Abstract
Trichomonas vaginalis is a protozoan parasite that causes trichomoniasis; a cosmopolitan sexually transmitted disease. Metronidazole is the drug of choice for T. vaginalis infections. The increase in metronidazole resistant parasites and undesirable side effects of this drug makes the search for an alternative a priority for the management of trichomoniasis. Pistacia lentiscus mastic and Ocimum basilicum oil are known for their antibacterial, antifungal, antiviral and antiprotozoal effects. The present study was carried out to investigate the in vitro effects of P. lentiscus mastic and O. basilicum oil on T. vaginalis trophozoites. The effects of different concentrations of P. lentiscus mastic (15, 10 and 5 mg/ml) and different concentrations of O. basilicum oil (30, 20 and 10 μg/ml) on multiplication of trophozoites at different time points (after 24, 48, 72 and 96 h) were determined, also morphological changes were reported by transmission electron microscopy (TEM). The results showed that both plants caused an inhibition of growth of T. vaginalis trophozoites. The minimal lethal concentration of P. lentiscus mastic was 15 mg/ml after 24 h incubation, 10 mg/ml after 48 h and 5 mg/ml after 96 h. The minimal lethal concentration of O. basilicum oil was 30 μg/ml after 24 h incubation, 20 μg/ml after 48 h and 10 μg/ml after 96 h. TEM study of trophozoites treated by P. lentiscus mastic or by O. basilicum oil showed considerable damage of the membrane system of the trophozoites, and extensive vacuolization of the cytoplasm. These results highly suggest that P. lentiscus mastic and O. basilicum oil may be promising phytotherapeutic agents for trichomoniasis treatment.
Keywords: Trichomonas vaginalis, Trichomoniasis, Pistacia lentiscus mastic, Ocimum basilicum oil
Introduction
Trichomonas vaginalis (T. vaginalis) is the causative agent of trichomoniasis; the most common nonviral sexually transmitted disease, with 174 million new infections each year (WHO 2011). Infection with this protozoan may have serious consequences, such as adverse pregnancy outcomes (Cotch et al. 1997), predisposition to cervical cancer (Viikki et al. 2000) and pelvic inflammatory disease (Cherpes et al. 2006). Trichomoniasis is also a cofactor in human immunodeficiency virus transmission and acquisition (Van Der Pol et al. 2008). Moreover, Sutcliffe et al. (2006) reported a relationship between trichomoniasis and prostate cancer.
Although metronidazole is the drug of choice recommended by food and drug administration (FDA, USA) for the treatment of human trichomoniasis, carcinogenic and teratogenic effects as well as clinical or laboratory generated drug resistant isolates of T. vaginalis have been reported (Upcroft et al. 2006). According to some reports, metronidazole resistance is a major problem in clinical isolates of T. vaginalis in the USA and worldwide which has limited the therapeutic effectiveness of metronidazole (Rasolson et al. 2002). In addition, some side effects such as headache, dry mouth, metallic taste, glossitis and urticaria have been described (Upcroft et al. 2006).
In this context, there is an urgent need for new trichomonacidal agents with high effectiveness and low toxicity. The study of plants used by traditional medicine is a mean for finding alternative treatment, and several anti parasitic properties of many new natural product groups have been identified with their surprising efficacy (Kayser et al. 2003).
Pistacia lentiscus mastic is a natural resin derived from the stem and leaves of the mastic tree, P. lentiscus Linn, native to Mediterranean areas (Takahashi et al. 2003). Traditional healers used it for the relief of upper abdominal discomfort, stomachaches, dyspepsia and peptic ulcer (Koo et al. 2000). Substantial evidence has also suggested that P. lentiscus mastic gum exhibits hepatoprotective, cardioprotective, anti-inflammatory and antioxidant properties (Giaginis and Theocharis 2011). Numerous studies showed that P. lentiscus mastic has impressive antibacterial and antimicrobial properties (Takahashi et al. 2003).
Balan et al. (2007) suggested that P. lentiscus mastic gum might be developed into a chemotherapeutic agent for the treatment of human cancer colon and other cancers. Several studies further evaluated the potential antiproliferative properties of mastic gum against several types of neoplasia (Giaginis and Theocharis 2011). Pistacia khinjuk mastic, another species of the genus Pistacia, showed an antihelminthic effect against protoscoleces of Echinococcus granulosus (Taran et al. 2009). Markovics et al. (2012) strongly suggested that feeding goats to P. lentiscus foliage alleviates coccidiosis. Furthermore, Taran et al. (2010) reported that topical rubbing of Pistacia atlantica mastic decreased skin lesion size in the BALB/c mice infected with Leishmania major compared with that in the control, and it was concluded that P. atlantica mastic can be used for controlling cutaneous leishmaniasis caused by L. major and inhibiting development of cutaneous leishmaniasis lesions.
