Abstract
Morphological characters of Setaria sp. collected from cattle and buffaloes were studied. Three species Setaria digitata, Setaria cervi and Setaria labiatopapillosa were identified in the present study. Out of the 500 cattle screened 187 were found to harbour worms. 56.8 % (106) of animals with S. digitata, 24.13 % (45) had S. cervi and 18.96 % (36) had S. labiatopapillosa. Morphological characters of male and female worms of all the three species were studied in detail.
Keywords: Setaria digitata, Setaria cervi, Setaria labiatopapillosa, Cattle, Morphology
Introduction
Filarial nematodes of Setaria sp. usually lives in the peritoneal cavity of cattle and the larvae called as microfilaria are found in the blood. Adult worms are generally considered to be non pathogenic although they may cause a mild fibrinous peritonitis, but the larval forms caused serious conditions. They migrate erratically into the central nervous system of unnatural hosts such as horses, sheep and goats leads to serious and often fatal neuropathologic disorder known as epizootic cerebrospinal setariosis or cerebrospinal nematodiasis or kumri or lumbar paralysis. This study was undertaken to morphologically characterize various species of Setaria of cattle based on the anterior and the posterior ends of the worms.
Materials and methods
Cattle slaughtered at the Karnataka Meat and Poultry Marketing Corporation Limited (KMPMCL) Slaughter house, Bangalore and animals subjected to post mortem examination at the Department of Veterinary Pathology, Veterinary College, Hebbal, Bangalore and other institutes in and around Bangalore were screened in the present study. A total number of 500 cattle were screened during the study.
Collection and processing of worms
The peritoneal cavities of animals were thoroughly searched during evisceration and the worms were collected in normal saline. The worms were counted and male and female worms were separated based on their length. For identification of species, the cephalic and caudal ends of the worms were mounted separately in a clearing and mounting medium Rubin’s mountant. Speciation was done based on the descriptions of Shoho (1958), Willard and Walker (1969), Sonin (1977) and Anderson (1992). The length and width of Setaria worms were also measured.
Results
Out of the 500 cattle screened, 187 (37.4 %) were found to harbour Setaria worms in the peritoneal cavity. Three species of Setaria viz., Setaria digitata, Setaria cervi and Setaria labiatopapillosa were observed in the present study. Out of the 187 cattle which were positive for Setaria, 106 (56.8 %) had S. digitata, 45 (24.13 %) had S. cervi and 36 (18.96 %) had S. labiatopapillosa. Mixed infection with all the three Setaria sp. was found in 30 animals (16.04 %). The worms were found freely in the peritoneal cavity or attached to the intestines, mesentery, walls of the peritoneum, lungs, liver, heart, urinary bladder, uterus and fascia. Some worms were found embedded in patches of inflammatory tissue attached to the visceral walls of the pelvic peritoneum.
The average body length and width of S. digitata female worms was 156 and 0.70 mm, respectively whereas that of S. digitata male worms was 82 and 0.50 mm, respectively. The average body length and width of S. cervi female worms was 142 and 0.48 mm, respectively whereas that of S. cervi male worms found to be 76 and 0.47 mm, respectively. The average body length and width of S. labiatopapillosa female worms was 150 and 0.60 mm, respectively whereas that of S. labiatopapillosa male worms was 80 and 0.40 mm, respectively. The gross female and male worms are depicted in Figs. 1 and 2, respectively.
Fig. 1.
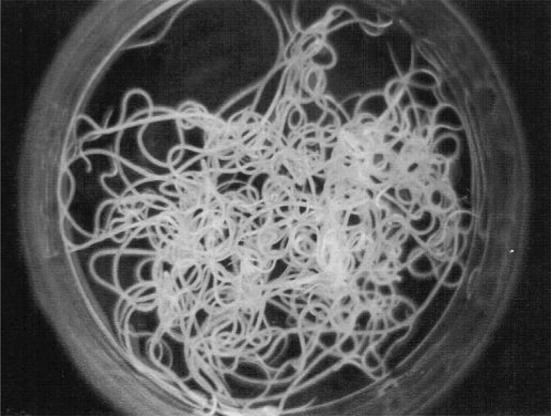
Setaria sp. female worms—Gross
Fig. 2.
