Abstract
Toxoplasma gondii is a unique intracellular parasite, which infect a large proportion of the world population, but uncommonly causes clinically significant disease. The present study was performed to estimate the prevalence of toxoplasmosis in 400 apparently healthy blood donor males, their ages were between 18 and 57 years using enzyme linked immunosorbent assay, and to examine the effects of infection on total testosterone, free testosterone and follicle stimulating hormone (FSH) levels in their sera. Seroprevalence showed 10 (2.5 %) and 121 (30 %) of them had IgM and IgG antibodies respectively. Both acute and chronic toxoplasmosis in males recorded higher significant (P < 0.05) mean concentration for total and free testosterone hormone, they were 12.188 ± 0.73, 7.837 ± 0.52 ng/ml and 44.121 ± 1.76, 27.984 ± 0.94 pg/ml respectively. The mean concentration of FSH revealed non-significant (P < 0.05) differences in both disease activities, they were 6.41 ± 0.47 and 6.515 ± 0.51 IU/ml respectively.
Keywords: Toxoplasma gondii, Toxoplasmosis, Total testosterone hormone, Free testosterone hormone, Follicle stimulating hormone
Introduction
Toxoplasmosis is a zoonotic disease caused by the protozoan parasite Toxoplasma gondii, human and other warm blooded animals are its hosts (Steven et al. 2008). Humans may remain infected for life and will stay asymptomatic unless immunosuppression occurs (Herrmann et al. 2010). Primary infection of toxoplasmosis in immunocompetent subject is usually asymptomatic or associated with self limited symptoms such as fever, malaise, and cervical lymphadenopathy. Infection acquired during pregnancy is frequently associated with transmission of T. gondii to the fetus, resulting in congenital disease. In immunocompromised patients, T. gondii infection causes severe manifestation, including splenomegaly, chorioretinitis, pneumonitis, encephalitis, multisystem organs failure, and even death (Montoya and Liesenfeld 2004). There is considerable evidence that steroid hormones affect the course of toxoplasmosis in humans and mice. Henry and Beverley (1976) demonstrated the differences in the immune and inflammatory responses of male and female mice following infection with T. gondii. In these studies, female mice developed more severe brain inflammation than male mice following infection. Moreover, a direct role for sex hormones was demonstrated in experiments which found that gonadectomy increased resistance, whereas estrogen administration exacerbated disease in mice. Similarly, simultaneous gonadectomy and estrogen treatment predisposed guinea pigs to increased parasite burdens compared with non treated control animals (Kittas and Henry 1980). The prevailing hypothesis for immunological differences between the sexes is that sex hormone, in particular, testosterone, 17 β-oestradiol, and progesterone, influences the immune system. The localization of sex hormone receptors in immune cells, including lymphocytes, macrophages, granulocytes, and mast cells, illustrates that there are direct connections between the endocrine and immune systems and that endocrine factors can directly modulate the expression of target genes in immune cells (Oktenli et al. 2004).This study was aimed to investigate any relationship between toxoplasmosis (Acute, chronic) and sex hormonal disturbances in male patients such as testosterone (total, free) and follicle stimulating hormone (FSH) levels.
Materials and methods
Blood samples were collected from 400 apparently healthy blood donor males; their ages were between 18 and 57 years. They were attended the National blood transfusion center in Baghdad between October 2011 and January 2012.
Before collection of samples, an information sheet was prepared and designed according to a questionnaire which covers different Information. 5 ml of venous blood were drown from redial vein from each person by using disposable syringe, blood was placed in plain tubes and centrifuged at 3,000 rpm for 10 min. Sera were dispensed into five eppendorf tubes by using micropipette and stored at −20 °C until serological and hormonal tests were performed.
Detection of anti-T. gondii antibody (IgG) and (IgM) by (ELISA) technique
Measurements of IgG and IgM antibodies were performed and interpreted according to the direction of the manufacturer of bioCheck Toxoplasma IgG ELISA (BC-1085) kit and bioCheck Toxoplasma IgM ELISA (BC-1087) kit respectively.
Purified T. gondii antigen is coated on the surface of micro wells. Diluted patient serum was added to the wells, and the T. gondii IgG-specific Ab, if present, will bind to the Ag. All unbound materials were washed away. Horse radish peroxidase (HRP) conjugate is added, which binds to the Ab–Ag complex. Excess HRP-conjugate is washed off and solution of tetra methyl benzidine reagent was added. The enzyme conjugated catalytic reaction is stopped at a specific time. The intensity of the color generated is proportional to the amount of IgG-specific Ab in the sample. The results were read by ELISA reader.
Estimation of serum total testosterone hormone levels by ELISA technique
The Monobind Testosterone Enzyme Immunoassay USA Test kit (3725-300) was used. The quantitative determination of total concentration in human sera by microplate Enzyme Immunoassay was done according to the manufacturer’s instructions.
