Abstract
Food-borne trematode infections, which are mainly transmitted through consumption of inadequately cooked or raw fish and crabs, affect a large section of population, particularly in Southeast Asian countries, thus eliciting a remarkable morbidity and causing serious damage to health. In India, centering in several mountainous regions of the Northeast, the natives have the habit of consuming such fish or crabs that still sustain viable infective larval stage (metacercaria) of trematode flukes in their muscle tissue. The present study was undertaken to ascertain the spectrum of metacercarial diversity in commonly edible freshwater fishes and crab species in the northeastern state of Manipur and to adjudge their zoonotic potential, if any. Commonly edible fishes belonging to 15 species from 12 localities and crabs belonging to 2 species from 11 localities across Manipur state were surveyed for the purpose. The study revealed that 3 species of fishes (Channa punctatus, C. straitus and Wallago attu) harboured 4 different types of metacercariae belonging to 4 trematode families—Euclinostomum heterostomum (Clinostomidae); Lophosicyadiplostomum sp. and Posthodiplostomum sp. (Diplostomidae); and Polylekithum sp. (Allocreadiidae) in addition to adult flukes of Isoparorchis hypselobagri (Isoparorchiidae). Among these, metacercariae of Posthodiplostomum showed the highest prevalence (2.33 %) though a low abundance, while for other species the prevalence ranged between 0.25 and 1.19 %. The crab species (Barythelphusa lugubris masoniana and Potamiscus manipuriensis) were found infected with 4 different types of metacercariae representing the genera Paragonimus (Troglotrematidae) and Microphallus (Microphallidae). The paragonimids showed a higher rate of occurrence (~4–25 %) compared to microphallids (~15 %). The crustaceans surveyed emerged as prospective intermediate hosts for lungflukes. Identifying the potent vectors for zoonotic parasites helps in control measures towards their transmission to higher mammals.
Keywords: Trematode, Metacercaria, Fish, Crustacea, Zoonosis, Manipur, Northeast India
Introduction
Trematodiases are caused by digenetic flukes (Platyhelminthes: Trematoda) and are a major public health problem world wide. Food-borne trematodiasis (FBT) is an important group of neglected tropical diseases, which are zoonotic as they are transmitted by the consumption of raw or undercooked aquatic foods that harbour the metacercaria (i.e., infective larval stage) of the fluke. Over 100 species of FBTs are known to cause infections in humans (WHO 2009). FBTs are endemic in various parts of the world, particularly Southeast Asian regions (Dixon and Flohr 1997). As estimated, more than 750 million people are at risk of infections with food-borne trematodes (Keiser and Utzinger 2009). Fishes and crustaceans may harbour the infective metacercarial stage of a large number of trematodes, which are responsible for FBT (WHO 1995; Lun et al. 2005; Bullard and Overstreet 2008), thus serving as vector for some human helminthic diseases. In India, like most other tropical countries, parasitic infections play a major role in public health. Particularly in Manipur, Northeast India, the native people have similar food habits to those of the Southeast Asian neighbours of consuming raw or inadequately cooked fish or crabs that still sustain viable infective stages (i.e., metacercaria) of trematode flukes in their tissues (Mahanta 1990). Thus, trematode infections of the lung and intestine are especially significant as potential zoonoses in the region.
Freshwater fish harbour infections caused by various trematode species belonging to different families viz., Phyllodistomidae, Monorchiidae, Zoogonidae, Callodistomidae, Homalometridae, Opecoelidae, Microphallidae, Opisthorchiidae, Heterophyidae, Isoparorchiidae, Clinostomatidae, Diplostomidae etc. (Yamaguti 1971). A pioneering amount of literature is available on metacercariae of various digenetic trematodes from fishes all over the world (Malek and Mobedi 2001; Arafa et al. 2005; Silva-Souza and Ludwig 2005; Vianna et al. 2005; Rim et al. 2008; Han et al. 2008; Sohn 2009; Skov et al. 2009; Sohn et al. 2009; Gustinelli et al. 2010; Thuy et al. 2010; Gholami et al. 2011), including that from India (Kumari 1994; Jhansilakshmibai and Madhavi 1997; Singh et al. 2003; Vankara et al. 2011; Shareef and Abidi 2012).
The Crustacea-borne trematode infections are caused by fluke parasites belonging to families Paragonimidae, Microphallidae, Lecithodendriidae, Brachylaimidae etc. (Yamaguti 1971; Anantaraman and Subramoniam 1976; Janardanan et al. 1987). Among these infections, Paragonimus spp causing paragonimiasis pose a continuing public health problem (WHO 1995; Blair et al. 1999; Nakamura-Uchiyama et al. 2002). They occur in a number of countries in several regions of Asia, Africa and Latin America (Toscano et al. 1995). In context of India, the focal transmission of paragonimiasis has been documented in north-eastern states of the country, such as Arunachal Pradesh, Manipur and Nagaland (WHO 2009). Several species of Paragonimus have been reported to occur in the commonly edible crab species prevailing in the mountainous ranges of Northeast India (Singh 2002, 2003; Narain et al. 2003; Singh et al. 2006, 2007, 2009; Tandon et al. 2007; Devi et al. 2010). The regions, where crabs are commonly consumed as part of their traditional cuisine are suspected foci for human infection (Tandon et al. 2007). However, there is scanty information available on the metacercarial infection status in commonly edible freshwater fishes and crabs in the Manipur region of Northeast India. So, the present study was undertaken to ascertain the spectrum of metacercarial diversity in these common components of the traditional cuisine in Manipur state and to adjudge their zoonotic potential, if any.