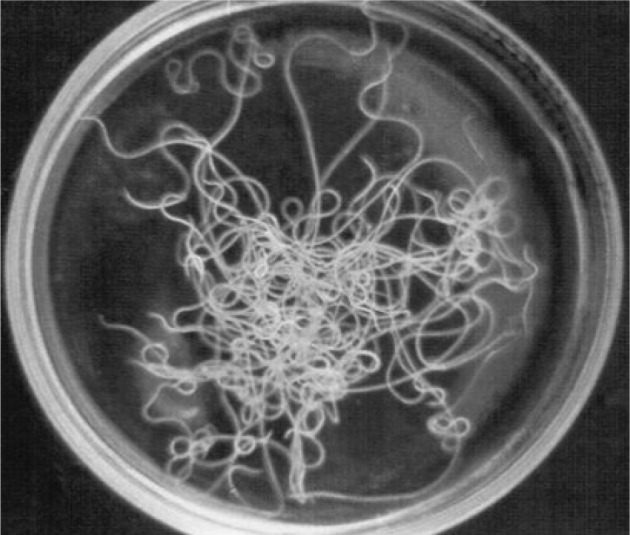
Setaria sp. male worms—Gross
Setaria digitata female worms had a peribuccal crown with a central “helmet” at the cephalic end and the lateral lips were triangular (Fig. 3) and the tail end terminated in a smooth knob with oval lateral appendages (Fig. 4). Cuticle was smooth in S. digitata female worms. S. digitata male worms also had cephalic ends similar to females but the cuticle was corrugated (Fig. 5). In S. digitata males, the right spicule was stout and thick with a narrow proximal end whereas the left spicule had a shaft and a blade (Fig. 6).
Fig. 3.
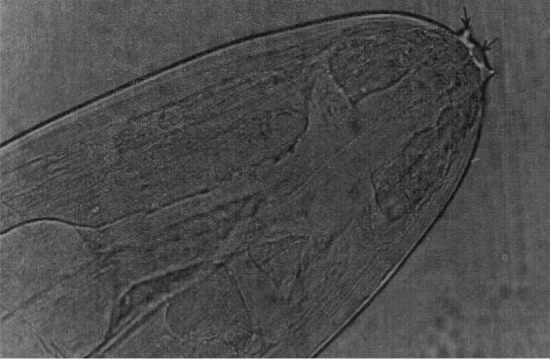
Setaria digitata female worms—Cephalic end
Fig. 4.
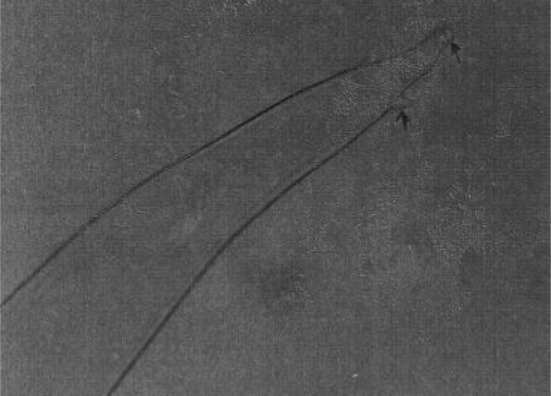
Setaria digitata female worms—Caudal end
Fig. 5.