A dose response curve is used to ascertain the concentration of testosterone hormone in unknown specimens.
The absorbance obtained from the printout of the microplate reader was recorded.
The absorbance for each duplicate serum reference versus the corresponding testosterone concentration in ng/ml on linear graph paper was plotted.
The best-fit curve through the plotted points was drawn.
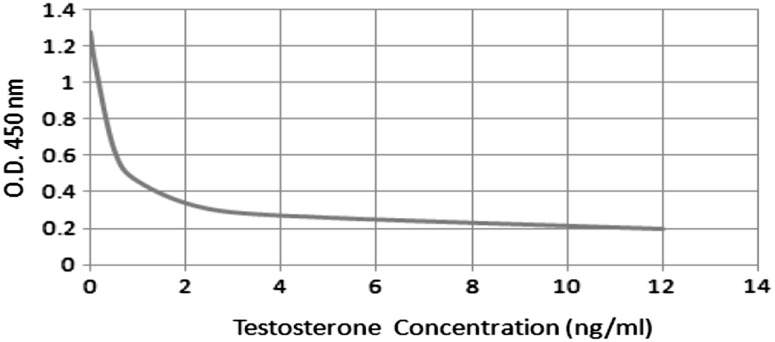
To determine the concentration of testosterone for an unknown, the average absorbance of the duplicates for each unknown was located on the vertical axis of the graph, and the concentration (in ng/ml) from the horizontal axis of the graph was read (Fig. 1).
Fig. 1.
Standard curve of total testosterone concentration
Estimation of serum free testosterone hormone levels by ELISA technique
DiaMetra Italy kit (DKO-015) was used for determination of free testosterone hormone concentration in human sera according to the manufacturer’s instructions. The mean value of absorbance of the standards against concentration was plotted. The best fit curve through the plotted points was drew.
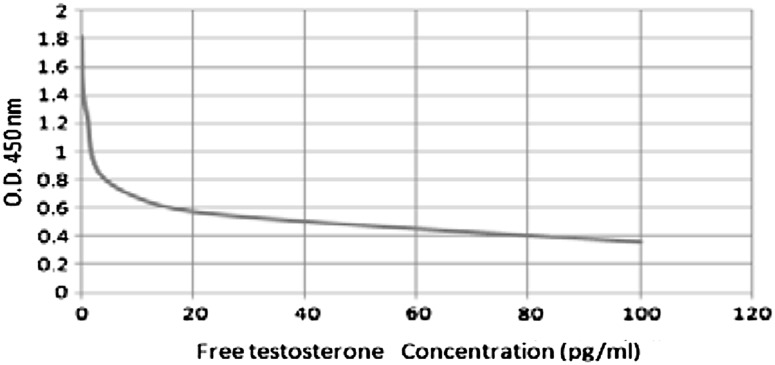
The values of the samples on the standard curve were interpolated to obtain the corresponding values of the concentration expressed in pg/ml (Fig. 2).
Fig. 2.
Standard curve of free testosterone concentration
Estimation of serum FSH levels by ELISA technique
Monobind USA kit (425-300) was intended, the quantitative determination of follicle stimulating hormone concentration in human serum by a microplate immunoenzymometric assay (IEMA/ELISA) was done according to the manufacturer’s instructions.
A dose response curve is used to ascertain the concentration follicle stimulating hormone in unknown specimens.
The absorbance obtained from the printout of the microplate reader was recorded.
The absorbance for each duplicate serum reference versus the corresponding FSH concentration in IU/ml on linear graph paper was plotted.
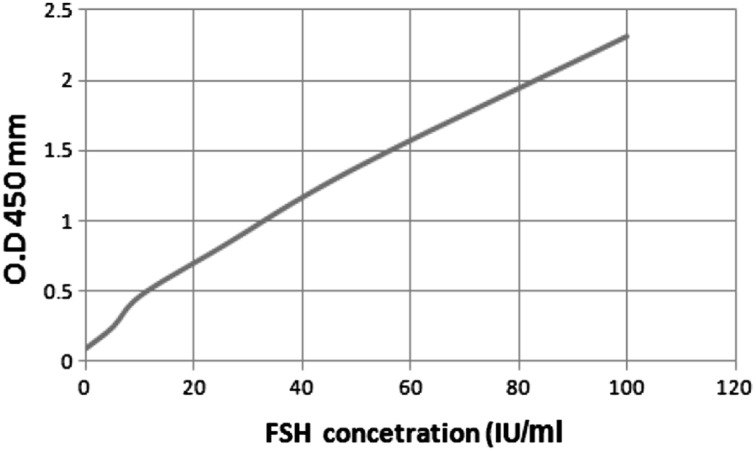
The best-fit curve through the plotted points was drawn (Fig. 3).
Fig. 3.