Ocimum basilicum Linn, a member of the Lamiaceae family, is an annual herb which grows in several regions around the world (Issazadeh et al. 2012). It is well known as a plant of a folk medicinal value. It is active against several species of bacteria and act as a natural powerful antifungal ingredient (Koba et al. 2009). O. basilicum oil contains rosmarinic acid, one of the most abundant caffeic acid esters; it has been reported to have antioxidant, anti-HIV and anti-inflammatory effects (Keita et al. 2000). Almeida et al. (2007) investigated the effects of O. basilicum essential oil on Giardia lamblia trophozoites, and they reported high antigiardial potential. In this context, the present in vitro study aimed to evaluate the anti-T. vaginalis activity of P. lentiscus mastic and O. basilicum oil. As far as we know, this is the first report demonstrating the potential anti-T. vaginalis activity of these natural products.
Materials and methods
In vitro culture
Trichomonas vaginalis trophozoites were isolated from female patients attending the outpatient clinic, Gynecology and Obstetrics Hospital, Ain Shams University. The trophozoites were cultured axenically in vitro at 37 °C in trypticase–yeast extract–maltose (TYM) medium (Diamond 1957), pH 6.0, supplemented with 1 ml heat-inactivated horse serum, crystalline penicillin (1,000,000 IU/ml), and streptomycin sulfate (100,000 μg/ml). Isolates were subcultured every 48 h in TYM medium and maintained in Parasitology Diagnostic and Research Unit, Faculty of Medicine, Ain Shams University.
Plant materials
Pistacia lentiscus mastic was obtained from Chios Gum Mastic Growers Association (Chios, Greece) and O. basilicum oil was obtained from Sigma-Aldrich (Germany), and each was dissolved in 5 % acetone (Duarte et al. 2009). T. vaginalis trophozoites were incubated in 5 ml TYM medium containing P. lentiscus mastic in different concentrations (5, 10, 15 mg/ml) for 24, 48, 72 and 96 h at 37 °C. Also, T. vaginalis trophozoites were incubated in 5 ml TYM medium containing O. basilicum oil in different concentrations (10, 20, 30 μg/ml) for 24, 48, 72 and 96 h at 37 °C.
Drug
The anti-T. vaginalis activity of the tested plant materials was compared with the drug metronidazole which was supplied as 250-mg tablets (Flagyl, Sanofi-Aventis, Egypt). Metronidazole tablets were dissolved in distilled water. T. vaginalis trophozoites were incubated in 5 ml TYM medium containing metronidazole in different concentrations (20 and 40 μg/ml) for 24, 48, 72 and 96 h at 37 °C.
Parasite control containing T. vaginalis trophozoites incubated in 5 ml TYM medium and solvent control containing T. vaginalis trophozoites incubated in 5 ml TYM medium with added solvent, were included in the study.
Each experiment was performed in duplicates
Evaluation of the P. lentiscus mastic and O. basilicum oil anti-T. vaginalis efficacy was done by:
- Detecting the inhibition of growth of T. vaginalis trophozoites by the tested plants materials. This was done by:
- Counting the number of trophozoites from culture tubes at time points of study using the improved Neubauer cell counting chamber and testing the viability of trophozoites using 0.3 % Trypan blue stain (Ali 2007).
- Calculation of motility percent of the trophozoites as: the ratio of motile number to total number of parasites counted per 10 high power field (Ali 2007).
- Calculation of percent of growth inhibition according to the equation: Percent of growth inhibition = a − b/a × 100 where a = mean number of trophozoites in parasite control tubes and b = mean number of trophozoites in tested tubes (Palmas et al. 1984).
Detecting the minimal lethal concentration of each of P. lentiscus mastic and O. basilicum oil according to Meri et al. (2000).
Transmission electron microscopy (TEM). The ultra-structure changes of T. vaginalis trophozoites were studied using TEM, after 24 h incubation period using P. lentiscus mastic (10 mg/ml) and O. basilicum oil (20 μg/ml). The trophozoites were fixed with 2.5 % (v/v) cold glutaraldehyde and processed for examination with JEOL–JEM-1010 transmission electron microscope (Cedillo-Rivera et al. 2002).
Statistical analysis
Data were analyzed using SPSS version 19 for Windows (SPSS Inc., Chicago, IL, USA). Data were presented as the mean ± SD of duplicates, percent of growth inhibition, percent of viability and percent of motility. The mean numbers were compared at the same time interval using student’s t test, the difference was considered significant when the p value was <0.05.
Ethical consideration
An informed consent was taken from all the patients after explaining the aim of the study to them. The study was approved by the Research Ethics Committee, Faculty of Medicine, Ain Shams University.
Results
The results of the present study are shown in Tables 1, 2, 3 and 4 and Figs. 1 and 2.
Table 1.