Materials and methods
Host collection and identification
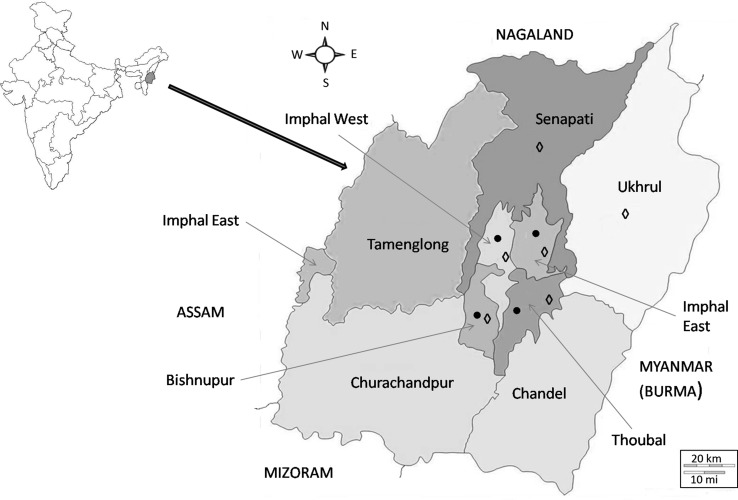
Manipur, located in the north-eastern region of India (22°16′54″N and 88°54′39″E), has a rich variety of piscine and crab hosts. For the collection of fishes, surveys covered localities under four districts in Manipur—Imphal East, Imphal West, Bisenpur and Thoubal; crabs were collected from additional two more districts (Senapati and Uhkrul) during a 2-year period beginning 2009 (Fig. 1; Tables 1, 2). The fishes collected represented 16 species under 15 genera and 11 families. They were identified following the standard literature (Vishwanath et al. 2007). The crab hosts were collected from various localities (hill streams and paddy fields) and rural markets in Manipur. The collection comprised two species (representing two genera under two families), which were identified with the help of the Zoological Survey of India, Kolkata.
Fig. 1.
Map of Manipur depicting the different locality sites surveyed for the collection of commonly edible fish (circle) and crabs (diamond). [Maps source: http://www.himalayanfootsteps.com/destinations/india/manipur/; http://d-maps.com/carte.php?num_car=31957&lang=en]
Table 1.
Freshwater piscine hosts examined from different localities in Manipur
| Name of the host | Locality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lamlong | Yaralpat | Lamphel | Mayang-imphal | Sekmaijing | Tera | Wangoi | Moirang | Nambol | Oinam | Tengtha | Thoubal | |
| Anabantidae | ||||||||||||
| Anabas testudineus | + | + | + | – | – | – | – | + | – | + | – | + |
| Bagridae | ||||||||||||
| Mystis bleekeri | – | + | – | + | – | – | – | + | – | – | – | – |
| Belontidae | ||||||||||||
| Colisa fasciatus | – | – | + | – | – | – | – | – | – | – | – | – |
| Channidae | ||||||||||||
| Channa punctatus | + | + | + | + | – | + | + | + | + | + | + | + |
| Channa striata | – | – | + | + | – | – | – | + | – | – | – | + |
| Cyprinidae | ||||||||||||
| Amblypharyngodon mola | – | – | – | + | – | – | – | – | – | – | – | + |
| Catla catla | – | – | – | – | – | – | + | – | – | – | – | – |
| Cirrhinus mrigala | – | – | – | + | – | – | + | – | – | – | – | – |
| Cyprinus carpio | – | – | – | – | – | + | + | – | – | + | – | – |
| Punctius jayarami | + | – | – | + | – | – | + | + | – | + | – | – |
| Clariidae | ||||||||||||
| Clarius batracus | + | – | + | – | – | – | – | – | – | – | – | – |
| Gobiidae | ||||||||||||
| Glossogobius giuris | – | – | – | – | – | – | – | + | – | – | – | – |
| Heteropneustidae | ||||||||||||
| Heteropneustus fossilus | – | + | + | + | + | – | + | + | + | – | – | – |
| Notopteridae | ||||||||||||
| Notopterus notopterus | – | – | – | – | – | – | – | + | – | – | – | – |
| Siluridae | ||||||||||||
| Wallago attu | – | – | + | – | – | – | – | – | – | – | – | – |
| Symbranchidae | ||||||||||||
| Monopterus cuchia | – | – | – | – | + | – | – | – | – | – | – | – |
“+” localities where collections were made
Table 2.
Crab hosts examined from different localities in Manipur
| Name of the host | Locality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Luwangsangbam | Takhel | Yeingangpokpi | Chajing | Kangchup | Khurkhul | Leimaram | Bishnupur | Thoubal | Motbung | Ukhrul | |
| Barythelphusa lugubris masoniana | + | + | + | + | – | + | + | + | + | – | + |
| Potamiscus manipuriensis | + | – | + | – | + | – | – | – | – | + | + |
Examination of the host for metacercariae
The gastrointestinal tract, other internal organs and muscle tissue of the fish hosts were teased in 0.7 % saline at room temperature and examined for recovery of the metacercarial and/or adult stages of the fluke parasites.
For isolation of metaceracaria from muscle tissue of the crab host, the minced tissue was digested overnight by incubating at 37 °C in artificial gastric juice following the earlier described procedure (Tandon et al. 2007). The digested material was filtered through a mesh-wire sieve, and the filterable sediment was then examined under a dissecting stereoscopic microscope for recovery of metacercariae.
The recovered metacercariae were duly processed for identification and preserved for further use.
Light microscopy (LM)
For the purpose of whole mount preparations, encysted metacercariae were made to excyst by providing a gentle mechanical pressure and flattened between a glass slide and cover glass. Specimens fixed in 70 % ethyl alcohol were processed with suitable whole mount preparation following the standard protocol. Observations were made with the help of Leitz Ortholux-2 research microscope.
Scanning electron microscopy (SEM)
Metacercariae were fixed in 10 % neutral buffered formalin and processed following the standard protocol for SEM (Roy and Tandon 1991). Observations were made with JSM 35CF (JEOL) and LEO 435 VP SE microscopes at electron-accelerating voltages ranging between 10 and 20 kV.
Spectrum of metacercariae
Preliminary identification, based on morphological criteria, was done following the standard literature (Yamaguti 1971; Bray et al. 2008; Gibson et al. 2002). The prevalence, mean intensity and abundance of metacercaria infections were calculated following Bush et al. (1997).
Results
Metacercariae in fishes
The status of metacercarial infections prevalent in commonly edible fish hosts from various localities of the study area is shown in Table 3. A total of 1737 fishes of sixteen species (belonging to eleven families) were surveyed (Table 1). Fishes from only one family, namely Channidae, were found to be infected with metacercariae, with a low infection rate of 1.44 %. Based on morphological and morphometric criteria (Rim et al. 2008; Sohn et al. 2009), the recovered metacercariae were identified as belonging to genera—Euclinostomum (Rudolphi 1809) Travassos 1928 (family Clinostomidae); Posthodiplostomum Dubois 1936 and Lophosicyadiplostomum Dubois 1936 (family Diplostomidae) and Polylekithum Arnold 1934 (family Allocreadiidae), all of which are briefly described with a mention of important characters as follows (Fig. 2).
