th days (mean of survival = 12.7 days) in ESA vaccinated group post infection, highly significant difference was found between survival curves of ESA vaccinated group and both control groups, agreeing with extended survival time with ESA up to 15th days reported by Daryani et al. (2003).
A significant increase in the survival rate was also reported by other workers using live attenuated vaccines (Yap et al. 1998), DNA vaccines (Fachado et al. 2003; Yan et al. 2011), or recombinant T. gondii nucleoside triphosphate hydrolase-II (Tan et al. 2011). On the other hand, Spencer et al. (2004) who used attenuated; temperature-sensitive mutant strain of Toxoplasma gondii and Martin et al. (2004) who used recombinant GRA4 or ROP2 vaccines, reported no significant difference in the survival rate between immunized and infected control mice.
Statistically significant reduction was found in the mean tachyzoites count in all organs in ATV vaccinated group agreeing with the results of Eissa et al. (2012) who reported significant decrease in parasite density in different organs when compared to the infected control group. ESA vaccinated group showed statistically significant reduction in the mean tachyzoites count in all organs when compared to the infected control group, but highly significant difference in the mean tachyzoites count of spleen in ESA vaccinated group, when compared to the infected control. ESA vaccinated exhibited a profound decrease in parasite load following parasite challenge with a higher percentage of reduction in all examined organs then the ATV vaccinated group, statistically significant difference was found between the ESA group and the ATV group in the mean tachyzoites count of brain. Costa-Silva et al. (2008) reported that ESA immunized mice had lower parasitaemia as well as longer survival then mice in the control group. Zenner et al. (1999) have reported that purified ESA, namely GRA2 and GRA5, had a significant degree of organs’ protection. Furthermore, a study conducted by Daryani et al. (2003) showed that the ESA-F2 antigens could be used as a good candidate for the development of new immunization strategy against toxoplasmosis.
In the present study, the pathological changes detected in liver sections of the infected control group were in accordance with those reported by Suzuki et al. (1993) and Eissa et al. (2012). Moreover, the described pathology for the spleen and lung was similar to the description of Abdel Wahab et al. (1989). The histopathological picture of the liver in both vaccinated groups, revealed marked reduction in the pathological changes observed as compared to controls, especially in ESA vaccinated group in which liver section showed almost normal hepatic tissue and according to the scoring criteria; 100 % of examined specimens were scored as (0), but that receiving ATV vaccine only 60 % were scored as (0) and the remaining specimens (40 %) was scored as (1). Eissa et al. (2012), demonstrated reduced pathological changes in the liver of mice receiving ATV compared to mice receiving Toxoplasma lysate vaccine. Ali et al. (2003) reported decreased pathological changes following vaccination by live cyst vaccines and irradiated cyst vaccine. Even though the tachyzoite challenge was lethal to the mice, it can be concluded that ESA vaccine showed more promising results versus ATV, as demonstrated by the survival rate of vaccinated mice; tachyzoites count and histopathological examination, this could be attributed to the fact that ESA of T. gondii tachyzoites, represent more than 90 % of the parasite circulating antigens, might be the first target of the host immune system (Daryani et al. 2003).
In a study conducted by Rahman and Anuar (1992) reported that ESA may be the best form of antigen for stimulation of the cell-mediated immune response. Moreover, Grover et al. (2012) reported decreased parasite load in vaccinated mice due to increased level of CD4+ T-lymphocytes in response to vaccination by single parasite peptide (AS15), which is one of the constituent of ESA. Vaccination with ESA showed no increase of IL-10 serum levels at the early phases, this cytokine when increase inhibits the proliferation of B and T lymphocytes and suppresses the killing of T. gondii by human macrophages (Abdollahi et al. 2013).
Although, Eissa et al. (2012) reported statistically significant increase in the mean percentage of CD8+ T-lymphocytes in ATV vaccinated group but that was attributed to the use of BCG as an adjuvant.
The options and combinations are so broad, and yet untested, that several years of research will be needed before we can decide which combination will be more adequate or what will be the most efficient immunization protocol (Romero et al. 2012).
This study suggested that significant protection was mounted following vaccination with ESA Vaccine.