Standard curve of follicle stimulating harmone concentration
Statistical analysis
Statistical analysis system-SAS (2004) program was used to effect of treatment in parameters study. Chi square test was used in significant compare between percentages. Least significant effect-LSD test was used to significant compare between means.
Results
The current study showed the actual percentage of toxoplasmosis in male blood donors attending the National blood transfusion center in Baghdad using ELISA IgM and IgG. Out of 400 male blood donors 10 (2.5 %) had acute toxoplasmosis characterized by the presence of positive IgM antibodies, while 121 (30.25 %) of them had chronic toxoplasmosis characterized by the presence of positive IgG antibodies only. There were highly significant differences between them (P < 0.01; Table 1).
Table 1.
Percentage distribution of males infected with toxoplasmosis by ELISA IgG and IgM test
| Test | ELISA test | χ2 | |||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | |||||
| Subject | N | % | N | % | N | % | |
| IgG | 121 | 30.25 | 279 | 69.75 | 400 | 100 | 10.363** |
| IgM | 10 | 2.50 | 390 | 97.50 | 400 | 100 | 12.826** |
** P < 0.01
Male blood donors infected with toxoplasmosis had a highest significant differences (P < 0.05) in the mean concentration of serum TTH and FTH hormones in both disease activities in comparison to the control group. Patients with acute toxoplasmosis scored a mean concentration values of 12.188 ng/ml and 44.542 pg/ml for both hormones respectively, while patients with chronic toxoplasmosis scored 7.837 ng/ml and 27.984 pg/ml respectively. However FSH hormone showed non-significant difference (P < 0.05) for the mean concentration between acute and chronic toxoplasmosis patient (Table 2).
Table 2.
The mean concentration of hormones (total testosterone, free testosterone and FSH) in sera of males infected with toxoplasmosis
| Subject | No. | Follicle stimulating hormone (FSH) (IU/ml) | Free testosterone hormone (FTH) (pg/ml) | Total testosterone hormone (TTH) (ng/ml) |
|---|---|---|---|---|
| Acute toxoplasmosis patients with(IgM) | 10 | 12.188 ± 0.73 | 44.121 ± 1.76 | 6.410 ± 0.47 |
| Chronic toxoplasmosis patients with(IgG) | 121 | 7.837 ± 0.52 | 27.984 ± 0.94 | 6.515 ± 0.51 |
| Control | 30 | 6.455 ± 0.48 | 18.375 ± 0.78 | 6.780 ± 0.61 |
| LSD Value | – | 3.812* | 12.683* | 1.679 NS |
Hormonal values: mean ± SD
NS non-significant
* P < 0.05
The current results revealed that acute infected patient with toxoplasmosis at the age group of 18–25 years showed the lowest mean concentration of both TTH and FTH hormones 1.750 ng/ml and 2.658 pg/ml respectively. They characterized by the absence of hair from the faces of all patients as well as patients with chronic toxoplasmosis at this age group scored the lowest mean concentration of both TTH and FTH hormones 1.996 ng/ml and 2.516 pg/ml respectively. They also characterized by few hair in their faces of 22 (73.33 %) patients while the other eight (26.66 %) showed thick hair in their faces (Table 3).
Table 3.
The mean concentration of (TTH and FTH) in sera of patients with acute and chronic toxoplasmosis according to age group and hair abundance
| Age group | Acute patients | Hair abundance | Chronic patients | Hair abundance | ||||
|---|---|---|---|---|---|---|---|---|
| TTH (ng/ml) | FTH (pg/ml) | Form | No. (%) | TTH (ng/ml) | FTH (pg/ml) | Form | No. (%) | |
| 18–25 | 1.75 ± 0.03 | 2.65 ± 0.0 | No hair | 3/3 100 |
1.99 ± 0.02 | 2.51 ± 0.08 | Few thick | 22/30–8/30 73.33–26.66 |
| 26–33 | 16.46 ± 1.79 | 62.16 ± 4.64 | Thick-few | 1/2–1/2 50 |
12.62 ± 1.26 | 45.0 ± 3.85 | Thick few | 25/30–5/30 83.33–16.66 |
| 34–41 | 14.06 ± 1.63 | 48.26 ± 3.51 | Thick | 2/2 100 |
10.75 ± 1.19 | 43.48 ± 3.69 | Thick | 29/29 100 |
| 42–49 | 11.40 ± 1.42 | 55.13 ± 4.69 | Thick | 1/2 100 |
11.68 ± 1.36 | 44.96 ± 4.17 | Thick few | 20/25–5/25 80–20 |
| 50–57 | 17.26 ± 2.04 | 52.38 ± 3.93 | Thick | 2/2 100 |
2.12 ± 0.09 | 3.96 ± 0.06 | Few thick | 6/7–1/7 85.7–14.28 |
| LSD value | 5.72* | 16.42* | – | – | 4.947* | 14.87* | – | – |
Hormonal values: mean ± SD
* P < 0.05
On the other hand the age group of 26–33 years has approximately the highest mean concentration 16.464 ng/ml and 62.161 pg/ml respectively for both TTH and FTH with the acute toxoplasmosis and characterized by normal hair abundance, also patient with chronic toxoplasmosis at this age group scored the highest mean concentration of both TTH and FTH 12.624 ng/ml and 45.003 pg/ml respectively. They were characterized by thick hair faces of 25 (83.33 %) patients while the other five (16.66 %) showed a few hair in their faces (Table 3).