Table 3.
Status of metacercarial infection in fishes surveyed from different localities in Manipur
| Name of host | Locality-numberwise of host examined | Location of parasite in host | Metacercaria recovered |
|---|---|---|---|
| Channa punctatus | Lamphel-101 | Body muscle | Polylekithum sp. & Posthodiplostomum sp. |
| Mayangimphal-69 | – | – | |
| Moirang-250 | Liver | Euclinostomum heterostomum | |
| Nambol-60 | – | – | |
| Porompat-65 | – | – | |
| Tengtha-51 | – | – | |
| Tera-30 | – | – | |
| Thoubal-56 | – | – | |
| Wangoi-26 | – | – | |
| Channa straita | Lamphel-3 | – | – |
| Mayangimphal-6 | – | – | |
| Moirang-69 | Intestinal lumen | Lophosicyadiplostomum sp. | |
| Thoubal-6 | – | – |
Fig. 2.
LM pictures of the encysted (b, d) and excysted (a, c, e, f) metacercariae recovered from fish hosts. a Euclinostomum heterostomum; b, c Polylekithum sp.; d, e Posthodiplostomum sp.; f Lophosicyadiplostomum sp.
Euclinostomum heterostomum
Excysted metacercaria body stout; 5.50 × 2.20 mm in size; oral sucker surrounded by collar-like folds, relatively smaller than ventral; ventral sucker large, highly developed; intestinal caeca long, with lateral diverticular branching; reproductive organs well developed, testes irregular in shape; cirrus sac intertesticular; ovary small, round, intertesticular; uterus reaching forward up to ventral sucker.
Polylekithum sp.
Metacercarial cyst oval in shape, 0.50 × 0.35 mm in size. Excysted metacercaria elongate, 0.88–1.08 × 0.35–0.41 mm in size; body unspined; oral sucker subterminal; prepharynx short; intestinal caeca wide, reaching posterior extremity; reproductive organs under-developed; excretory bladder showing presence of granules.
Posthodiplostomum sp.
Metacercarial cyst oval in shape, 0.64–0.67 × 0.53–0.56 mm. Excysted metacercaria with distinctly bipartite body, 0.50–0.68 mm long; forebody lanceolate, 0.40–0.50 × 0.32–0.57 mm, hindbody oval, 0.10–0.18 × 0.14–0.20 mm; oral and ventral suckers feebly developed; holdfast almond-shaped; testes tandem; ovary ellipsoidal, pretesticular.
Lophosicyadiplostomum sp.
Metacercaria nonencysted, body bipartite, 0.77–0.89 mm in length; forebody 0.52–0.54 × 0.23–0.29 mm, hindbody 0.26–0.28 × 0.16–0.18 mm; oral sucker elliptical, with equatorial muscular rings surrounding it dorsally and laterally; pseudosuckers present; ventral sucker small, located in forebody; holdfast organ circular, with central cavity; testes tandem, globular; ovary oval, pretesticular.
Metacercariae in crab hosts
The status of metacercarial infection prevalent in crab hosts (represented by Barytelphusa lugubris masoniana (Gecarcinucidae) and Potamiscus manipuriensis (Potamidae)) from the study area is shown in Table 4. Both species were found to be infected with metacercariae, with an infection rate of 28.90 %. Based on morphological and morphometric criteria the recovered metacercariae were identified as two species of the genus Paragonimus (family Paragonimidae) and two more types representing the genus Microphallus (family Microphallidae) (Fig. 3), as briefly described below.
Table 4.
Status of metacercarial infection in crab species surveyed from different localities in Manipur
| S. no. | Host species | Locality surveyed | No. of hosts examined | Status of metacercarial infection |
|---|---|---|---|---|
| 1. | Barythelphusa lugubris masoniana | Bishenpur | 10 | – |
| Chajing | 30 | – | ||
| Kangchup (Singda) | 16 | – | ||
| Khurkhul | 40 | – | ||
| Leimaram | 35 | – | ||
| Luwangsangbam | 20 | – | ||
| Takhel | 60 | – | ||
| Thoubal | 46 | – | ||
| Ukhrul | 16 | – | ||
| Yeingangpokpi | 326 (total = 599) | P. westermani | ||
| 2. | Potamiscus manipuriensis | Kangchup (Singda) | 30 | – |
| Luwangsangbam | 35 | – | ||
| Motbung | 175 | Microphallus sp. 1 and Microphallus sp. 2 | ||
| Ukhrul | 50 | P. heterotremus | ||
| Yeingangpokpi | 45 (total = 335) | – |
Fig. 3.
LM pictures of the encysted (a, c, e) and excysted (b, d, f, g) metacercariae recovered from crab hosts (Scale bar 50 μm). a, b Paragonimus westermani. c, d Paragonimus heterotremus. e, f Microphallus sp.-type 1. g Microphallus sp.-type 2
Paragonimus westermani
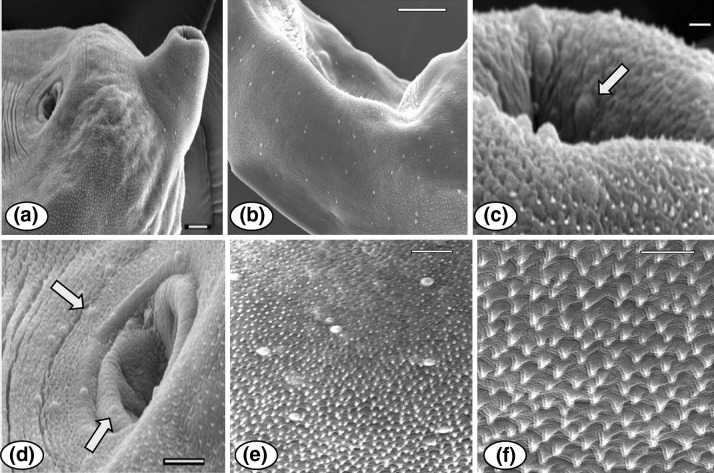
Encysted metacercaria round in shape, 0.81 mm in diameter. Excysted metacercaria 0.47–0.61 × 0.24–0.36 mm in size; whole body covered with single pointed spines and small dome shaped papillae, latter also dispersed around oral and ventral suckers (Fig. 4); oral sucker terminal; ventral sucker pre equatorial, larger than oral sucker; pharynx present, oesophagus very short; intestinal bifurcation in anterior forebody, caeca undulating terminating at posterior end; reproductive anlagen poorly developed.