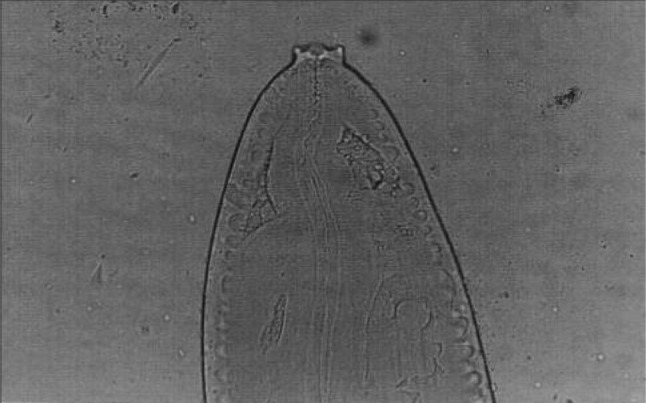
Setaria digitata male worms—Cephalic end
Fig. 6.
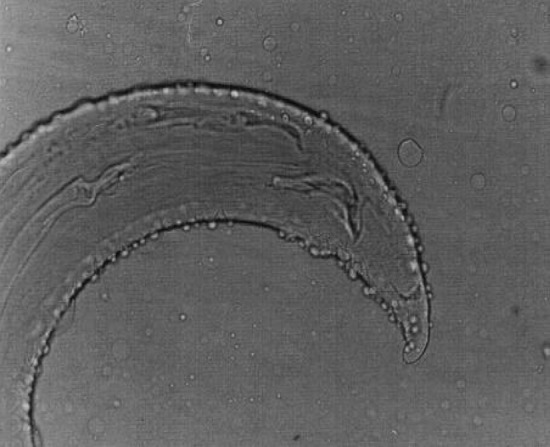
Setaria digitata male worms—Caudal end
Setaria cervi females had crescentic lateral lips (Fig. 7) with tails studded with spines (Fig. 8). In S. cervi males, the cephalic end was similar to females with corrugated cuticle (Fig. 9) and in the caudal end, the right spicule was shorter and the left spicule had a tubular chitinous part with an expanded proximal end with narrow and bifid distal end (Fig. 10).
Fig. 7.
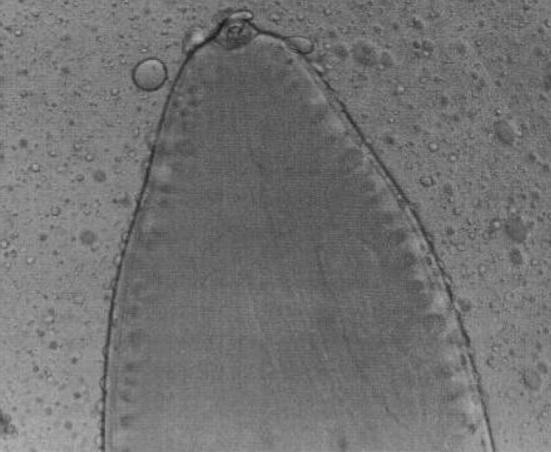
Setaria cervi female worms—Cephalic end
Fig. 8.
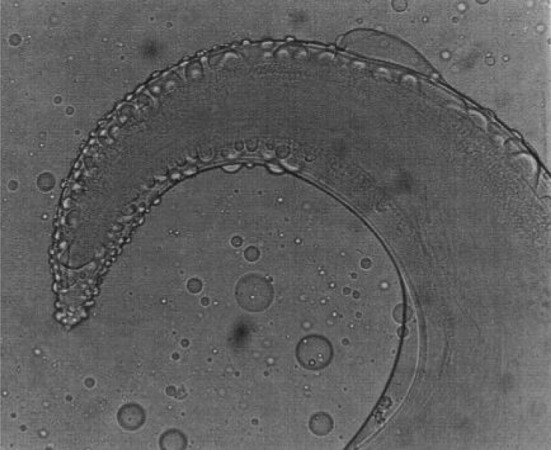
Setaria cervi female worms—Caudal end
Fig. 9.
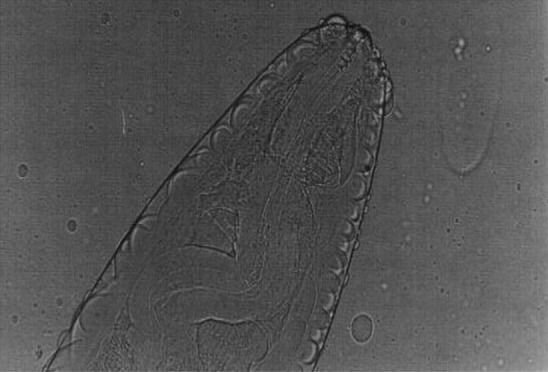
Setaria cervi male worms—Cephalic end
Fig. 10.