Effect of P. lentiscus mastic on the in vitro growth T. vaginalis trophozoites (106) at different incubation periods
| Dosage of treatment | After 24 h | After 48 h | After 72 h | After 96 h | ||||
|---|---|---|---|---|---|---|---|---|
| (Trophozoites number) Mean ± SD |
% of growth inhibition | (Trophozoites number) Mean ± SD |
% of growth inhibition | (Trophozoites number) Mean ± SD |
% of growth inhibition | (Trophozoites number) Mean ± SD |
% of growth inhibition | |
| Parasite control | (2.10–2.36) 2.23 ± 0.13 |
0 | (3.96–4.36) 4.16 ± 0.20 |
0 | (11.28–11.52) 11.4 ± 0.12 |
0 | (4.94–5.16) 5.05 ± 0.11 |
0 |
| Metronidazole, 20 μg | (0.78–0.82) 0.8 ± 0.02* |
64.1 | (0.07–0.73) 0.4 ± 0.33* |
90.4 | (0.03–0.17) 0.1 ± 0.07* |
99.1 | 0.00* | 100 |
| Metronidazole, 40 μg | 0.00* | 100 | 0.00* | 100 | 0.00* | 100 | 0.00* | 100 |
| Solvent control | (2.05–2.35) 2.2 ± 0.15 |
1.3 | (4.00–4.22) 4.11 ± 0.11 |
1.2 | (11.2–11.4) 11.3 ± 0.1 |
0.9 | (4.91–5.09) 5.0 ± 0.09 |
1 |
| Pistacia lentiscus mastic, 5 mg/ml | (1.18–1.32) 1.25 ± 0.07*,• |
44 | (0.58–0.62) 0.6 ± 0.02*,• |
85.6 | (0.25–0.55) 0.4 ± 0.15*,• |
96.4 | 0.00* | 100 |
| Pistacia lentiscus mastic,10 mg/ml | (0.1–0.35) 0.225 ± 0.125*,• |
90 | 0.00*,• | 100 | 0.00*,• | 100 | 0.00* | 100 |
| Pistacia lentiscus mastic, 15 mg/ml | 0.00*,** | 100 | 0.00*,• | 100 | 0.00*,• | 100 | 0.00* | 100 |
SD standard deviation
* p < 0.05, statistically significant difference in comparison to parasite control in the same time interval; ** p < 0.05, statistically significant difference in comparison to metronidazole 20 μg in the same time interval; • no statistically significant difference (p > 0.05) in comparison to metronidazole 20 μg
Table 2.
Percentage of viability and motility of T. vaginalis trophozoites in culture after exposure to P. lentiscus mastic at different incubation periods
| Dosage of treatment | After 24 h (%) | After 48 h (%) | After 72 h (%) | After 96 h (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Viability (%) | Motility (%) | Viability (%) | Motility (%) | Viability (%) | Motility (%) | Viability (%) | Motility (%) | |
| Parasite control | 100 | 98 | 100 | 76.2 | 100 | 21 | 100 | 9.6 |
| Metronidazole, 20 μg | 35.9 | 33.1 | 9.6 | 7.1 | 0.9 | 0.2 | 0 | 0 |
| Metronidazole, 40 μg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Solvent control | 98.7 | 97 | 98.8 | 76 | 99.1 | 20 | 99 | 9.5 |
| Pistacia lentiscus mastic, 5 mg/ml | 56 | 41 | 14.4 | 10.8 | 5.4 | 0.9 | 0 | 0 |
| Pistacia lentiscus mastic, 10 mg/ml | 10 | 8.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pistacia lentiscus mastic, 15 mg/ml | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 3.
Effect of O. basilicum oil on the in vitro growth T. vaginalis trophozoites (106) at different incubation periods
| Dosage of treatment | After 24 h | After 48 h | After 72 h | After 96 h | ||||
|---|---|---|---|---|---|---|---|---|
| (Trophozoites number) Mean ± SD |
% of growth inhibition | (Trophozoites number) Mean ± SD |
% of growth inhibition | (Trophozoites number) Mean ± SD |
% of growth inhibition | (Trophozoites number) Mean ± SD |
% of growth inhibition | |
| Parasite control | (2.10–2.36) 2.23 ± 0.13 |
0 | (3.96–4.36) 4.16 ± 0.20 |
0 | (11.28–11.52) 11.4 ± 0.12 |
0 | (4.94–5.16) 5.05 ± 0.11 |
0 |
| Metronidazole, 20 μg | (0.78–0.82) 0.8 ± 0.02* |
64.1 | (0.07–0.73) 0.4 ± 0.33* |
90.4 | (0.03–0.17) 0.1 ± 0.07* |
99.1 | 0.00* | 100 |
| Metronidazole, 40 μg | 0.00* | 100 | 0.00* | 100 | 0.00* | 100 | 0.00* | 100 |
| Solvent control | (2.05–2.35) 2.2 ± 0.15 |
1.3 | (4.00–4.22) 4.11 ± 0.11 |
1.2 | (11.2–11.4) 11.3 ± 0.1 |
0.9 | (4.91–5.09) 5.0 ± 0.09 |
1 |
| Ocimum basilicum oil, 10 μg/ml | (1.6–1.8) 1.7 ± 0.1*,• |
23.8 | (0.88–0.92) 0.9 ± 0.02*,• |
78.4 | (0.45–0.55) 0.5 ± 0.05*,** |
95.6 | 0.00* | 100 |
| Ocimum basilicum oil, 20 μg/ml | (0.5–0.7) 0.6 ± 0.1*,• |
73.1 | 0.00*,• | 100 | 0.00*,• | 100 | 0.00* | 100 |
| Ocimum basilicum oil, 30 μg/ml | 0.00*,** | 100 | 0.00*,• | 100 | 0.00*,• | 100 | 0.00* | 100 |
SD standard deviation
* p < 0.05, statistically significant difference in comparison to parasite control in the same time interval; ** p < 0.05, statistically significant difference in comparison to metronidazole 20 μg in the same time interval; • no statistically significant difference (p > 0.05) in comparison to metronidazole 20 μg
Table 4.
