Abstract
Gross and histopathological changes were recorded in a pregnant cattle died of clinical anaplasmosis, a tick transmitted economically important disease caused by Anaplasma marginale. Grossly emaciated carcass along with pale visible mucous membranes and pale serosal surface, splenomegaly and hepatomegaly was observed. Microscopically, in lungs variable extend of interstitial pneumonia, emphysema along with infiltration of mononuclear cells was seen. Spleen showed extensive increase in red pulp area with massive proliferation of lymphocytes. In liver marked thickening of capsule with fatty changes along with retention of bile was seen. Gall bladder showed congestion, glandular hyperplasia and thickening wall. Myocardium showed degeneration and necrosis.
Keywords: Anaplasma marginale, Histopathology, Lungs, Heart, Liver, Gall bladder
Introduction
Bovine anaplasmosis caused by Anaplasma marginale, an intra erythrocytic rickettsial organism (Dumler et al. 2001) transmitted by ticks, or mechanically by biting flies or blood-contaminated fomites. A. marginale invades and multiplies within mature erythrocytes and during acute anaplasmosis, rickettsemia levels may exceed 109 infected erythrocytes per ml (Kieser et al. 1990). Clinical signs include fever, anaemia, weakness, constipation, jaundice, loss of appetite, dehydration, depression, laboured breathing, abortion in pregnant animals and often death (Richey and Palmer 1990). Anaemia and icterus are usually observed without haemoglobinaemia and haemoglobinuria (Rymaszewska and Grenda 2008) as in place of intravascular haemolysis, extra vascular erthrophagocytosis occurs in disease. In severely affected animals urine often becomes dark brown due to the presence of bile pigments. While comparing parasitaemia with haemolytic indices, positive correlation has been observed in level of parasitaemia and mean corpuscular fragility (Nazifi et al. 2008) in naturally infected crossbred cattle. Degenerative changes are observed in different organs due to hypoxic conditions created by anemia. Further changes may be due to immunological reactions produced by parasite. Very few published reports are available in literature on histopathological changes in bovine anaplasmosis. Here in this communication gross and histopathological changes observed in various organs of an animal died of clinical anaplasmosis are recorded.
Materials and methods
Leishman stained thin blood smears were prepared and examined from pregnant cattle suspected for anaplasmosis based on history and clinical signs. Clinical examination of animal revealed presence of fever, pale mucus membranes, strenuous breathing and decrease in appetite. Later on animal died of disease. Systematic necropsy was performed on the animal and gross lesions were recorded. The tissue samples from various vital organs viz; lungs, heart, liver, kidneys and spleen were collected in 10 % neutral buffered formalin and processed for histopathological examination as per the standard procedure (Luna 1968). Thin sections of 5 μm thickness were prepared and stained with routine haematoxylin and eosin. Sections were thoroughly examined under light microscope for various histopathological changes.
Results and discussion
Microscopic examination of Leishman stained blood smears revealed presence of typical A. marginale organisms in the erythrocytes (Fig. 1).
Fig. 1.
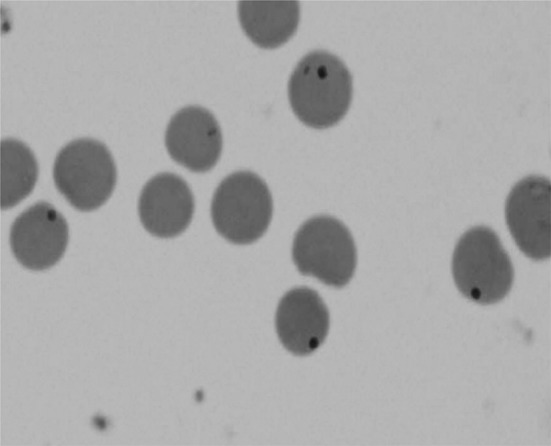
Showing Anaplasma marginale in erythrocytes
Gross lesions
Gross lesions included pale mucus membranes with emaciated carcass. Visible mucous membranes and serosal surfaces of the abdominal organs had yellowish discoloration indicating icterus (Fig. 2).
Fig. 2.
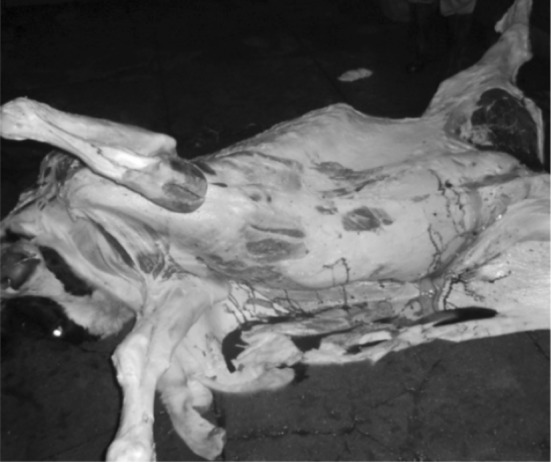
Yellow discoloration of fascia with emaciated carcass. (Color figure online)
Splenomegaly along with congestion and discoloration of spleen was seen. Splenomegaly may be due to excessive destruction of erythrocytes by phagocytic cells. In anaplasmosis, anaemia develops due to extensive erythrophagocytosis initiated by parasite-induced damage of red blood cells and anti-erythrocytic antibodies (DeVos et al. 2006). Lungs showed pulmonary congestion, haemorrhage, oedema and emphysema. Hepatomegaly (Fig. 3) with thickening of bile duct was an observation as also reported previously (Egbe-Nwiyi et al. 1997). Gall bladder was distended with dark colored bile (Fig. 4). Jaundice and anemia in anaplasmosis may be due to the destruction of the blood cells and their contents being released into the blood stream. Dead foetus was seen in uterus of the animal presented in the study. Oxygen deprivation can result in death of foetus and abortion in pregnant cow (De-Whittier et al. 2007). Abortion has been reported previously in clinical anaplasmosis in cattle (Correa et al. 1978).