References
- Abdel Wahab RM, Morsy TA, Bahagat AB, Abdel Rahim MI, Eissa MH, Al-Alfy YE. The histopathological picture of concomitant infection with Leishmania major and Toxoplasma gondii in albino mice. J Egypt Soc Parasitol. 1989;19:1–11. [PubMed] [Google Scholar]
- Abdollahi SH, Ayoobi F, Khorramdelazad H, Hassanshahi G, Ahmadabadi BN, Rezayati M, Ravary A, Shamsizadeh A, Arababadi MK. Interleukin-10 serum levels after vaccination with in vivo prepared T. gondii excreted/secreted antigens. Oman Med J. 2013;28(2):112–115. doi: 10.5001/omj.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SM, Allam SR, Negm AY, EL-Zawawy LA. Vaccination against congenital toxoplasmosis. J Egypt Soc Parasitol. 2003;33:863–874. [PubMed] [Google Scholar]
- Araujo FG, Remington JS. Partially purified antigen preparations of Toxoplasma gondii protect against lethal infection in mice. Infect Immun. 1984;45(1):122–126. doi: 10.1128/iai.45.1.122-126.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo FG, Prockocimer P, Lin L, Remington SJ. Activity of clarithromycin alone or in combination with other drugs for treatment of murine toxoplasmosis. Antimicrob Agents Chemother. 1992;36:2454–2457. doi: 10.1128/AAC.36.11.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts T, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1419. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Berenhaum MC, Brown IN. Dose response relationships for agents inhibiting the immune response. Immunology. 1964;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- Bout DT, Mevelec MN, Vegle-Roussel FV, Dimier-Poisson ID, Lebrun M. Prospects for a human toxoplasmosis vaccine. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2:227–234. doi: 10.2174/1568008023340488. [DOI] [PubMed] [Google Scholar]
- Bullow R, Boothroyd J. Protection of mice from fatal Toxoplasma gondii infection by immunization with P30 antigen in liposomes. J Immunol. 1991;147:3496–3500. [PubMed] [Google Scholar]
- Buxton D. Toxoplasmosis; the first commercial vaccine. Parasitol Today. 1993;9:335–337. doi: 10.1016/0169-4758(93)90236-9. [DOI] [PubMed] [Google Scholar]
- Casciotti LK, Ely H, Williams ME, Khan IA. CD8_-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4_T cells. Infect Immun. 2002;70:434–443. doi: 10.1128/IAI.70.2.434-443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WY, Nam HW, Kwak NH, Huh W, Kim YR, Kang MW, Cho SY, Dubey JP. Foodborne outbreaks of human toxoplasmosis. J Infect Dis. 1997;175:1280–1282. doi: 10.1086/593702. [DOI] [PubMed] [Google Scholar]
- Coombs GH, Muller S. Recent advances in the search for new anti-coccidial drugs. Int J Parasitol. 2002;32:497–508. doi: 10.1016/S0020-7519(01)00354-X. [DOI] [PubMed] [Google Scholar]
- Costa-silva TA, Meira CS, Ferreira IM, Hiramoto RM, Pereira-Chioccola VL. Evaluation of immunization with tachyzoite excreted–secreted proteins in a novel susceptible mouse model (A/Sn) for Toxoplasma gondii. Exp Parasitol. 2008;120:227–234. doi: 10.1016/j.exppara.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Cox JD, Coulter AR. Adjuvants: a classification and review of their mode of action. Vaccine. 1997;15:248–253. doi: 10.1016/S0264-410X(96)00183-1. [DOI] [PubMed] [Google Scholar]
- Darcy F, Maes P, Gras-Masse H. Protection of mice and nude rats against toxoplasmosis by a multiple antigenic peptide contraction derived from Toxoplasma gondii P30 antigen. J Immunol. 1992;149:3636–3641. [PubMed] [Google Scholar]
- Daryani A, Hosseini AZ, Dalimi A. Immune responses against excreted/secreted antigens of Toxoplasma gondii tachyzoites in the murine model. Vet Parasitol. 2003;113:123–134. doi: 10.1016/S0304-4017(03)00044-X. [DOI] [PubMed] [Google Scholar]
- Dimier-Poisson I, Aline F, Me′ve′lec MN, Beauvillain C, Buzoni-Gatel D, Bout D. Protective mucosal Th2 immune response against Toxoplasma gondii by murine mesenteric lymph node dendritic cells. Infect Immunol. 2003;71(9):5254–5265. doi: 10.1128/IAI.71.9.5254-5265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury RAB, Wallington EA. Carleton’s histological technique. 5. Oxford, New York, Toronto: Oxford University Press; 1980. pp. 140–142. [Google Scholar]