Percentage of viability and motility of T. vaginalis trophozoites in culture after exposure to O. basilicum oil at different incubation periods
| Dosage of treatment | After 24 h (%) | After 48 h (%) | After 72 h (%) | After 96 h (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Viability (%) | Motility (%) | Viability (%) | Motility (%) | Viability (%) | Motility (%) | Viability (%) | Motility (%) | |
| Parasite control | 100 | 98 | 100 | 76.2 | 100 | 21 | 100 | 9.6 |
| Metronidazole, 20 μg | 35.9 | 33.1 | 9.6 | 7.1 | 0.9 | 0.2 | 0 | 0 |
| Metronidazole, 40 μg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Solvent control | 98.7 | 97 | 98.8 | 76 | 99.1 | 20 | 99 | 9.5 |
| Ocimum basilicum oil, 10 μg/ml | 76.2 | 70 | 21.6 | 15 | 4.4 | 0.9 | 0 | 0 |
| Ocimum basilicum oil, 20 μg/ml | 26.9 | 19 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocimum basilicum oil, 30 μg/ml | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
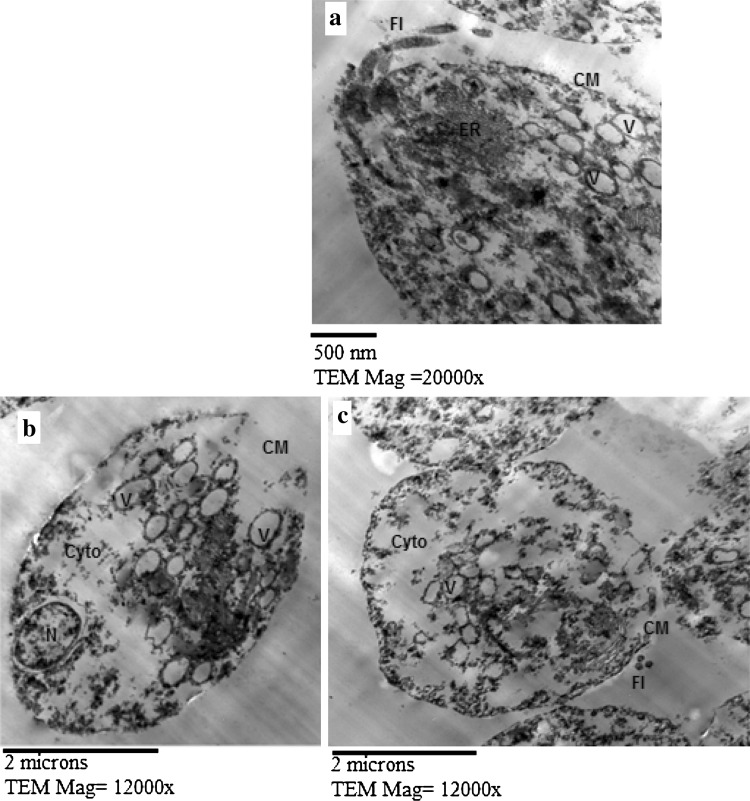
Fig. 1.
TEM of T. vaginalis trophozoites from P. lentiscus mastic (10 mg/ml) treated culture at 24 h incubation period showing abnormal vacuolization (V) (a–c), defects in the cell membrane (CM) with extensive cytoplasmic destruction (Cyto) (b, c). N nucleus, Fl flagella, ER endoplasmic reticulum
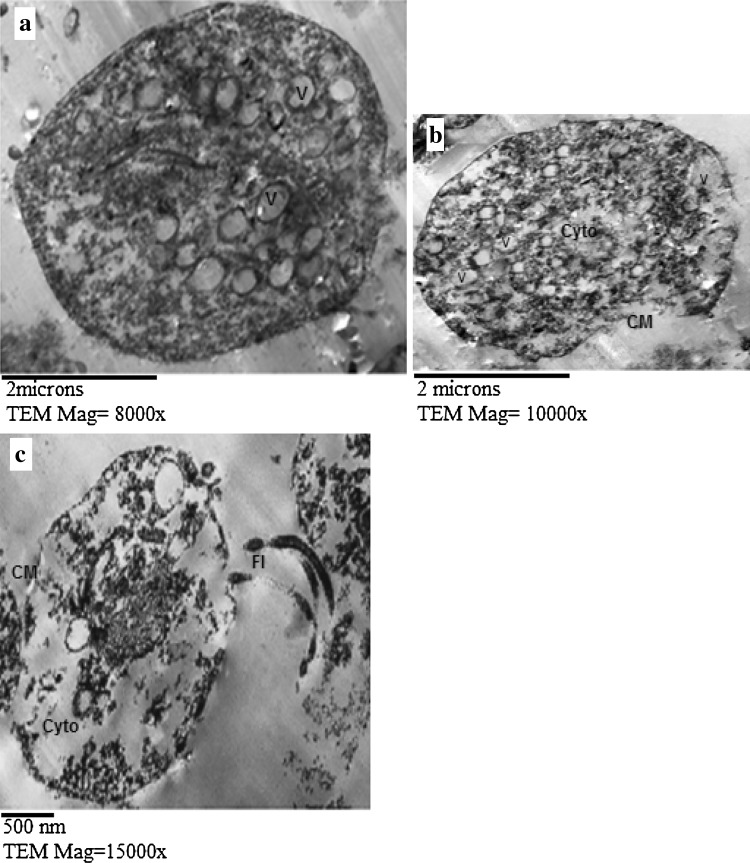
Fig. 2.