Fig. 4.
SEM views of Paragonimus westermani metacercaria: a Excysted metacercaria, showing the oral and ventral suckers, also the presence of papillae in anterior end of the body (Scale bar 20 μm), b showing the distribution of papillae on the lateral side of the body (Scale bar 50 μm), c view of the anterior region near the oral sucker, showing the presence of randomly distributed papillae (arrow) along with body spines (Scale bar 2 μm), d ventral sucker region, showing the presence of papillae around the sucker (arrows) (Scale bar 10 μm), e tegumental surface showing the single-pointed, backwardly directed spination pattern and randomly scattered papillae (Scale bar 10 μm), f tegument spination a magnified view (Scale bar 5 μm)
P. heterotremus
Encysted metacercaria rounded, 0.43 mm in diameter. Excysted form 0.41–0.52 × 0.15–0.23 mm in size; single-pointed tegumental spines distributed all over body surface, pointing towards posterior side; oral sucker terminal; ventral sucker pre-equatorial, distinct, exhibiting rings of papillae; pharynx present, oesophagus very short; intestinal bifurcation at anterior forebody, caeca undulating, terminating at posterior end; reproductive organs not yet developed (Fig. 5).
Fig. 5.
SEM of P. heterotremus metacercaria: a Excysted metacercaria, showing the oral and ventral suckers (Scale bar 100 μm), b the oral end in a magnified view (Scale bar 10 μm); c tegumental spination in anterior region (Scale bar 5 μm); (d, e) ventral sucker, showing circum-sucker rows of papillae (Scale bar 20 and 10 μm), (f) posterior end of the body, showing the backwardly directed spination pattern (Scale bar 10 μm)
Microphallus spp
Metacercaria Microphallus-type 1: cyst form rounded, thin-walled, 0.83 mm in diameter. Excysted form having globular body, 0.42–0.52 × 0.37–0.50 mm in size; ventral sucker post equatorial; oesophagus long; caeca short, divergent just anterior to ventral sucker; testes postovarian, symmetrical; ovary dextral to ventral sucker, vitellarian clusters present in groups of 6–7, posterior to each testis.
Metacercaria Microphallus-type 2: excysted form elongated, 0.46–0.54 mm in length, 0.20–0.24 mm in width; thick muscular structure in anterior body surrounding oral sucker; ventral sucker large-sized; ovary dextral to ventral sucker; testes postovarian, cylindrical in shape, arranged on either posteriolateral side of body.
The host fish Channa punctatus was found to harbour 3 metacercarial types, whereas C. striatus was found infected with only one metacercarial type. All metacercarial cysts were recovered from the muscle tissue except for Lophosicyadiplostomum sp., which was found in the intestinal lumen (Table 3). Posthodiplostomum sp. showed the highest prevalence (2.33 %), though a low abundance (0.27) and Euclinostomum heterostomum, the lowest prevalence (0.25 %); for other species the prevalence ranged between 0.51 and 1.19 % (Table 5; Fig. 6a). Among the paragonimids, Paragonimus westermani showed a higher prevalence (25.04 %) and abundance (1.50) compared to P. heterotremus. The microphallids also showed a prevalence ranging between 15 and 16 % (Table 5; Fig. 6b).
Table 5.
Spectrum of metacercarial infections in the commonly edible fish and crabs species in the respective foci
| Host species surveyed | Metacercaria | Location | No. of host | Prevalence (%) | No. of metacercaria recovered per host | Mean intensity | Abundance | |
|---|---|---|---|---|---|---|---|---|
| Examined | Infected | |||||||
| Fishes | ||||||||
| Channa punctatus | Euclinostomum heterostomum | Liver | 771 | 2 | 0.25 | 1 | – | – |
| Polylekithum sp. | Muscle | 771 | 4 | 0.51 | 1–3 | 2.25 | 0.01 | |
| Posthodiplostomum sp. | Muscle | 771 | 18 | 2.33 | 2–30 | 11.50 | 0.27 | |
| Channa straita | Lophosicyadiplostomum sp. | Intestine | 84 | 1 | 1.19 | 6 | 6.00 | 0.08 |
| Crabs | ||||||||
| Barythelphusa lugubris masoniana | Paragonimus westermani | Muscle | 599 | 150 | 25.04 | 5–20 | 6.00 | 1.50 |
| Potamiscus manipuriensis | Paragonimus heterotremus | Muscle | 335 | 15 | 4.47 | 1–3 | 1.67 | 0.08 |
| Microphallus sp. 1 | Muscle | 335 | 55 | 16.41 | 2–7 | 3.27 | 0.54 | |
| Microphallus sp. 2 | Muscle | 335 | 50 | 14.92 | 1–3 | 0.48 | 0.08 | |
Fig. 6.