According to the marital status and fertility the present results showed that male blood donors infected with acute toxoplasmosis had a significant difference between married and unmarried patients in the amount of hormones levels (P < 0.05). The mean concentration of TTH and FTH hormones in married patients was (6.982 ng/ml) and 62.474 ng/ml respectively compared to 17.394 and 25.768 ng/ml in unmarried patients respectively and the result revealed that fertile married patients had significantly higher level of TTH (11.72 ng/ml) than infertile one (2.244 ng/ml) as well as the FTH hormone, fertile male patients had higher mean concentration (64.424 pg/ml) in comparison to infertile one (60.524 pg/ml) (Table 4). On the other hand patients infected with chronic toxoplasmosis showed different results between married and unmarried in the amount of hormones levels. The mean concentration of TTH and FTH hormones in married patients was 2.372 ng/ml and 52.468 pg/ml respectively, compared to 13.302 and 3.5 ng/ml in unmarried patients respectively. The result revealed that fertile married chronic patients had moderately higher mean concentration of TTH (2.5 ng/ml) in comparison to infertile (2.244 ng/ml) while chronic fertile infected patients had higher mean concentration of FTH (60.524 pg/ml) in comparison to infertile one (44.412 pg/ml) (Table 4).
Table 4.
The mean concentration of (TTH and FTH) in sera of patients with acute and chronic toxoplasmosis according to marital status and fertility
| Marital status and fertility | Acute patients | Chronic patients | ||||
|---|---|---|---|---|---|---|
| TTH (ng/ml) | FTH (pg/ml) | No. (%) | TTH (ng/ml) | FTH (pg/ml) | No. (%) | |
| Married | 6.98 ± 0.08 | 62.74 ± 2.73 | 7/10 70 |
2.37 ± 0.06 | 52.46 ± 2.47 | 101/121 83.4 |
| Un-married | 17.39 ± 0.85 | 25.76 ± 0.92 | 3/10 13.3 |
13.30 ± 0.75 | 3.50 ± 0.06 | 20/121 16.5 |
| Fertile | 11.72 ± 0.73 | 64.42 ± 2.81 | 5/7 71.4 |
2.50 ± 0.05 | 60.52 ± 2.71 | 31/101 30.6 |
| Infertile | 2.24 ± 0.03 | 60.52 ± 2.07 | 2/7 29.5 |
2.24 ± 0.06 | 44.41 ± 1.83 | 70/101 69.3 |
| LSD value | 5.80* | 11.65* | 9.48* | 4.37* | 12.74* | 12.92* |
Hormonal values: mean ± SD
* P < 0.05
The present results showed that male blood donors infected with toxoplasmosis had a normal mean concentration of serum FSH hormone in both disease activities in comparison to the control group, as shown in Table 2.
The mean concentration of FSH according to the age group and hair abundance (mustache and beard) in the faces of the current subject revealed that acute infected patients at the age group of 18–25 years showed the highest mean concentration of FSH hormone (7.734 IU/ml). This characterized by the absence of hair from the faces of all patients, as well as patients with chronic toxoplasmosis at this age group which scored the highest mean concentration of FSH hormone (7.121 IU/ml) and characterized by few hair in face of 22 (73.33 %) patients, while the other eight (26.66 %) showed thick hair (Table 5).
Table 5.
The mean concentration of FSH in sera of patients with acute and chronic toxoplasmosis according to the age group and hair abundance
| Age group | Acute patients | Hair abundance | Chronic patients | Hair abundance | ||
|---|---|---|---|---|---|---|
| FSH (IU/ml) | Form | No. (%) | FSH (IU/ml) | Form | No. (%) | |
| 18–25 | 7.734 ± 0.52 | No hair | 3/3 100 |
7.121 ± 0.50 | Few-thick | 22/30–8/30 73.3–26.6 |
| 26–33 | 5.252 ± 0.18 | Thick-few | 1/2 50 |
6.162 ± 0.45 | Thick-few | 25/30–5/30 83.3–16.6 |
| 34–41 | 5.521 ± 0.21 | Thick | 2/2 100 |
6.432 ± 0.42 | Thick | 29/29 100 |
| 42–49 | 7.411 ± 0.49 | Thick | 1/1 100 |
6.241 ± 0.52 | Thick-few | 20/25–5/25 80–20 |
| 50–57 | 6.132 ± 0.17 | Thick | 2/2 100 |
6.619 ± 0.41 | Few-thick | 6/7–1/7 85.7–14.2 |
| Total | 32.050 ± 1.65 | – | – | 32.575 | – | – |
| LSD value | 2.872 NS | – | – | 2.089 NS | – | – |
Hormonal values: mean ± SD
NS non-significant
On the other hand the age group of 26–33 years showed lower mean concentration (5.252 IU/ml) of FSH with the acute toxoplasmosis and characterized by normal hair abundance. However, patient with chronic toxoplasmosis at this age group scored the lowest mean concentration of FSH (6.162 IU/ml) which characterized by thick hair abundance on faces of 25 (83.33 %) patients while the other five (16.66 %) showed a few hair in their faces (Table 5).