TEM of T. vaginalis trophozoites from O. basilicum oil (20 μg/ml) treated culture at 24 h incubation period showing abnormal vacuolization (V) (a–c), membranous defects (CM) with extensive cytoplasmic destruction (Cyto) (b, c). Fl flagella
Growth inhibition and minimal lethal concentration
As regards P. lentiscus mastic treated culture, it showed 100 % inhibition of the parasitic growth with concentration of 15 mg/ml after 24 h incubation. 90 % inhibition of the parasitic growth was observed with concentration of 10 mg/ml after 24 h and complete inhibition of growth (100 %) after 48 h. 44 % inhibition of the parasitic growth was observed with concentration of 5 mg/ml after 24 h, 85.6 % after 48 h and 96.4 % after 72 h till complete inhibition of growth (100 %) after 96 h (Table 1).
Each concentration showed statistical significant difference (p < 0.05) when compared to parasite control and no statistical significant difference (p > 0.05) when compared to metronidazole 20 μg/ml except for the concentration of 15 mg/ml after 24 h which showed significant difference (p < 0.05) when compared to metronidazole 20 μg/ml. Minimal lethal concentration of P. lentiscus mastic was 15 mg/ml after 24 h, 10 mg/ml after 48 h and 5 mg/ml after 96 h (Table 1).
Concerning the viability and motility of P. lentiscus mastic treated culture, the higher the concentration and the longer the time of exposure the less the viability and motility percentage. Concentration of 15 mg/ml showed 0 % viable and motile trophozoites after 24 h incubation. Concentration of 10 mg/ml showed 10 % viable and 8.7 % motile trophozoites after 24 h decreased to 0 % for viability and motility after 48 h. Concentration of 5 mg/ml showed 56 % viable and 41 % motile trophozoites after 24 h, 14.4 % viable and 10.8 % motile trophozoites after 48 h, 5.4 % viable and 0.9 % motile trophozoites after 72 h, and decreased to 0 % for viability and motility after 96 h (Table 2).
As regards O. basilicum oil treated culture, it showed 100 % inhibition of the parasitic growth with concentration of 30 μg/ml after 24 h incubation. Decrease of the trophozoites growth by 73.1 % was observed with concentration of 20 μg/ml after 24 h and complete inhibition (100 %) of growth after 48 h. Decrease of the trophozoites growth by 23.8 % was observed with concentrations of 10 μg/ml after 24 h, 78.4 % after 48 h and 95.6 % after 72 h till complete inhibition (100 %) of growth after 96 h (Table 3).
Each concentration showed statistical significant difference (p < 0.05) when compared to parasite control and no statistical significant difference (p > 0.05) when compared to metronidazole 20 μg/ml except for the concentration of 30 μg/ml after 24 h and 10 μg/ml after 72 h which showed significant difference (p < 0.05) when compared to metronidazole 20 μg/ml at the same time interval (Table 3).
Concerning the viability and motility of O. basilicum oil treated culture, the higher the concentration and the longer the time of exposure the less the viability and motility percentage, concentration of 30 μg/ml showed 0 % viable and motile trophozoites after 24 h incubation. 20 μg/ml showed 26.9 % viable and 19 % motile trophozoites after 24 h, and decreased to 0 % for viability and motility after 48 h. 10 μg/ml showed 76.2 % viable and 70 % motile trophozoites after 24 h, 21.6 % viable and 15 % motile trophozoites after 48 h, 4.4 % viable and 0.9 % motile trophozoites after 72 h, and decreased to 0 % for viability and motility after 96 h (Table 4).
Transmission electron microscopy study (TEM)
As regards P. lentiscus mastic treated culture, TEM showed that the membrane system of T. vaginalis trophozoites was considerably damaged (Fig. 1b, c), with abnormal vacuolization of the cytoplasm (Figure 1a–c), and extensive cytoplasmic destruction (Fig. 1b, c).
As regards O. basilicum oil treated culture, TEM showed that the membrane system of the trophozoites was considerably damaged (Fig. 2b, c), with abnormal vacuolization of the cytoplasm (Fig. 2a–c), and extensive cytoplasmic destruction (Fig. 2b, c).