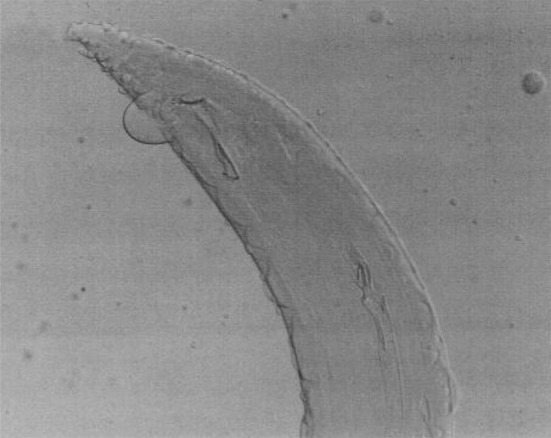
Setaria cervi male worms—Caudal end
Setaria labiatopapillosa females had a prominent peribuccal crown with rectangular lateral lips (Fig. 11) and tail end has a smooth button with pointed lateral appendages (Fig. 12). Cephalic end of male S. labiatopapillosa worms also had a prominent peribuccal crown and triangular lateral lips as in females (Fig. 13). Caudal end of S. labiatopapillosa males was highly coiled with less prominent right spicule and left spicule was wider in the middle and ending indistinctly (Fig. 14).
Fig. 11.
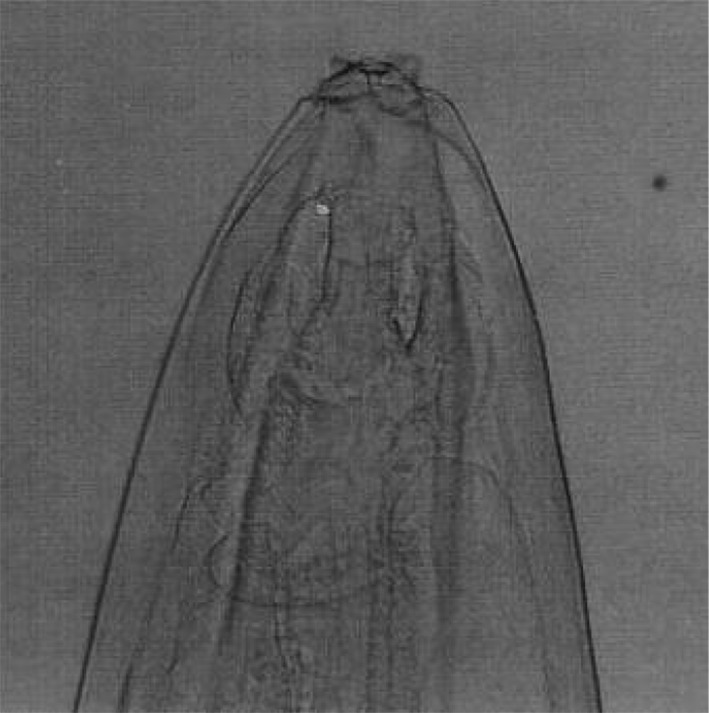
Setaria labiatopapillosa female worms—Cephalic end
Fig. 12.