According to the marital status and fertility the present results showed that male blood donors infected with acute toxoplasmosis had non-significant differences (P < 0.05) between married and unmarried patients in hormones levels. Fertile married patients had lower level of FSH (5.78 IU/ml) than infertile one (7.20 IU/ml) (Table 6). On the other hand, patients infected with chronic toxoplasmosis also showed non-significant differences (P < 0.05) in the amount of FSH hormone. The mean concentration of FSH hormones in married patients was (6.72 IU/ml) compared to (6.31 IU/ml) in unmarried patients, and the result revealed that fertile married patients had a lower mean concentration of FSH (6.18 IU/ml) in comparison to infertile (7.26 IU/ml) (Table 6).
Table 6.
The mean concentration of FSH in sera of patients with acute and chronic toxoplasmosis according to marital status and fertility
| Marital status and fertility | Acute patients | Chronic patients | ||||
|---|---|---|---|---|---|---|
| FSH (IU/ml) | No. | % | FSH (IU/ml) | No. | % | |
| Married | 6.49 ± 0.12 | 7 | 70.00 | 6.72 ± 0.09 | 101 | 83.47 |
| Un-married | 6.33 ± 0.08 | 3 | 30.00 | 6.31 ± 0.09 | 20 | 16.52 |
| Fertile | 5.78 ± 0.09 | 5 | 71.43 | 6.18 ± 0.07 | 31 | 30.64 |
| Infertile | 7.20 ± 0.15 | 2 | 29.56 | 7.26 ± 0.09 | 70 | 69.30 |
| LSD Value | 2.734 NS | – | 11.64* | 2.264 NS | – | 13,219* |
Hormonal values: mean ± SD
* P < 0.05
Discussion
Out of 400 apparently healthy males, only 10 (2.5 %) have acute toxoplasmosis, while 121 (30.25 %) of them have chronic toxoplasmosis. This result was in line with previous result obtained by Jassam (2010) who recorded 6 (6 %) and 49 (49 %) for acute and chronic toxoplasmosis infections in males respectively. Toxoplasmosis is asymptomatic disease. However, it may cause severe complications in pregnant women and immunocompromised patients (Espinoza 2005). Accordingly, Klien (2004) showed that immunological differences exist between the sexes, and genetic and behavioral differences may explain some variability in response to infection.
The present study showed that Toxoplasma infected men had a higher concentration of testosterone than Toxoplasma free controls. This result was in agreement with previous study by (Flegr et al. 2008). High concentrations of TTH and FTH have immunosuppressive effects characterized by lower cellular immunity (Roberts et al. 2001). Moreover, it is possible that men with increased levels of testosterone had greater chance of being infected by toxoplasmosis either due to impaired immunity or due to changed behavior and personality profile. Their tendency to disregard rules of their society which can result in lower hygienic standards and, corresponding increased risk of contact with a source of infection (Kanková et al. 2011). On the other hand Flegr et al. (2008) showed that Toxoplasma infected men had a higher concentration of testosterone while women had a lower concentration in comparison to control group. He explained such opposite direction of the testosterone shift in men compared to women to the gender specificity of behavioral shifts in Toxoplasma infected subjects. The current result was fit with James (2010) idea that hypothesized that many parasites and pathogens change the concentration of steroid hormone and he showed that infected hosts which often result in a shift in the sex ratio, namely the increase of proportion of males in the offspring. Recently, a disagreement result with a previous study by Kanková et al. (2011) whom observed a decreased in testosterone levels (TTH and FTH) in both laboratory female and male mice infected with virulent strain of Toxoplasma at a latent phase in comparison to uninfected controls. This disagreement may result from the type of parasite strain, which differs in virulence and epidemiological pattern of occurrence (Peyron et al. 2006). Thus, the parasite genotype appears to be an important factor influencing the outcome of clinical illness in human (Vallochi et al. 2005).