Prevalence of metcercariae in a fish hosts: 1, Euclinostomum heterostomum; 2, Polylekithum sp.; 3, Posthodiplostomum sp.; 4, Lophosicyadiplostomum sp. b crab hosts: 1, Paragonimus westermani; 2, P. heterotremus; 3, Microphallus sp.-type 1; 4, Microphallus sp.-type 2
Discussion
As per the reports of WHO fish-borne trematode zoonoses are a serious health hazard (WHO 1995) and the subject has been recently reviewed (Chai et al. 2005). In the present study, among all the fish species studied, only the members of the family Channidae were found to harbour metacercariae. The channid fishes are reported to harbour metacercariae of several trematodes, e.g. Atrophecaecum hindusthanensis, Clinostomum sp., E. heterostomum, Diplostomulum cerebralis, Neascus gussevi, Metaclinostomum srivastavi and Tetracotyl szidati, to name a few (Chakrabarti 1974; Jhansilakshmibai and Madhavi 1997; Thapa et al. 2008; Vankara et al. 2011). Several species of Euclinostomum have been reported from India; these include E. indicum and E. heptacaecum from C. punctatus and E. channi from C. marulius (Bhalerao 1942; Jaiswal 1957). In the present study E. heterostomum, found encysted in the liver of C. punctatus, showed a low prevalence (0.25 %). In a similar study carried out in Meghalaya (another state in northeast India), C. striatus and C. punctatus also showed a low prevalence of E. heterostomum (1.44 and 0.74 %, respectively) (Thapa et al. 2008). Metacercarial infection of Clinostomum sp. that causes considerable damage to the viscera and muscles of many fish species has been reported from C. punctatus and Heteropneustus fossilis (Kalantan et al. 1987; Thapa et al. 2008; Vankara et al. 2011; Shareef and Abidi 2012). Besides affecting the nutritional value and/or mortality rates of the infected fish, infections by metacercariae of Clinostomum species are also important as potential fish-borne zoonoses (Kamo et al. 1962; Chung et al. 1995; Kitagawa et al. 2003; Dzikowski et al. 2004; Park et al. 2009). These parasites have been reported to cause laryngopharyngitis or even asphyxia and ocular parasitosis in human subjects (Eiras 1994; Tiewchaloern et al. 1999). However, in the present study, the Clinostomum infection was not detected in the fishes examined, though C. punctatus was found infected with three other types of metacercariae. Species of Polylekithum (= Procreadium) are known to occur in cyprinid fishes and have been reported from birds also ( Verma 1936; Vidyarthi 1938; Jaiswal 1957; Kakaji 1969). It is for the first time that Polylekithum sp. infection has been reported from C. punctatus through the present study. The same host also harboured the metacercaria of Posthodiplostomum sp. as coinfection with Polylekithum sp. Many species of Posthodiplostomum (namely P. austral, P. oblongum, P. opisthosicye, P. botauri, P. grayii and P. milvi) have been reported from birds (Dubois 1937, 1969; Vidyarthi 1938; Verma 1936; Fotedar and Raina 1965); a few reports are there from crucian carp and common carp (Ishii 1951; Nagasawa et al. 1989) and from Channa argus as well (Nguyen et al. 2012). Channa striata also harbours metacercariae of Haplorchis sp., Clinostomum complanatum and E. heterostomum (Chakrabarti 1974; Thapa et al. 2008). In the present study, C. striata was found to be infected with the metacercaria of Lophosicyadiplostomum (family Diplostomidae)—the first report from the region.
As in the present study, natural infections of P. westermani metacercariae have been reported in B. masoniana in several foci of Arunachal Pradesh (North-east India) (Tandon et al. 2007; Devi et al. 2010). Potamiscus manipurensis, Alcomon superciliosum and Barytelphusa lugubris are identified as common potential second intermediate hosts for other Paragonimus species as well including P. heterotremus, P. hueit’ungensis, and P. skrjabini in Northeast India (Singh and Singh 1997; Singh 2002, 2003; Singh et al. 2006, 2007, 2009, 2012). In India P. westermani has been reported from various carnivorous mammalian hosts such palm civet cat, domestic dogs, panther, cat, tiger and mongoose (Rao 1935; Srivastava 1938; Dutta and Gupta 1978; Singh and Somvanshi 1978; Gaur et al. 1980; Parihar and Shrivastava 1988; Blair et al. 1999). An epidemiological survey carried out (during 1980s) in the Manipur region revealed the prevalence of paragonimiasis in human subjects in the region (Singh et al. 1993). In the present survey, P. manipuriensis, also harboured other metacercariae of the microphallid trematode genus—Microphallus, beside P. heterotremus. Metacercarial stages of Microphallus have been earlier reported from sand crabs and brackish-water prawns (Anantaraman and Subramoniam 1976; Jayasree et al. 2001). Metacercariae of Microphallus indicus have been reported from B. lugubris in Meghalaya (Goswami et al. 2013). However, the two microphallid metacercaria types recovered during the present study were morphologically very different from M. indicus. So far, from India only a few microphallid taxa have been identified, namely Basantisia ramai, Levinseniella indica and Pseudospeloterma indicum from birds (Lal 1936; Pande 1938; Murhar 1960), Mehraformes jabalpurensis and M. indicus from reptiles (Bharadwaj 1963; Mukherjee and Ghosh 1967), Megalatriotrema hispidum from the common frog (Rao 1969), and Spelotrema narii from jackals (Rao 1965).
The overall prevalence of metacercarial infections in the crustacean hosts was found to be optimally high (35.8 %). Among the crab species surveyed, Barythelphusa emerged as the potent vector and transmitter host for P. westermani, a high prevalence (25.04 %) of which was recorded in the region. Paragonimiasis caused by P. westermani is one of the medically important food-borne trematodiases in the tropical, subtropical, and some temperate countries (Miyazaki 1991; Blair et al. 1999).
As evident from the foregoing account, amongst the metacercarial infections prevalent in the region only two—Clinostomum sp. occurring in channid fishes, and Paragonimus spp in crustacean hosts—are known to be having zoonotic implications. However, Clinostomum infections were not encountered in the present study. Therefore, in view of prevalent culinary practices and food habits of the natives, paragonimiasis emerged as the only (crab-borne) potential zoonosis and warrants a thorough epidemiological study in suspected focal areas of infection in the region.
Acknowledgments
The study was carried out under the “North-East Parasite Information and Analysis Centre (NEPIAC)” sanctioned to VT and funded by Department of Information Technology (Ministry of Communication and Information Technology, GOI). Financial support in the form of ‘Junior Research Fellowship (NEPIAC)’, ‘University Grants Commission Research Fellowship in Science for Meritorious Students’ and ‘Council of Scientific & Industrial Research (CSIR) Senior Research Fellowship’ to VDA is gratefully acknowledged.