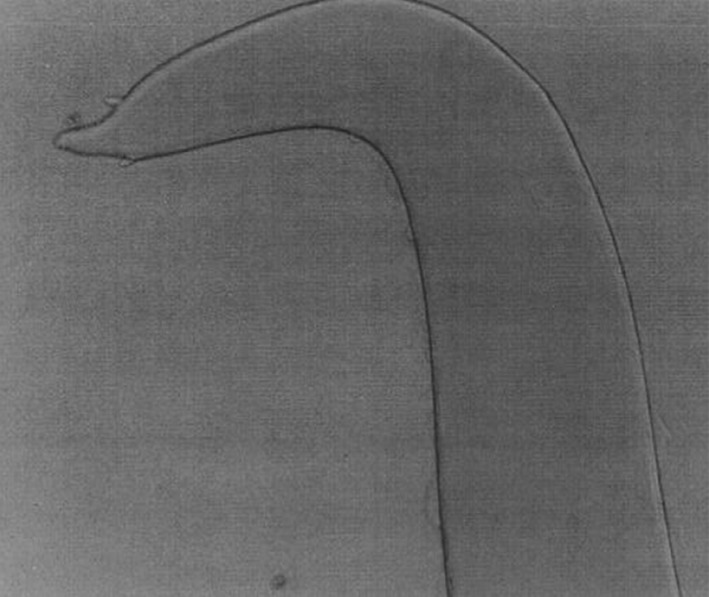
Setaria labiatopapillosa female worms—Caudal end
Fig. 13.

Setaria labiatopapillosa male worms—Cephalic end
Fig. 14.
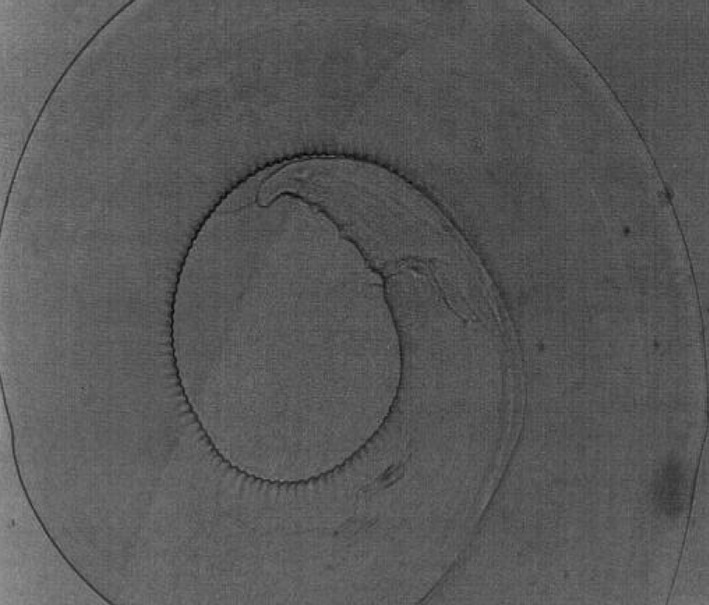
Setaria labiatopapillosa male worms—Caudal end
Discussion
Reports on prevalence and location of various species of Setaria worms in cattle from India and abroad were recorded. S. digitata adult worms found in the urinary bladder (Alwar and Lalitha 1954; Yoshikawa et al. 1976; Thirumurthy et al. 1995), eye (Otake 1980, Ohtake et al. 1989), epicardium of the heart (Fujita et al. 1985) and in the lungs and mesenteric lymph nodes (Ashizawa and Moritomo 1989). S. cervi worms observed in patches of inflammatory reaction in the visceral peritoneum (Sarwar 1945; Chauhan and Pande 1980), liver (Vasudev 1955) and spinal cord (Pachauri 1972). S. labiatopapillosa has been reported from the anterior chamber of the eye (Rao 1941), peritoneal cavity (Osipov 1972) and diaphragm (Pelligrini et al. 1980) where these worms were found to be associated with inflammatory reactions. In the present study, several worms were found embedded in areas of inflammation attached to the visceral walls of the pelvic peritoneum. Haemorrhagic inflammation was also observed in the peritoneal wall and noticed in bulls. These findings are in accordance with the reports of Sarwar (1945) and Chauhan and Pande (1980) indicating that these worms can cause inflammatory reactions in their normal sites also.