Oktenli et al. (2004) showed that acute Toxoplasma infection may cause temporary hypogonadotropic gonadal insufficiency regardless of the cause of the disease. Toxoplasmosis is one of classical conditions known to have profound adverse effect on human reproductive function (Zighelboim et al. 1968). Experimental evidence has established that mice undergo acquired hypogonadotropic hypogonadism secondary to hypothalamic dysfunction after chronic infection with T. gondii (Antonios et al. 2000). In addition; male patient was previously reported with transient hypogonadotropic and hypogonadism due to Toxoplasma infection (Oktenli et al. 2001). Hypogonadal patients present with the classic signs and symptoms of androgen deficiency, complaining of decreased libido or decreased frequency of shaving. Theoretically low testosterone levels cause loss of libido, reduced beard and body hair growth (Oktenli et al. 2004). This result in line with the current data, the age group of 18–25 years showed the lowest mean concentration of testosterone levels accompanied with reduced beard and mustache from most patients. Spartt et al. (1992) suggested that primary hypogonadism occurs in acute illness. Second hypogonadism in mean with critical illness, surgery (Wang et al. 1978) or respiratory failure (Semple et al. 1981) is attributed to hypogonadotropism. Reproductive axis suppression in acute illness is related to disease severity (Dong et al. 1992).
Abbasian (2011) showed significant correlation between Toxoplasma infection and testosterone increase in men. However Shirbazou et al. (2011) demonstrated that there is a significant correlation between increased of these hormone levels and their complication such as hair reduction and hairiness. The other age groups showed high levels of both hormone with normal hair abundance (thick mustache and beard) in both disease activity (acute and chronic).This is result accepted especially when this hormones responsible for the growth of body hair (Mooradian et al. 1987), since high concentration of TTH correlated with high hair growth. Previously the increased level of testosterone in men explained by positive association between testosterone and dopamine (Hull et al. 2004) and that can increase in response to local inflammatory processes in the infected brain (Flegr et al. 2007). The mean concentration of TTH and FTH decreased at the age group 50–57 years, it was 2.126 ng/ml and 3.966 pg/ml, respectively, in chronic infected patients due to the normal decline of testosterone hormone with age. This probably happened due to the normal decline with age and this drop in testosterone is partially responsible for the significant physiologic changes seen in aging men (Bassil et al. 2009).
There is little information about the possible involving of T. gondii in the pathogenicity of male infertility. Previously, Zhou et al. (2002) found that Toxoplasma infection in infertile human couples was higher than that in fertile couples, possibly related to the anti-sperm antibodies which were higher in Toxoplasma infected couples. This explanation concerned with this study that showed the most chronic Toxoplasma infected patients scored in infertile patient (n = 70) 69.30 %. As well as Qi et al. (2005) investigated 100 cases of man’s sterility, 36 % of them were serologically Toxoplasma-IgG, IgM positive and concluded that T. gondii infection may affect man’s fertility and cause sterility. In addition Lu et al. (2005) found pathological changes in the testes, epididymis, vas deferens, prostate and thalamus of male mice with experimentally induced acute T. gondii infection and they concluded that acute infection can cause infertility; also, Sun et al. (2008) concluded that acute T. gondii infection can affect the reproductive function of experimentally infected male mice.
Dongmei et al. (2005) investigated that apoptosis of spermatogenic cells happened in all different people (fertile and infertile) males, and there was a negative correlation between apoptosis rate and sperm density percentage of forward motility. There is very close relationship between sperm apoptosis and male infertility. Thus, Toxoplasma infection may cause the apoptosis of spermatogenic cells.
Toxoplasma infected men had equal concentration of FSH with Toxoplasma free controls. This result was in agreement with previous result by Makker et al. (2009). While other study by Bopple et al. (2008) demonstrated a disagree result characterized by a significant increase of FSH (P < 0.05), LH (P < 0.05) and prolactin (P < 0.01) levels in Toxoplasma infected patients men when compared with the control group, and he explained this increase to impaired feedback of anterior pituitary by leydig cells.
Khan et al. (1999) demonstrated the elevated levels of FSH and LH with decreased levels of free and total testosterone in the Toxoplasma infected men. This finding agreed with the present result only in this age group while other age groups disagreed with this finding. Babu et al. (2004) had reported elevated levels of FSH and LH hormones with low testosterone concentration in toxoplasmosis patients and they attributed this finding with a primary hypogonadism that showed in those patients.
Primary hypogonadism involves failure of the testes to respond to FSH and LH, when primary hypogonadism affects testosterone production, testosterone is insufficient to inhibit production of FSH and LH here FSH and LH levels are elevated (Alwachi 2003).
Patients with 26–33 years showed lower mean concentration of FSH in both disease activities. These results disagreed with AL-Karboli (2011) who revealed that no significant difference found in FSH hormonal level and impact of age of Toxoplasma infected patients in comparison to control. This disagreement results may related to the different age group limits used in both studies or to the different hormonal kits used.