References
- Anantaraman S, Subramoniam T. On a microphallid metacercaria occurring in the ovaries of the sand crabs Emerita asiatica and Albunea symnista on the Madras coast. Proc Indian Acad Sci B. 1976;84(5):192–199. [Google Scholar]
- Arafa MI, Shaheen MS, Monib MEM. Studies on some clinostomatid metacercariae from Tilapia nilotica in Assiut Governorate. Assiut Vet Med J. 2005;51(107):218–227. [Google Scholar]
- Bhalerao GD. Some metacercarial forms of Clinostomatidae (Trematoda) from India. Proc Indian Acad Sci. 1942;16:67–71. [Google Scholar]
- Bharadwaj ON. The morphology of a new trematode Mehraformes jabalpurensis n.g., n. sp. (Microphallidae) Proc Natl Acad Sci India. 1963;33:245–250. [Google Scholar]
- Blair D, Xu ZB, Agatsuma T. Paragonimiasis and the genus Paragonimus. Adv Parasitol. 1999;42:174–178. doi: 10.1016/s0065-308x(08)60149-9. [DOI] [PubMed] [Google Scholar]
- Bray RA, Gibson DI, Jones A. Keys to the Trematoda. UK: Natural History Museum, CABI; 2008. [Google Scholar]
- Bullard SA, Overstreet RM. Digeneans as enemies of fishes. In: Eiras J, Segner H, Wahil T, Kapoor BG, editors. Fish diseases. US: Science; 2008. pp. 817–976. [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–583. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- Chai JY, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Chakrabarti KK. Studies on some metacercariae of the Indian freshwater fishes Channa punctatus (Bloch) and C. striatus (Bloch) Rev Iber Parasitol. 1974;34:1–2. [Google Scholar]
- Chung DI, Moon CH, Kong HH, Choi DW, Lim DK. The first human case of Clinostomum complanatum (Trematoda: Clinostomidae) infection in Korea. Korean J Parasitol. 1995;33:219–223. doi: 10.3347/kjp.1995.33.3.219. [DOI] [PubMed] [Google Scholar]
- Devi KR, Narain K, Agatsuma T, Blair D, Nagataki M, Wickramasinghe S, Yatawara L, Mahanta J. Morphological and molecular characterization of Paragonimus westermani in northeastern India. Acta Trop. 2010;116:31–38. doi: 10.1016/j.actatropica.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Dixon BR, Flohr RB. Fish and shellfish-borne trematode infections in Canada. Southeast Asian J Trop Med Public Health. 1997;28:58–64. [PubMed] [Google Scholar]
- Dubois G. Contribution à ľétude des Diplostomes ďoiseaux (Diplostomidae Poirier, 1886) du Musée de Vienne. Bull Soc Neuchât Sc Nat. 1937;62:99–128. [Google Scholar]
- Dubois G. Notes helminthologiques. II: Diplostomatidae Poirier et Cyathocotylidae Poche (Trematoda) Rev Suisse Zool. 1969;76(2):3–21. doi: 10.5962/bhl.part.146029. [DOI] [PubMed] [Google Scholar]
- Dutta S, Gupta PP. Paragonimiasis in a bear cat (Articus binturong) Ann Trop Med Parasitol. 1978;72:391–393. doi: 10.1080/00034983.1978.11719335. [DOI] [PubMed] [Google Scholar]
- Dzikowski R, Levy MG, Poore MF, Flowers JR, Paperna I. Clinostomum complanatum and Clinostomum marginatum (Rudolphi, 1819) (Digenea: Clinostomidae) are separate species based on differences in ribosomal DNA. J Parasitol. 2004;90:413–414. doi: 10.1645/GE-159R. [DOI] [PubMed] [Google Scholar]
- Eiras JC. Elementos de ictioparasitologia. Porto: Fundação Eng. António de Almeida; 1994. [Google Scholar]
- Fotedar DN, Raina MK. On a new species of the trematode genus Posthodiplostomum Dubois, 1936, from Milvus migrans lineatus (gray), common Kite in Kashmir. Kashmir Sc. 1965;1(1–2):64–69. [Google Scholar]
- Gaur SNS, Tewari HC, Sethi MS, Prakash OM. Helminth parasites from tiger (Panthera tigris) in India. Indian J Parasitol. 1980;4:71–72. [Google Scholar]
- Gholami Z, Mobedi I, Esmaeili HR, Kia EB. Occurrence of Clinostomum complanatum in Aphanius dispar (Actinoptrygii: Cyprinodontidae) collected from Mehran River, Hormuzgan Province, South of Iran. Asian Pac J Trop Biomed. 2011;1:189–192. doi: 10.1016/S2221-1691(11)60025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DI, Jones A, Bray RA. Keys to the Trematoda. UK: Natural History Museum, CABI; 2002. [Google Scholar]
- Goswami LM, Prasad PK, Biswal DK, Chatterjee A, Tandon V. Crustacean-borne infections with microphallid metacercariae (Digenea: Microphallidae) from focal areas in Meghalaya, north-east India. J Helminthol. 2013;87(2):222–229. doi: 10.1017/S0022149X12000260. [DOI] [PubMed] [Google Scholar]
- Gustinelli A, Caffara M, Florio D, Otachi EO, Wathuta EM, Fioravanti ML. First description of adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey heron Ardea cinerea L. and a redescription of the metacercaria from Nile tilapia Oreochromis niloticus niloticus L. in Kenya. Syst Parasitol. 2010;76:39–51. doi: 10.1007/s11230-010-9231-5. [DOI] [PubMed] [Google Scholar]
- Han ET, Shin EH, Phommakorn S, Sengvilaykham B, Kim JL, Rim HJ, Chai JY. Centrocestus formosanus (Digenea: Heterophyidae) encysted in the freshwater fish, Puntius brevis, from Lao PDR. Korean J Parasitol. 2008;46:49–53. doi: 10.3347/kjp.2008.46.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S. Diplostomiasis of crucian carp in Japan. Dobutsugaku-zasshi. 1951;27(321):410–411. [Google Scholar]
- Jaiswal GP. Studies on the trematode parasites of fishes and birds found in Hyderabad State. Zool Jahrb Abt Syst Part I–IV. 1957;85(12):1–72. [Google Scholar]
- Janardanan KP, Ramanandan SK, Usha NV. On the progenetic metacercaria of Pleurogenoides ovatus Rao, 1977 Trematoda Pleurogenitinae from the freshwater crab, Paratelphusa hydrodromous Herbst, with observations on its in vitro excystment. Zool Anz. 1987;219(5–6):313–320. [Google Scholar]