In cattle particularly prevalence of S. digitata has been found to be more common than S. cervi and S. labiatopapillosa. Shoho (1958) from Ceylon, Mohan (1975) from Andhra Pradesh, Patnaik (1989) and Mohanty et al. (2000) from Orissa reported the prevalence of S. digitata in cattle ranging from 77 to 95 %. However, in the present study although the prevalence of S. digitata was the highest, i.e., 56.8 % compared to S. cervi, 24.13 % and S. labiatopapillosa, 18.96 %, the overall prevalence was lower compared to the above reports. This variation could be possibly due to variations in climatic conditions, geographical location, and susceptibility of the animals and other factors.
Prevalence of S. cervi in cattle as observed by Frickers (1948) from Surinam and McFadzean (1955) from Gambia was 42 and 32.69 %, respectively, which is less compared to the prevalence of S. digitata in the present study. Mohan (1975) concluded that S. cervi was the least common species occurring in South India among cattle. The present study is also in agreement with the observation of Mohan (1975) wherein the prevalence of S. cervi was 24.13 %. Apart from few prevalence reports (Rao 1941; Sarwar 1946), In India, cattle has been found to be less affected by species of S. labiatopapillosa. However, Patnaik (1989) from Orissa recorded 19.3 % of the cattle to be infected with this species. But several reports from abroad indicated a higher infection rate, viz., Green and Trueman (1971) from Australia (75 %), Brengues and Gidel (1972) from France (50 %) and Ogbogu et al. (1990) from Nigeria (58.6 %). Also, this species has been reported to be the most common species among cattle in the United States and Canada (Willard and Walker 1969). In India, Rao (1941) from Andhra Pradesh and Sarwar (1946) from New Delhi reported the prevalence of this species in cattle, but in the present study, 18.96 % of the cattle found revealed S. labiatopapillosa which is low compared with other countries wherein S. labiatopapillosa species occurred in the regions with temperate weather conditions except for Nigeria which implied the adoption of the species for cooler climatic conditions.
All the morphological characters of the three species of Setaria encountered in the present study were in accordance with the description of Shoho (1958), Willard and Walker (1969), Sonin (1977) and Anderson (1992).
References
- Alwar VS, Lalitha CM. A check list of the helminth parasites in the Department of Parasitology, Madras Veterinary College. Indian Vet J. 1954;31:142–148. [Google Scholar]
- Anderson RC. Nematode parasites of vertebrates: their development and transmission. 1. Wallingford: CAB International; 1992. pp. 448–451. [Google Scholar]
- Ashizawa H, Moritomo Y (1989) Erratic parasitism by Setaria sp. in the lungs and mesenteric lymph nodes of cattle. In: Proceedings of the Faculty of Agriculture, Kyushu Tokal University, vol 8. pp 69–76. (Helmn. Abstr. 58:3695)
- Brengues J, Gidel R. [Research on Setaria labiatopapillosa (Perroncito, 1882) in Western Africa] Dynamics of this filariasis under natural conditions. Annals de Parassitologie Humane et Comparee. 1972;7:597–611. [PubMed] [Google Scholar]
- Chauhan PPS, Pande BP. On the occurrence of Setaria labiatopapillosa in the intestinal lining of buffalo calf. Indian J Parasitol. 1980;4:89–91. [Google Scholar]
- Frickers J. Occurrence of Setaria cervi in cattle and Stephanurus dentatus in swine in Suirinam. Tijdschr, Diergeneesk. 1948;73:888–890. [PubMed] [Google Scholar]