Fertile men had lower FSH levels than infertile men infected with toxoplasmosis. This result agreed with Andersen et al. (2004). FSH, LH and testosterone hormones are prime regulators of germ cell development. The quantitative production of spermatozoa generally requires the presence of FSH, LH and testosterone (Anderson et al. 1997). The role of FSH for the initiation of spermatogenesis is more important for female than for male fertility (Radu et al. 2010).
References
- Abbasian L. Role of Toxoplasma gondii infection in serum level of testosterone. Kowsar Med J. 2011;16:123–127. [Google Scholar]
- AL-Karboli AR (2011) Immunological and physiological study of women infected with Toxoplasma. Dissertation, College of Science, University of Baghdad, pp 144
- Alwachi SN. Biology. Amman: Dar alfiker; 2003. p. 270. [Google Scholar]
- Andersen A, Ziebe G, Jorgensen S, Petersen N, Skakkebaek J, Andersen N. Time to pregnancy in relation to semen quality assessed by CASA before and after sperm separation. Hum Reprod. 2004;17:173–177. doi: 10.1093/humrep/17.1.173. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Wallace EM, Groome NP. Physiological relations between inhibin B, follicle stimulating hormone secretion and spermatogenesis in normal men and response to gonadotrophin suppression by exogenous testosterone. Hum Reprod. 1997;12:746–753. doi: 10.1093/humrep/12.4.746. [DOI] [PubMed] [Google Scholar]
- Antonios SN, Ismail HI, Essa T. Hypothalamic origin of reproductive failure in chronic experimental toxoplasmosis. J Egypt Soc Parasitol. 2000;30:593–599. [PubMed] [Google Scholar]
- Babu M, Sadhnani M, Swarna M, Padmavathi P, Reddy P. Evaluation of FSH, LH, and testosterone levels in different subgroups of infertile males. Indian Clin Biochem. 2004;19(1):45–49. doi: 10.1007/BF02872388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil N, Alkaade S, Morley GE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5(3):427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boepple PA, Hayes FJ, Dwyer AA, Hang Lee TR, William F, Crowley JR, Pitteloud N. Relative roles of inhibin B and sex steroids in the negative feedback regulation of follicle-stimulating hormone in men across the full spectrum of seminiferous epithelium function. J Clin Endocrinol Metab. 2008;93:1809–1814. doi: 10.1210/jc.2007-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Hawker F, McWilliam D, Bangah M, Burger H, Handelsman D. Circulating immunoreactive inhibin and testosterone level in men with critical illness. Clin Endocrinol. 1992;36:399–404. doi: 10.1111/j.1365-2265.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Dongmei X, Zhou Y, Diao W. Preliminary investigation on relationship between spermatogenic cells apoptosis and infection of Toxoplasma gondii in male in fertility. Chinese J Schistomiasis. 2005;6:1–20. [Google Scholar]
- Espinoza LA (2005) Toxoplasmosis. Caribbean AIDS education and training center. In: HIV primary care guide. Chap 11, Sect 6. Florida, USA, pp 1–4
- Flegr J, Lindova J, Kodym P, Machala L, Rohacova H, Sirocka B, Maly M. Evaluation of a commercial IgE ELISA in comparison with IgA and IgM ELISAs, IgG avidity assay and complement fixation for the diagnosis of acute toxoplasmosis. Clin Microbiol Infect. 2007;13:40–47. doi: 10.1111/j.1469-0691.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- Flegr J, Lindova J, Kodym P. Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology. 2008;135:427–431. doi: 10.1017/S0031182007004064. [DOI] [PubMed] [Google Scholar]
- Henry L, Beverley JK. Age and sex differences in the response of lymph node post-capillary venules in mice infected with Toxoplasma gondii. J Exp Pathol. 1976;57:274–281. [PMC free article] [PubMed] [Google Scholar]
- Herrmann DC, Pantchev N, Globokar-Vrhovec M, Barutzki DH, Wilking A, Luder CG, Contraths FJ, Schrres G. Typical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. Int J Parasitol. 2010;40:285–292. doi: 10.1016/j.ijpara.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- James WH. Potential solutions to problems posed by the offspring sex ratio of people with parasitic and viral infection. Folia Parasitol. 2010;57(2):114–120. doi: 10.14411/fp.2010.014. [DOI] [PubMed] [Google Scholar]
- Jassam FS (2010) Relationship between toxoplasmosis and testosterone hormone among schizophrenic patients in Baghdad. Dissertation, College Council of Health and Medical Technology. Baghdad, Iraq