Discussion
The present in vitro study was carried out to evaluate the effects of P. lentiscus mastic and O. basilicum oil on T. vaginalis. Taking into account that some clinical cases of trichomoniasis are caused by T. vaginalis isolates resistant to metronidazole and its side effects specially with higher doses, the treatment constitutes a major challenge, and the options are limited (Cudmore et al. 2004). In the present study, different concentrations of P. lentiscus mastic and O. basilicum oil were tested, on the axenic culture of T. vaginalis in comparison to parasite control and metronidazole control. P. lentiscus mastic and O. basilicum oil had lethal effects on growth of the trophozoites, with ultrastructure destructive changes.
In this study the effect of P. lentiscus mastic and Ocimum bacilicum oil on the inhibition of trophozoites growth showed statistically significant difference when compared to parasite control, inhibition of trophozoites growth and trophozoites motility were in relation to concentration and incubation time.
The minimal inhibitory concentration at 24 h interval of P. lentiscus mastic was 15 mg/ml, and of O. bacilicum oil was 30 μg/ml, showing potency comparable to that of metronidazole 40 μg/ml. P. lentiscus 15 mg/ml and O. bacilicum oil 30 μg/ml showed statistically significant difference when compared to metronidazole 20 μg/ml after 24 h incubation. These findings were in accordance with the results of Ali (2007) who reported similar inhibitory effect when using Propolis, and also those of Rocha et al. (2012) when using saponins from Quillaja, Passiflora and Ilex species on the axenic culture of T. vaginalis. The similarity between the effects of natural products in this study and other studies on T. vaginalis trophozoites can be attributed to the possibility that different plants may share the same active ingredients.
With lower concentrations of P. lentiscus mastic and O. bacilicum oil longer incubation period was required to obtain complete loss of viability and motility. The study recorded that a 90 % inhibition of growth with concentration of 10 mg/ml P. lentiscus mastic after 24 h incubation period increased to 100 % inhibition after 48 h which represents the minimal inhibitory concentration at that point of time, while concentration of 5 mg/ml needed up to 96 h to completely inhibit the trophozoites growth. Also, a 73.1 % inhibition of growth with concentration of 20 μg/ml of O. bacilicum oil after 24 h incubation period reached 100 % inhibition after 48 h, while concentration of 10 μg/ml needed up to 96 h to completely inhibit the trophozoites growth. These results were comparable to that of metronidazole 20 μg/ml whose inhibitory effect progressed over time to reach complete inhibition after 96 h, however being natural products, they have the advantage over metronidazole of being more safe.
The anti T. vaginalis effect of P. lentiscus mastic reported in this study can be attributed to the ability of P. lentiscus mastic to induce apoptosis (li et al. 2011), and to a variety of phytochemicals constituents which are medicinally important such as α-pinene, β-pinene, β-myrcene, limonene, trans-caryophyllene, camphene, and to phenolic compounds which revealed considerable antimicrobial activity and particular antifungal activity (Ansari and Siddiqui 2012). These activities are probably in relationship with the structure of the phenolic compound gallic acid, which is a component of P. lentiscus mastic. Gallic acid has been tested for its ability to inhibit the proliferation of Trypanosoma cruzi trypomastigotes in vitro (Koide et al. 1998). Also, it was suggested that the anti Trichomonas tenax and anti-T. vaginalis effect of pomegranate peel extract can be attributed to its gallic acid content (El-Sherbini and Shoukry 2012). It would be of interest to determine which of the constituents of P. lentiscus mastic, alone or in synergy with another constituent, have the ability to produce the anti T. vaginalis activities reported in this study.
The ability of the crude extracts of O. bacilicum oil to inhibit the growth of bacteria and fungi is an indication of its broad-spectrum antimicrobial potential, which may be employed for the management of microbial infections. Primary phytochemical screening of O. bacilicum oil, revealed the presence of saponin, steroids, tannins, glycosides, alkaloids and flavonoids (Issazadeh et al. 2012). In general, isolated plant’s substances such as flavonoids, alkaloids, cumarins, saponins and glycosides have demonstrated activity against different flagellates such as G. lamblia (Barbosa et al. 2007), Trichomonas gallinae (Adebajo et al. 2006), T. vaginalis (Arthan et al. 2008), and Leishmania infantum (González-Coloma et al. 2011). Anti-T. vaginalis activity of other plant’s saponins was reported (Rocha et al. 2012). Thus, the anti T. vaginalis activity reported in this study can be attributed to the saponin content of O. bacilicum oil as well as the biologically active flavonoids.
Almeida et al. (2007) reported high antigiardial potential of O. basilicum essential oil by inhibition of proteolytic activity of cysteine proteinases of G. lamblia trophozoites. Cysteine proteinases are present in a wide range of parasitic protozoa, including T. vaginalis and appear to be important in several aspects of the life cycle and of the parasite-host relationships (Gradoni and Ascenzi 2004). So we can suggest that the inhibitory effect of O. basilicum oil on the growth of T. vaginalis trophozoites is, also possibly, through its ability to inhibit cysteine proteinase’s activity of T. vaginalis.