- Dubey JP. Toxoplasmosis—a waterborne zoonosis. Vet Parasitol. 2004;126(1–2):57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Eissa MM, Allam SR, El-Azzouni MZ, Maged HR, Dessouky IS. Further studies on autoclaved cercarial vaccine against Schistosomiasis safety, longevity and stability. J Egypt Soc Parasitol. 2003;33:541–560. [PubMed] [Google Scholar]
- Eissa MM, El-Azzouni MZ, Bolous ML. Vaccination trial against experimental trichinosis using autoclaved Trichinella spiralis larva vaccine. J Egypt Soc Parasitol. 2003;33:219–228. [PubMed] [Google Scholar]
- Eissa MM, El-Azzouni MZ, Mady RF, Fathy FM, Baddour NM. Initial characterization of an autoclaved Toxoplasma vaccine in mice. Exp Parasitol. 2012;131:310–316. doi: 10.1016/j.exppara.2012.05.001. [DOI] [PubMed] [Google Scholar]
- El-Temsahy M, El-Kerdany ED, Abou-Shamaa AM. Study of the role of antioxidants in experimental toxoplasmosis. J Med Res Inst. 2002;23:59–69. [Google Scholar]
- Fachado A, Rodriguez A, Molina J, Silverio JC, Marrino AP, Pinto LM, Angel SO, Infante JF, Traub-cseko Y, Amendoeira RR, Lannes-Vreira J. Long term protective immune response elicited by vaccination with an expression genomic library of Toxoplasma gondii. Infect Immunol. 2003;71:5407–5411. doi: 10.1128/IAI.71.9.5407-5411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Hakim FT, Hieny S, Cheever A, Sher A. Synergistic role of CD4_ and CD8_ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- Grover HS, Blanchard N, Gonzalez, Chan S, Robey EA, Shastri N. T. gondii peptide elicits CD4 T cells that can control parasite burden. Infect Immun. 2012;80(9):3279–3288. doi: 10.1128/IAI.00425-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes HPA, Vanknapen F. Characterization of a secretory antigen from Toxoplasma gondii and its role in circulating antigen production. Int J Parasitol. 1982;12:433–437. doi: 10.1016/0020-7519(82)90073-X. [DOI] [PubMed] [Google Scholar]
- Innes EA. Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? Expert Rev Vaccines. 2010;9:1117–1119. doi: 10.1586/erv.10.113. [DOI] [PubMed] [Google Scholar]
- Innes EA, Vermeulen AN. Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora. Parasitol. 2006;133:145–168. doi: 10.1017/S0031182006001855. [DOI] [PubMed] [Google Scholar]
- Innes EA, Bartley PM, Maley S, Katzer F, Buxton D. Veterinary vaccines against Toxoplasma gondii. Mem Inst Oswaldo Cruz. 2009;104(2):246–251. doi: 10.1590/S0074-02762009000200018. [DOI] [PubMed] [Google Scholar]
- Khan IA, Ely KH, Kasper LH. A purified antigen (P30): mediated CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- Kim MH, Choi YK, Park YK, Nam HW. A toxoplasmic uveitis case of a 60-year-old male in Korea. Korean J Parasitol. 2000;38:29–31. doi: 10.3347/kjp.2000.38.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl JL, Ruskin J, Remington JS. The use of killed vaccines in immunization against an intracellular parasite: Toxoplasma gondii. J Immunol. 1972;108:425–431. [PubMed] [Google Scholar]
- Liu CH, Fan Y, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wei F, Gao S, Jiang L, Lian H, Yuan B, Yuan Z, Xia Z, Liu B, Xu X, Zhu XQ. Toxoplasma gondii infection in pregnant women in China. Trans R Soc Trop Med Hyg. 2009;103:162–166. doi: 10.1016/j.trstmh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Martin V, Supanitsky A, Echeverria P, Litwn S, Tanos T, De Roodt A, Guarnera ER, Angel SO. Recombinant GRA4 or ROP2 Protein combined with alum or the GRA4 gene provides partial protection in chronic murine models of toxoplasmosis. Clin Vaccine Immunol. 2004;11:704–710. doi: 10.1128/CDLI.11.4.704-710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gómez F, Garcia-González LF, Mondragón-Flores R, Bautista-Garfias CR. Protection against Toxoplasma gondii brain cyst formation in mice immunized with Toxoplasma gondii cytoskeleton proteins and Lactobacillus casei as an adjuvant. Vet Parasitol. 2009;160:311–315. doi: 10.1016/j.vetpar.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Modabber F. Vaccines against leishmaniasis. Ann Trop Med Parasitol. 1995;89:83–88. doi: 10.1080/00034983.1995.11813017. [DOI] [PubMed] [Google Scholar]