Fig. 3.

Hepatomegaly with distended gall bladder. Yellow discoloration of omentum. (Color figure online)
Fig. 4.

Gall Bladder distended with dark green coloured thick bile. (Color figure online)
Histopathology
Lungs
Histophological changes in the lungs showed variable extents of interstitial pneumonia (Fig. 5), marked thickening of inter alveolar septa characterized by proliferation of type-I pneumocytes and mild infilteration of mononuclear cells. The emphysema (Fig. 6) observed may be compensatory to terminal dyspnea. Progressively, these changes in the pulmonary tissues may cause respiratory distress which was evident clinically.
Fig. 5.
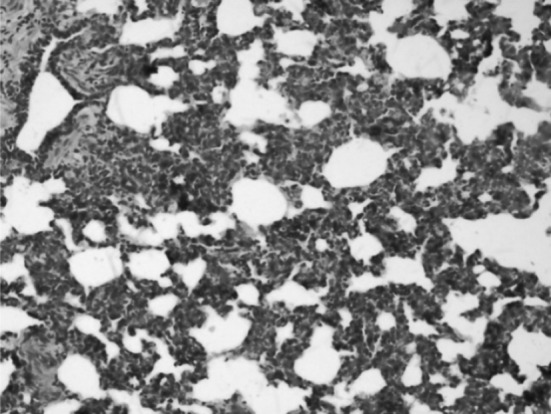
Lungs showing interstitial pneumonia H&E × 20X
Fig. 6.
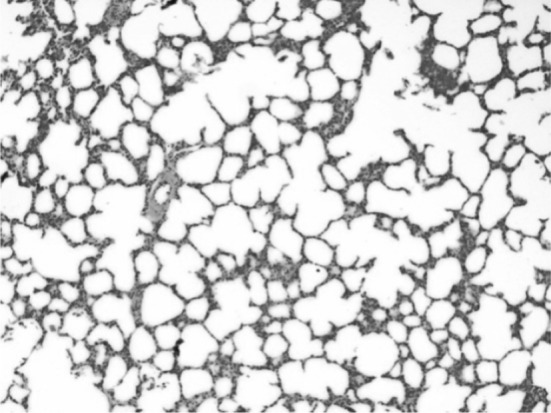
Lungs showing emphysema H&E × 10X
Heart
Histological examination of heart revealed disorientation of myocardial fibers. Mild to moderate multifocal myocardial degeneration and coagulative necrosis was seen which may be due to severe anaemia observed in the present case (Fig. 7).
Fig. 7.

Myocardial degeneration and necrosis. H&E × 20X
Spleen
Spleen showed increased red pulp with massive proliferation of lymphocytes. Red pulp contained numerous histiocytes showing erythrophagocytosis (Fig. 8). Splenic changes are similar to those observed in babesiosis except that there is generally more extensive accumulation of plasma cells in the red pulp in cases of anaplasmosis (DeVos et al. 2006).
Fig. 8.
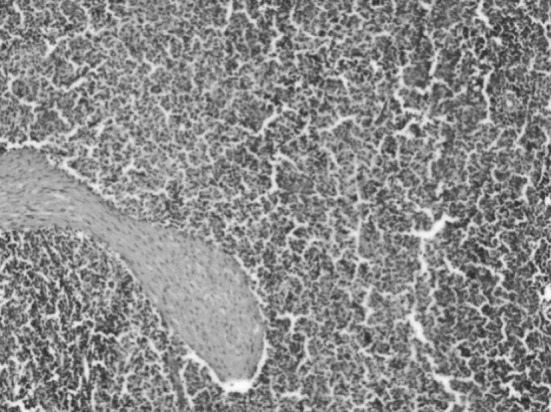
Section of spleen showing increased red pulp. H&E x 10 X. (Color figure online)
Gall bladder
Gall bladder showed congestion and thickening of gall bladder wall with glandular hyperplasia. (Fig. 9) which may be due to retention of bile in the gall bladder.
Fig. 9.