- Jayasree L, Janakiram P, Madhavi R. Epibionts and parasites of Macrobrachium rosenbergii and Metapenaeus dobsoni from Gosthani estuary. J Nat Hist. 2001;35:157–167. doi: 10.1080/00222930150215297. [DOI] [Google Scholar]
- Jhansilakshmibai K, Madhavi R. Euclinostomum heterostomum (Rudolphi, 1809) (Trematoda): life-cycle, growth and development of the metacercaria and adult. Syst Parasitol. 1997;38:51–64. doi: 10.1023/A:1005829625739. [DOI] [Google Scholar]
- Kakaji VL. Studies on helminth parasites of Indian fishes. Part III. On some species of the genus Allocreadium Looss, 1900. Ann Parasitol. 1969;44:131–146. doi: 10.1051/parasite/1969442131. [DOI] [PubMed] [Google Scholar]
- Kalantan AMN, Arfin M, Nizami WA. Seasonal incidence and pathogenicity of the metacercariae of Clinostomum complanatum in Aphanius dispar. Jpn J Parasitol. 1987;36:17–23. [Google Scholar]
- Kamo H, Ogino K, Hatsushika ARA. Unique infection of man with Clinostomum sp., a small Trematoda causing acute laryngitis. Yonago Acta Med. 1962;6:37–40. [Google Scholar]
- Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22(3):466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa N, Oda M, Totoki T, Washizaki S, Oda M, Kifune T. Lidocaine spray used to capture a live Clinostomum parasite causing laryngitis. Am J Otolaryngol. 2003;24:341–343. doi: 10.1016/S0196-0709(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kumari DCH. A new species of strigeid metacercaria, Neascus punctatusi (Trematoda: Diplostomatidae) from an Indian freshwater fish, Channa punctatus (Bloch) Riv di Parassitol. 1994;11(2):245–249. [Google Scholar]
- Lal MB. A new species of genus Levinseniella from the jack snipe, Gallinago gallinule. Proc Indian Acad Sci. 1936;4:92–96. [Google Scholar]
- Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- Mahanta MC. Paragonimiasis. In: Parija CS, editor. Reviews of parasitic zoonoses. Delhi: AITBS; 1990. pp. 196–203. [Google Scholar]
- Malek M, Mobedi I. Occurrence of Clinostomum Complanatum (Rudolphi, 1819) (Digenea: Clinostomatidae) in Capoeta capoeta gracilis (Osteichthys: Cyprinidae) from Shiroud River, Iran. Iran J Public Health. 2001;30:95–98. [Google Scholar]
- Miyazaki I. An illustrated book of helminthic zoonoses. 1. Tokyo: International Medical Foundation of Japan; 1991. [Google Scholar]
- Mukherjee RP, Ghosh RK. On two new trematodes of the genus Microphallus. Zool Anz. 1967;178:342–347. [Google Scholar]
- Murhar BM. On a new host record of the trematode Basantisia ramai Pande from the pigeon ColumbaDomestica Gmel at Nagpur. Bull Zool Soc. 1960;3:79–81. [Google Scholar]
- Nagasawa K, Awakura T, Urawa S. A checklist and bibliography of parasites of freshwater fishes of Hokkaido. Sci Rep Hokkaido Fish Hatch. 1989;44:1–49. [Google Scholar]
- Nakamura-Uchiyama F, Mukae H, Nawa Y. Paragonimiasis: a Japanese perspective. Clin Chest Med. 2002;23:409–420. doi: 10.1016/S0272-5231(01)00006-5. [DOI] [PubMed] [Google Scholar]
- Narain K, Devi KR, Mahanta J. Paragonimus and paragonimiasis—a new focus in Arunachal Pradesh, India. Curr Sci. 2003;84:985–987. [Google Scholar]
- Nguyen TC, Li YC, Makouloutou P, Jimenez LA, Sato H. Posthodiplostomum sp. metacercariae in the trunk muscle of northern snakeheads (Channa argus) from the Fushinogawa River, Yamaguchi, Japan. J Vet Med Sci. 2012;74(10):1367–1372. doi: 10.1292/jvms.12-0025. [DOI] [PubMed] [Google Scholar]
- Pande BP. On a new genus of the Pleurogenetinae (Lecithodendriidae) from a kingfisher. Ann Mag Nat Hist. 1938;2:199–203. doi: 10.1080/03745481.1938.9755452. [DOI] [Google Scholar]
- Parihar NS, Shrivastava SN. Bronchial hyperplasia in a tiger (Panthera tigris) Indian J Anim Sci. 1988;58:230–233. [Google Scholar]
- Park CW, Kim JS, Joo HS, Kim J. A human case of Clinostomum complanatum infection in Korea. Korean J Parasitol. 2009;47:401–404. doi: 10.3347/kjp.2009.47.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MAN. Lung flukes in two dogs in Madras Presidency. Indian J Vet Sci Anim Husb. 1935;5(1):30–32. [Google Scholar]
- Rao BV. Helminth parasites of an Indian jackal (Canis aureus naria): Ancylostoma braziliense (Gomez 1910) Leiper 1915, Rectularia affinis (Jagerskiold, 1904) (Nematoda); and Spelotrema narii n. sp. (Trematoda) Indian J Helminthol. 1965;17:68–84. [Google Scholar]
- Rao R. On Megalatriotrema hispidum, a new genus and new species of microphallid trematode from frogs in Andhra Pradesh, India. Ann Parasitol. 1969;44:125–130. doi: 10.1051/parasite/1969442125. [DOI] [PubMed] [Google Scholar]
- Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane municipality and Savannakhet Province, Lao PDR. Korean J Parasitol. 2008;46(4):253–260. doi: 10.3347/kjp.2008.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Tandon V. Usefulness of tetramethylsilane in the preparation of helminth parasites for scanning electron microscopy. Riv Parasitol. 1991;8:405–413. [Google Scholar]
- Shareef PAA, Abidi SMA. Incidence and histopathology of encysted progenetic metacercarial of Clinostomum complanatum (Digenea: Clinostomidae) in Channa punctatus and its development in experimental host. Asian Pac J Trop Biomed. 2012;2(6):421–426. doi: 10.1016/S2221-1691(12)60068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Souza AT, Ludwig G. Parasitism of Cichlasoma paranaense Kullander, 1983 and Gymnotus carapo Linnaeus, 1814 by Clinostomum complanatum (Rudolphi, 1814) metacercariae in the Taquari River. Braz J Biol. 2005;65:513–519. doi: 10.1590/S1519-69842005000300017. [DOI] [PubMed] [Google Scholar]