TEM of each of P. lentiscus mastic and O. bacilicum treated culture showed considerable damage of the membrane system of the trophozoites, abnormal vacuolization and extensive destruction of the cytoplasm, after incubation period of 24 h with the concentration of 10 and 20 μg/ml, respectively. Those findings are in accordance with results of Oxberry et al. (1994) who reported loss of the cytoplasmic material, vacuolization and disruption of cytoplasmic membrane after usage of metronidazole. Once this occurs other degenerative changes associated with cell death and decline growth are also likely to occur ending up by complete loss and disintegration of trophozoites.
In conclusion, the results from this study support our perspective for the possibility of using P. lentiscus mastic and O. basilicum oil as anti-T. vaginalis agents.
References
- Adebajo AC, Ayoola OF, Iwalewa EO, Akindahunsi AA, Omisore NOA, Adewunmi CO, Adenowo TK. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine. 2006;13(4):246–254. doi: 10.1016/j.phymed.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Ali NM. In vitro activity of commercially available Egyptian propolis on Trichomonas vaginalis. N Egypt J Med. 2007;36:7–15. [Google Scholar]
- Almeida I, Alviano DS, Vieira DP, Alves PB, Blank AF, Lope AH, Alviano CS, Rosa MS. Antigiardial activity of Ocimum basilicum essential oil. Parasitol Res. 2007;101:443–452. doi: 10.1007/s00436-007-0502-2. [DOI] [PubMed] [Google Scholar]
- Ansari S, Siddiqui AN. Pistacia lentiscus: a review on phytochemistry and pharmacological properties. Int J Pharm Sci. 2012;4(4):16–20. [Google Scholar]
- Arthan D, Sithiprom S, Thima K, Limmatvatirat C, Chavalitshewinkoon-Petmitr P, Svasti J. Inhibitory effects of Thai plants β-glycosides on Trichomonas vaginalis. Parasitol Res. 2008;103(2):443–448. doi: 10.1007/s00436-008-0996-2. [DOI] [PubMed] [Google Scholar]
- Balan KV, Prince J, Han Z, Dimas K, Cladaras M, Wyche JH, Sitaras NM, Pantazis P. Antiproliferative activity and induction of apoptosis in human colon cancer cells treated in vitro with constituents of a product derived from Pistacia lentiscus L. Phytomedicine. 2007;14:263–272. doi: 10.1016/j.phymed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Barbosa E, Calzada F, Campos R. In vivo antigiardial activity of three flavonoids isolated from some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J Ethnopharmacol. 2007;109(3):552–554. doi: 10.1016/j.jep.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Cedillo-Rivera R, Chavez B, Gonzalez-Robles A, Tapia A, Yepez-Mulia L. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. J Eukaryot Microbiol. 2002;49:201–208. doi: 10.1111/j.1550-7408.2002.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Cherpes T, Wiesenfeld H, Melan M, Kant JA, Consentino LA, Meyn LA, Hillier SL. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33:747–752. doi: 10.1097/01.olq.0000218869.52753.c7. [DOI] [PubMed] [Google Scholar]
- Cotch MF, Pastorek JG, Nugent RP, Hillier SL, Gibbs RS, Martin DH, Eschenbach DA, Edelman R, Carey JC, Regan JA, Krohn MA, Klebanoff MA, Rao AV, Rhoads GG. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity study group. Sex Transm Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Cudmore SL, Delgaty KL, Hayward-McClelland SF, Petrin DP, Garber GE. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin Microbiol Rev. 2004;17:783–793. doi: 10.1128/CMR.17.4.783-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LS. The establishment of various trichomonds of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. doi: 10.2307/3274682. [DOI] [PubMed] [Google Scholar]
- Duarte M, Giordani RB, De Carli GA, Zuanazzi JA, Macedo AJ, Tasca T. A quantitative resazurin assay to determinate the viability of Trichomonas vaginalis and the cytotoxicity of organic solvents and surfactant agents. Exp Parasitol. 2009;123:195–198. doi: 10.1016/j.exppara.2009.07.002. [DOI] [PubMed] [Google Scholar]
- El-Sherbini GT, Shoukry NM. In Vitro effect of pomegranate peel extract on Trichomonas tenax. Life Sci J. 2012;9(3):791–797. [Google Scholar]
- Giaginis C, Theocharis S. Current evidence on the anticancer potential of Chios mastic gum. Nutr Cancer. 2011;63(8):1174–1184. doi: 10.1080/01635581.2011.607546. [DOI] [PubMed] [Google Scholar]
- González-Coloma A, Reina M, Sáenz C, Lacret R, Ruiz-Mesia L, Arán VJ, Sanz J, Martínez-Díaz RA. Antileishmanial, antitrypanosomal and cytotoxic screening of ethnopharmacologically selected Peruvian plants. Parasitol Res. 2011;110(4):1381–1392. doi: 10.1007/s00436-011-2638-3. [DOI] [PubMed] [Google Scholar]