- Fujita J, Imai S, Ishii TN, Nunoya T, Takahashi K, Tomita T, Oikawa R. Heterotropic parasitism of Setaria digitata (Linstow, 1906) in the heart of a cattle. Jpn J Vet Sci. 1985;47:999–1002. doi: 10.1292/jvms1939.47.999. [DOI] [PubMed] [Google Scholar]
- Green DJ, Trueman KF. The occurrence of Setaria labiatopapillosa in Queensland. Aust Vet J. 1971;47:624. doi: 10.1111/j.1751-0813.1971.tb02099.x. [DOI] [PubMed] [Google Scholar]
- McFadzean JA. Setarial infections in the Gambia, British West Africa. Ann Trop Med Parasitol. 1955;49:417–418. doi: 10.1080/00034983.1955.11685692. [DOI] [PubMed] [Google Scholar]
- Mohan RN. A note on Setaria digitata in cattle and buffaloes and cells of the peritoneal exudate. Indian J Anim Sci. 1975;45:914–915. [Google Scholar]
- Mohanty MC, Sahoo PK, Satapathy AK, Ravindran B. Setaria digitata infections in cattle: parasite load, microfilaraemia status and relationship to immune response. J Helminthol. 2000;74:343–347. doi: 10.1017/s0022149x00000500. [DOI] [PubMed] [Google Scholar]
- Ogbogu VC, Bablis JM, Ajanusi OJ. Prevalence of microfilariae in cattle at slaughter in Zaria, Nigeria. Vet Parasitol. 1990;36:171–175. doi: 10.1016/0304-4017(90)90107-M. [DOI] [PubMed] [Google Scholar]
- Ohtake O, Sonoda M, Matsukawa K, Fukumoita S, Takahashi K, Kurosawa T. Clinical studies on bovine autumnal conjunctivitis in Japan. Jpn J Vet Sci. 1989;51:618–620. doi: 10.1292/jvms1939.51.618. [DOI] [PubMed] [Google Scholar]
- Osipov AN. Observations on the microfilarial numbers and on the life span of Setaria labiatopapillosa in cattle. Bylleten vsesoyuzwogo Gelmintologii im ki Stryabina. 1972;9:52–54. [Google Scholar]
- Otake O. Bovine conjunctivitis probably caused by filaria. J Vet Med. 1980;70:91–92. [Google Scholar]
- Pachauri SP. Cerebrospinal nematodiasis in a buffalo. A case report. Indian J Anim Res. 1972;6:17–19. [Google Scholar]
- Patnaik MM. On filarial nematodes in domestic animals in Orissa. Indian Vet J. 1989;66:573–574. [Google Scholar]
- Pelligrini N, Ramboli B, Poli A, Renzoni G, Gioli G. Eosinophilic granuloma caused by adult Setaria labiatopapillosa in the bovine diaphragm. Anatamo-histopathological findings and pathogenic considerations. Annali Facolta di Medicina Veterinari di pisa. 1980;33:103–112. [Google Scholar]
- Rao KSP. Worm in the anterior chamber of the eye of a bullock. Indian Vet J. 1941;18:35. [Google Scholar]
- Sarwar MM. On the pathogenicity of Setaria cervi (Rud, 1819) in buffaloes. Curr Sci. 1945;14:107. [Google Scholar]
- Sarwar MM. Two species of the nematode Genus Setaria Vibrog. Indian Vet J. 1946;22:405–409. [PubMed] [Google Scholar]
- Shoho C. Studies on Cerebrospinal nematodiasis in Ceylon. Ceylon Vet J. 1958;6:10–15. [Google Scholar]
- Sonin MD. Fundamentals of nematology. Moscow: Nauka; 1977. pp. 30–150. [Google Scholar]
- Thirumurthy CC, Senthilvel K, Pillai KM. Setaria digitata in bullock’s urine. J Vet Anim Sci. 1995;26:72. [Google Scholar]
- Vasudev T. Occurrence of Setaria cervi in the liver of cattle. Indian Vet J. 1955;32:233. [Google Scholar]
- Willard WB, Walker L. Taxonomy, hosts and geographic distribution of the Setaria (Nematoda: Filarioidea) in the United States and Canada. J Parasitol. 1969;55:359–368. doi: 10.2307/3277411. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Oyamada T, Yoshikawa M. Eosinophilic granulomas caused by adult Setarial worms in the bovine urinary bladder. Jpn J Vet Sci. 1976;38:105–116. doi: 10.1292/jvms1939.38.105. [DOI] [PubMed] [Google Scholar]