- Singh TS. Occurence of the Lung Fluke Paragonimus hueit’ungensis in Manipur, India. Chin Med J. 2002;65:426–429. [PubMed] [Google Scholar]
- Singh TS. Occurence of the lung fluke, Paragonimus heterotremus in Manipur, India. Chin Med Sci J. 2003;18(1):20–25. [PubMed] [Google Scholar]
- Singh TS, Singh YI. Three types of Paragonimus metacercariae isolated from Potamiscus manipurensis, Manipur. Indian J Med Microbiol. 1997;15(4):159–162. [Google Scholar]
- Singh NP, Somvanshi R. Paragonimus westermani in tigers (Panthera tigris) in India. J Wildl Dis. 1978;14:322–324. doi: 10.7589/0090-3558-14.3.322. [DOI] [PubMed] [Google Scholar]
- Singh TS, Mutum S, Razaque MA, Singh YI, Singh EY. Paragonimiasis in Manipur. Indian J Med Res. 1993;97:247–252. [PubMed] [Google Scholar]
- Singh HS, Rakhi RM, Priyavrat A, Kumar Y. Euclinostomum Srivastavi (Pandey and Baugh, 1970) n. comb., a rare larval trematode from Meerut, with a note on the genus Metaclinostomum. J Exp Zool. 2003;6(1):169–173. [Google Scholar]
- Singh TS, Singh DS, Sugiyama H. Possible discovery of Chinese lung fluke, Paragonimus skrjabini in Manipur, India. Southeast Asian J Trop Med Public Health. 2006;37(3):53–56. [PubMed] [Google Scholar]
- Singh TS, Sugiyama H, Rangsiruji A, Devi KR. Morphological and molecular characterizations of Paragonimus heterotremus, the causative agent of human paragonimiasis in India. Southeast Asian J Trop Med Public Health. 2007;38(1):82–86. [Google Scholar]
- Singh TS, Sugiyama H, Umehara A, Hiese S, Khalo K. Paragonimus heterotremus infection in Nagaland: a new focus of paragonimiasis in India. Indian J Med Microbiol. 2009;27(2):123–127. doi: 10.4103/0255-0857.49424. [DOI] [PubMed] [Google Scholar]
- Singh TS, Sugiyama H, Rangsiruji A. Paragonimus & paragonimiasis in India. Indian J Med Res. 2012;136(2):192–204. [PMC free article] [PubMed] [Google Scholar]
- Skov J, Kania P, Dalsgaard A, Jorgensen TR, Buchmann K. Life cycle stages of heterophyid trematodes in Vietnamese freshwater fishes traced by molecular and morphometric methods. Vet Parasitol. 2009;160(1–2):66–75. doi: 10.1016/j.vetpar.2008.10.088. [DOI] [PubMed] [Google Scholar]
- Sohn WM. Fish-borne zoonotic trematode metacercariae in the Republic of Korea. Korean J Parasitol. 2009;47(Suppl):S103–S113. doi: 10.3347/kjp.2009.47.S.S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn WM, Eom KS, Min DY, Rim HJ, Hoang EH, Yang Y, Li X. Fishborne trematode metacercariae in freshwater fish from Guangxi Zhuang autonomous region, China. Korean J Parasitol. 2009;47(3):249–257. doi: 10.3347/kjp.2009.47.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava HD. The occurrence of Paragonimus westermani in the lungs of cats in India. Indian J Vet Sci Anim Husb. 1938;8:157–255. [Google Scholar]
- Tandon V, Prasad PK, Chatterjee A, Bhutia PT. Surface fine topography and PCR-based determination of metacercaria of Paragonimus sp. from edible crabs in Arunachal Pradesh, Northeast India. Parasitol Res. 2007;102:21–28. doi: 10.1007/s00436-007-0715-4. [DOI] [PubMed] [Google Scholar]
- Thapa S, Jyrwa DB, Tandon V (2008) Platyhelminth parasite spectrum in edible freshwater fishes of Meghalaya. In: Tandon V, Yadav AK, Roy B (eds) Current trends in parasitology, proceedings of the 20th National Congress of Parasitology: 03–05 November 2008: India, Panima Publishing Corporation, New Delhi, pp 113–125
- Thuy DT, Kania P, Buchmann K. Infection status of zoonotic trematode metacercariae in Sutchi catfish (Pangasianodon hypophthalmus) in Vietnam: associations with season, management and host age. Aquaculture. 2010;302:19–25. doi: 10.1016/j.aquaculture.2010.02.002. [DOI] [Google Scholar]
- Tiewchaloern S, Udomkijdecha S, Suvouttho S, Chunchamsri K, Waikagul J. Clinostomum trematode from human eye. Southeast Asian J Trop Med Public Health. 1999;30:382–384. [PubMed] [Google Scholar]
- Toscano C, Yu SH, Nunn P, Mott KE. Paragonimiasis and tuberculosis, diagnostic—a review of the literature. Trop Dis Bull. 1995;92:R1–R26. [Google Scholar]
- Vankara AP, Vijayalakshmi C, Mani G. A report on various digenetic metacercariae from the freshwater fishes of River Godavari, Rajahmundry. J Parasit Dis. 2011;35(2):177–185. doi: 10.1007/s12639-011-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC. Notes on trematode parasites of Indian birds. Part I. Allahabad Univ Stud. 1936;12:147–188. [Google Scholar]
- Vianna RT, Pereira JJ, Brandao DA. Clinostomum complanatum (Digenea, Clinostomidae) density in Rhamdia quelen (Siluriformes, Pimelodidae) from south Brazil. Braz Arch Biol Technol. 2005;48:635–642. doi: 10.1590/S1516-89132005000500016. [DOI] [Google Scholar]
- Vidyarthi RD. New avian trematodes (family: Diplostomidae) from Indian birds. Proc Natl Acad Sci India. 1938;8(3):76–84. [Google Scholar]
- Vishwanath W, Lakra WS, Sarkar UK (2007) Fishes of North East India. National Bureau of Fish Genetic Resources, Lucknow, India, 264 pp
- World Health Organization (1995) Control of food borne trematode infections. WHO technical report series no. 849, pp 1–157 [PubMed]
- World Health Organization (2009) Foodborne trematode infections and taeniasis/cysticercosis. Report of the WHO expert consultation, Vientiane, Lao PDR, pp 1–50
- Yamaguti S. Synopsis of digenetic trematodes of vertebrates. Tokyo: Keigaku; 1971. [Google Scholar]