- Gradoni L, Ascenzi P. Nitric oxide and anti-protozoan chemotherapy. Parasitologia. 2004;46(1–2):101–103. [PubMed] [Google Scholar]
- Issazadeh K, Pahlaviani MR, Massiha A, Bidarigh S, Giahi M, Muradov PZ. Analysis of the phytochemical contents and anti-microbial activity of Ocimum basilicum L. Int J Mol Clin Microbiol. 2012;1:141–147. [Google Scholar]
- Kayser O, Kiderlen AF, Croft SL. Natural products as antiparasitic drugs. Parasitol Res. 2003;90(2):55–62. doi: 10.1007/s00436-002-0768-3. [DOI] [PubMed] [Google Scholar]
- Keita SM, Vincent C, Schmit J, Belanger A. Essential oil composition of Ocimum basilicum L., O. gratissimum L. and O. suave L. in the Republic of Guinea. Flavour Frag J. 2000;15:339–341. doi: 10.1002/1099-1026(200009/10)15:5<339::AID-FFJ922>3.0.CO;2-H. [DOI] [Google Scholar]
- Koba K, Poutouli PW, Christine RC, Sanda K. Antifungal activity of the essential oils from Ocimum gratissimum L. grown in Togo. J Sci Res. 2009;1:164–171. [Google Scholar]
- Koide T, Nose M, Inoue M, Ogihara Y, Yabu Y, Ohta N. Trypanocidal effects of gallic acid and related compounds. Planta Med. 1998;64(1):27–30. doi: 10.1055/s-2006-957360. [DOI] [PubMed] [Google Scholar]
- Koo H, Gomes FA, Rosalen PL, Ambrosano GMB, Park YK, Cury JA. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch Oral Biol. 2000;45:141–148. doi: 10.1016/S0003-9969(99)00117-X. [DOI] [PubMed] [Google Scholar]
- Li S, Cha I, Nam W. Chios mastic gum extracts as a potent antitumor agent that inhibits growth and induces apoptosis of oral cancer cells. Asian Pac J Cancer Prev. 2011;12:1877–1880. [PubMed] [Google Scholar]
- Markovics A, Cohen I, Muklada H, Glasser TA, Dvash L, Ungar ED, Azaizeh H, Landau SY. Consumption of Pistacia lentiscus foliage alleviates coccidiosis in young goats. Vet Parasitol. 2012;186:165–169. doi: 10.1016/j.vetpar.2011.11.072. [DOI] [PubMed] [Google Scholar]
- Meri T, Jokiranta S, Suhonen L, Meri S. Resistance of Trichomonas vaginalis to metronidazole: report of the first three cases from Finland and optimization of in vitro susceptibility testing under various oxygen concentrations. J Clin Microbiol. 2000;38(2):763–767. doi: 10.1128/jcm.38.2.763-767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxberry ME, Thompson RCA, Reynoldson JA. Evaluation of the effects of albendazole and metronidazole on the ultrastructure of Giardia duodenalis, Trichomonas vaginalis and Spironucleus muris using transmission electron microscopy. Int J Parasitol. 1994;24(5):695–703. doi: 10.1016/0020-7519(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Palmas C, Wakelin D, Gabriele F. Transfer of immunity against Hymenolepis nana in mice with lymphoid cells or serum from infected donors. Parasitology. 1984;89(2):287–293. doi: 10.1017/S0031182000001311. [DOI] [PubMed] [Google Scholar]
- Rasolson M, Vanacova S, Tomkova E, Rezga J, Hardy IJ, Kulda J. Mechanisms of in vitro development of resistance to metronidazole in Trichomonas vaginalis. Microbiology. 2002;148:2467–2477. doi: 10.1099/00221287-148-8-2467. [DOI] [PubMed] [Google Scholar]
- Rocha TD, Vieira PB, Gnoatto SC, Tasca T, Gosmann G. Anti-Trichomonas vaginalis activity of saponins from Quillaja, Passiflora, and Ilex species. Parasitol Res. 2012;110:2551–2556. doi: 10.1007/s00436-011-2798-1. [DOI] [PubMed] [Google Scholar]
- Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, De Marzo AM, Willett WC, Platz EA. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:939–945. doi: 10.1158/1055-9965.EPI-05-0781. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Fukazawa M, Motohia H, Ochiai K, Nishikawa H, Miyata TA. Pilot study on antiplaque effects of mastic chewing gum in the oral cavity. J Periodontol. 2003;74:501–505. doi: 10.1902/jop.2003.74.4.501. [DOI] [PubMed] [Google Scholar]
- Taran M, Azizi E, Shikhvaisi A, Asadi N. The antihelmintic effect of Pistacia khinjuk against protoscoleces of Echinococcus granulosus. World J Zool. 2009;4(4):291–295. [Google Scholar]
- Taran M, Mohebali M, Esmaeli J. In vivo efficacy of gum obtained Pistacia atlantica in experimental treatment of cutaneous leishmaniasis. Iranian J Publ Health. 2010;39:36–41. [PMC free article] [PubMed] [Google Scholar]
- Upcroft JA, Dunn LA, Wright JM, Benakli K, Upcroft P, Vanelle P. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardiaduodenalis. Antimicrob Agents Chemother. 2006;50:344–347. doi: 10.1128/AAC.50.1.344-347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, Van de Wijgert J, Mmiro F, Mugerwa R, Chipato T, Morrison CS. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- Viikki M, Pukkala E, Nieminen P, Hakama M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. Geneva: WHO Press; 2011. [Google Scholar]