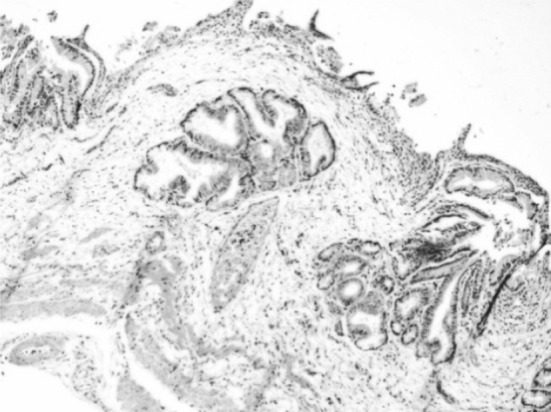
Congestion and thickening of gall bladder wall with glandular hyperplasia. H&E × 10X
Liver
Fatty changes were observed in the hepatocytes along with retention of bile which may be due to cholestasis (Fig. 10) along with mild infiltration of mononuclear cells in portal triad and marked thickening of glission capsule or perihepatitis. Coagulative necrosis of the heaptocytes and the bile ducts hyperplasia has been reported earlier (Egbe-Nwiyi et al. 1997). Hepatic necrosis is a feature of this disease. Anderson and Hurtado (1989) also reported centrilobular hypoxic hepatic necrosis and splenic hemosiderosis which may be due to erythrocyte destruction. DeVos et al. (2006) also reported biliary retention in most cases of anaplasmosis as there is accumulation of haemosiderin in cells of mono-nuclear phagocytic system.
Fig. 10.
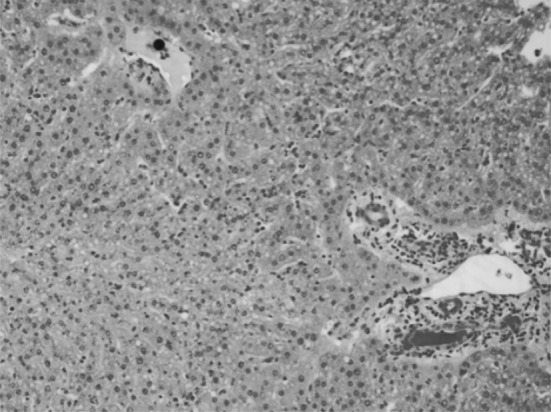
Section of liver showing retention of bile, mild fatty change and infiltration of mononuclear cells in portal triad along with stasis of bile in the bile ductule. H&E × 20X
Necropsy findings revealed splenomegaly, hepatomegaly along with anemia and jaundice as prominent gross observations where as microscopic findings include bile duct hyperplasia, bile retention, increase in liver capsule size, fatty changes in liver, lungs emphysema and myocardial degeneration.
Contributor Information
M. S. Bal, Email: bal_epi@rediffmail.com
L. D. Singla, Email: ldsingla@gmail.com
References
- Anderson ML, Hurtado BJ. Diagnosis of anaplasmosis in formalin-fixed tissue using the Wolbach’s Giemsa stain. J Vet Diagn Invest. 1989;1:185–186. doi: 10.1177/104063878900100221. [DOI] [PubMed] [Google Scholar]
- Correa WM, Correa CN, Gottschalk AF. Bovine abortion associated with Anaplasma marginale. Can J Comp Med. 1978;42(2):227–228. [PMC free article] [PubMed] [Google Scholar]
- DeVos AJ, Brock R, Molly JB (2006) Tick borne diseases of cattle. In: Australian and New Zealand Standard Diagnostic Procedures. Sub Committee on Animal Health Laboratory Standards, pp 1–29. http://www.scahls.org.au/home
- De-Whittier D, Currin N, Currin J. Anaplasmosis in Beef Cattle. Blacksburg: Virginia Tech Cooperative Extension; 2007. pp. 400–465. [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisia Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: uni-fication of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorick-ettsia, descriptions of six new species combinations and designation of Ehrlichia equi and’HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Egbe-Nwiyi TN, Salako MA, Otukonyong EE. Observations on naturally occurring bovine anaplasmosis in arid zone of North-Eastern Nigeria: prevalence, haematological and pathological changes. Stud Res Vet Med. 1997;5:95–99. [Google Scholar]
- Kieser ST, Eriks IS, Palmer GH. Cyclic rickettsemia during persistent Anaplasma marginale infection in cattle. Infect Immun. 1990;58:1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna LG. Manual of histological staining method of the armed forces institute of pathology. 3. New York: McGraw-Hill; 1968. [Google Scholar]
- Nazifi S, Razavi SM, Mansourian M, Nikahval B, Moghaddam M. Studies on correlations among parasitaemia and some hemolytic indices in two tropical diseases (theileriosis and anaplasmosis) in Fars province of Iran. Trop Anim Health Prod. 2008;40:47–53. doi: 10.1007/s11250-007-9052-y. [DOI] [PubMed] [Google Scholar]
- Richey EJ, Palmer GH. Bovine anaplasmosis. Compend Contin Educ Pract Vet. 1990;12:1661–1668. [Google Scholar]
- Rymaszewska A, Grenda S. Bacteria of the genus Anaplasma – characteristics of Anaplasma and their vectors: a review. Vet Med. 2008;53(11):573–584. [Google Scholar]