- Kanková S, Kodym P, Flegr J. Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp Parasitol. 2011;128:181–183. doi: 10.1016/j.exppara.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Khan AA, Arujo GF, Brighty KE. Anti-Toxoplasma gondii activities and structure activity relationships of novel fluoroquinolones related to trovaproxacin. Antimicrob Agents Chemother. 1999;43(7):1783–1787. doi: 10.1128/aac.43.7.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittas C, Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Brit J Exp Pathol. 1980;61(6):590–600. [PMC free article] [PubMed] [Google Scholar]
- Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Lu M, Yang L, Chen C, Wu X, Gong F. Infertility experiment on male mice infected with Toxoplasma. Chin J Zoonoses. 2005;21:592–594. [Google Scholar]
- Makker K, Agarwal A, Sharma R. Oxidative stress and male infertility. Indian J Med Res. 2009;129:357–367. [PubMed] [Google Scholar]
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8(1):1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Oktenli C, Koc B, Allis M, Tasar M, Doganci L. Unilateral renal agenesis and hypogonadotropic hypogonadism: an interesting coincidence. Clin Nephrol. 2001;55:340–342. [PubMed] [Google Scholar]
- Oktenli C, Dogansi L, Ozgurtas T. Transient hypogonadotrophic hypogonadism in males with acute toxoplasmosis: suppressive effect of interleukin-1β on the secretion of GnRH. Human Reprod. 2004;19(4):859–866. doi: 10.1093/humrep/deh161. [DOI] [PubMed] [Google Scholar]
- Peyron F, Lobry JR, Musset K, Ferradiz B, Gomez-Marin JE, Petersen E, Meroni V, Rausher B, Mercier C, Picot S, Cesbron-Delauw MF. Serotyping of Toxoplasma gondii in chronically infected pregnant women: predominance of type I in Europe and types II and III in Colombia (South America) Microbes Infect. 2006;8(9–10):2333–2340. doi: 10.1016/j.micinf.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Qi R, Su XP, Gao XL, Liang XL. Study on Toxoplasma infection in male with sterility in Shenyang China. Zhonghua Nan Ke Xue. 2005;11:503–504. [PubMed] [Google Scholar]
- Radu A, Pichon C, Camparo P, Antoine M. Expression of follicle stimulating hormone receptor in tumor blood vessels. N Eng J Med. 2010;363(17):1621–1630. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- Roberts F, Mets M, Ferguson N, Grady R. Histopathological features of ocular toxoplasmosis in fetus and infant. J Opthal. 2001;119(1):51–58. [PubMed] [Google Scholar]
- SAS . Statistical analysis system, user’s guide. Statistical. 7. Cary, NC: SAS. Inst. Inc.; 2004. [Google Scholar]
- Semple P, Beastall G, Watson W, Hume R. Hypothalamic pituitary dysfunction in respiratory hypoxia. Thorax. 1981;36:605–609. doi: 10.1136/thx.36.8.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirbazou S, Abasian L, Talebi MF. Effect of Toxoplasma gondii infection on plasma testosterone and cortisol level and stress index on patients referred to Sina hospital, Tehran. Jundishapur. J Microbiol. 2011;4(3):167–173. [Google Scholar]
- Spratt D, Bigos S, Beitins I, Cox P, Longcope C, Orav J. Both hyper- and hypogonadotropic hypogonadism occur transiently in acute illness: bio- and immunoactive gonadotropins. J Clin Endocrinol Metab. 1992;75:1562–1570. doi: 10.1210/jcem.75.6.1464665. [DOI] [PubMed] [Google Scholar]
- Steven E, Schmitt B, Golovko A, Mehdi E, and Santanu K (2008) Toxoplasmosis, Chap 2.9.10. In: Barry ON (ed) Terrestrial Manual. OIE Scientific Publications, Paris
- Sun L, Fan F, Wang J, Gong J. Acute Toxoplasma gondii infection affects the reproductive function of male mice. Zhonghua Nan Ke Xue. 2008;14:55–57. [PubMed] [Google Scholar]
- Vallochi AL, Muccioli M, Martins C, Silveira C, Belfort R. The genotype of Toxoplasma gondii strains causing ocular toxoplasmosis in humans in Brazil. Am J Ophthalmol. 2005;139:350–351. doi: 10.1016/j.ajo.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Wang C, Chan V, Yeung R. Effect of surgical stress on pituitary testicular function. Clin Endocrinol. 1978;9:255–266. doi: 10.1111/j.1365-2265.1978.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Song ML, Jiang QZ, Zhao QS, Gao WU, Shen QF, Huang CY, Wang CG. Serological survey of Toxoplasma gondii infection in invalid children. Chinese J Zoonoses. 2002;18:129–130. [Google Scholar]
- Zighelboim I, Maekelt GA, Teppa P, Perera JR, Garran DT, Maneiro P. Reproductive wastage and Toxoplasma antibodies. Am J Obstet Gynecol. 1968;101:839–843. doi: 10.1016/0002-9378(68)90041-0. [DOI] [PubMed] [Google Scholar]