- Rahman N, Anuar AK. Comparison of three forms of antigens in the demonstration of cell-mediated immune response in murine toxoplasmosis. Biochem Biophys Res Commun. 1992;189(2):640–644. doi: 10.1016/0006-291X(92)92248-V. [DOI] [PubMed] [Google Scholar]
- Romero O, Oliveira DM, Andrade-Neto V. Toxoplasmosis: advances and vaccine perspectives. In: Alfonso R, editor. Current topics in tropical medicine. Shanghai: InTech; 2012. pp. 169–184. [Google Scholar]
- Saadatnia G, Mohamed Z, Ghaffarifar F, Osman E, Moghadam ZK, Noordin R. Toxoplasma gondii excretory/secretory antigenic proteins of diagnostic potential. APMIS. 2012;120(1):47–55. doi: 10.1111/j.1600-0463.2011.02810.x. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Mordue DG, Su C, Robben PM, Howe DK. Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii. Philos Trans R Soc Lond B. 2002;357:81–88. doi: 10.1098/rstb.2001.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JA, Smith BF, Guarino AJ, Blaghurn BL, Baker HJ. The use of CPG as an adjuvant to Toxoplasma gondii vaccination. Parasitol Res. 2004;92:313–316. doi: 10.1007/s00436-003-1039-7. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Wong SY, Conley FK, Remington JS (1993) Susceptibility to chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection in mice. Infect Immun 61:2284–2288 [DOI] [PMC free article] [PubMed]
- Tafaghodi M, Tabassi AS, Amiri N. PLGA nanospheres loaded with autoclaved Leishmania major (ALM) and CPG-ODN; preparation and in vitro characterization. Iran J Basic Med Sci. 2008;11:112–119. [Google Scholar]
- Tan F, Hu X, Luo FJ, Pan CW, Chen X. Induction of protective Th1 immune responses in mice by vaccination with recombinant T. gondii nucleoside triphosphate hydrolase-II. Vaccine. 2011;29:2742–2748. doi: 10.1016/j.vaccine.2011.01.089. [DOI] [PubMed] [Google Scholar]
- Verma R, Khanna P. Development of T. gondii vaccine: a global challenge. Hum Vaccin Immunother. 2012;30:291–293. doi: 10.4161/hv.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto YI, Mineo JR, Meneghisse CS, Guimaraes ACS, Kawarabayashi M. Detection in human sera of IgG, IgM and IgA to excreted/secreted antigens from Toxoplasma gondii by use of dot-ELISA and immunoblot assay. Ann Trop Med Parasitol. 1998;92(1):23–30. doi: 10.1080/00034989860139. [DOI] [PubMed] [Google Scholar]
- Yan HK, Yuan ZG, Petersen E, Zhang X, Zhou DH, Liu Q, He Y, Lin RQ, Xu MJ, Chen XL, Zhong XL, Zhu XQ. Toxoplasma gondii: protective immunity against experimental toxoplasmosis induced by a DNA vaccine encoding the perforin-like protein 1. Exp Parasitol. 2011;128:38–43. doi: 10.1016/j.exppara.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Yap G, Kersten TS, Ferguson DJ. Partially protective vaccination permits the development of latency in a normally virulent strain of Toxoplasma gondii. Infect Immun. 1998;66(9):4382–4388. doi: 10.1128/iai.66.9.4382-4388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Gao S, Liu Q, Xia X, Liu X, Liu B, Hu R. Toxoplasma gondii antibodies in cancer patients. Cancer Lett. 2007;254:71–74. doi: 10.1016/j.canlet.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Zenner L, Estaquier J, Darcy F, Maes P, Capron A, Cesbron-Delauw MF. Protective immunity in the rat model of congenital toxoplasmosis and the potential of excreted secreted antigens as vaccine components. Parasite Immunol. 1999;21:261–272. doi: 10.1046/j.1365-3024.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Chen J, Wang M, Petersen E, Zhu X. Vaccines against T. gondii: new developments and perspectives. Expert Rev Vaccines. 2013;12:1287–1299. doi: 10.1586/14760584.2013.844652. [DOI] [PubMed] [Google Scholar]