Abstract
Haemoproteus columbae is the major protozoan infection reported in pigeon and appeared in the erythrocyte of the peripheral circulation. Incidence and parasitaemia of H. columbae in pigeon was studied in different localities of Jammu, India for a period from April to September 2010 using thin blood smear examination. Of the 150 pigeons (wild: 70, domestic: 80), 92 (61.33 %) were found to be infected with H. columbae. Domestic pigeon showed higher incidence rate (74.28 %) than the wild (50 %). Mature and immature gametocyte encircled the erythrocyte nucleus to form a halter shaped appearance were characteristic feature of the parasite. Pseudolynchia canariensis, the vector for H. columbae was also recovered from beneath the feathers of pigeons. No other haemoprotozoan parasite was observed in the blood smear of examined pigeon. Counting of infected erythrocyte revealed higher affection of 1–2 erythrocytes indicative of milder infection in the birds. A long term study within bird population is essential in order to disclose seasonal variation in parasite, vector density and age of infection such as nesting area.
Keywords: Haemoproteus columbae, Jammu, North-western India, Pigeon
Introduction
Pigeon occur worldwide, are reared as a symbol of peace, love, gentleness and spirit messengers and now-a-days also for meat, ornamental pet bird or as an experimental animal. Pigeon suffers from many metazoan and protozoan infections among which Haemoproteus columbae, transmitted by Pseudolynchia canariensis, affects domestic as well as wild pigeon. These infections were long considered to be non- pathogenic (Ashford 1971; Benett et al. 1988), although now they are known to negatively impact the reproductive success of wild birds (Allander 1997; Merino et al. 2000). The disease caused by H. columbae in pigeon is called as pseudomalaria or pigeon malaria and is fatal to young pigeons. In pigeon, gametocytes occur in erythrocytes and possess a halter or crescent shaped or horse shoe shaped partially encircling the nucleus of the host cell (Soulsby 1982). There are a very limited number of studies on H. columbae in pigeon from India (Mandal 2002; Shinde et al. 2008). The present study was directed towards the investigating the incidence of H. columbae in pigeon reared in purely domesticated and in free ranging/wild habitation in Jammu district of Jammu and Kashmir state of India.
Materials and methods
Study area
The present study was carried out in Jammu (74° 50′ E and 30° 40′ N), a semi-urban area located in the North-western part of India at 332 m above mean sea level. The study region has a subtropical climate, with an annual rainfall of about 1,069 mm. The mean annual minimum and maximum temperature is 16.36 and 30.18 °C, respectively.
Collection of blood samples
A total of 150 pigeons (70 domestic and 80 wild) were caught using net from April 2010 to September 2010 from different localities of Jammu. After catching, birds were inked and blood samples were collected by vein-puncture method from wing vein in vials containing EDTA. Thin blood smears were prepared from collected samples; air dried, fixed with methanol and were stained with 5 % Giemsa stain and examined under oil immersion. Blood smears were differentially counted for H. columbae and level of parasitaemia was reported as number of parasite per 1,000 erythrocytes and each smear were counted until at least 10,000 uninfected red blood cells were seen before they were declared negative. The birds were also examined for the presence/absence of ectoparasites.
Results
Of the 150 birds, 92 (61.33 %) were found to be infected with H. columbae and 9 (6.0 %) birds were found to be infested with Pseudolynchia canariensis, which is vector for this parasite. On stained blood smears gametocytes were detected in the cytoplasm of the erythrocytes (Fig. 1). The gametocyte encircled the erythrocyte nucleus and formed a halter shaped appearance. Incidences of H. columbae in wild and domesticated pigeons were recorded 50 % (40/80) and 74.28 % (52/70), respectively. The infected erythrocytes were counted in microscopic field for a total of ~1,000 numbers of erythrocytes in all cases. Of the 92 positive blood smears, 45 (48.91 %) showed 1–2 infected erythrocytes, 33 (35.86 %) on 3–5 erythrocytes and 14 (15.21 %) more than 5 erythrocytes with gametocytes of H. columbae. The infected erythrocyte contained only single gametocytes on examination of all positive cases. While examining the body of the pigeons, 11 numbers of hippoboscids flies were caught from 9 infested birds and later they were identified as P. canariensis (Fig. 2), a dipteran parasite of pigeon.
Fig. 1.
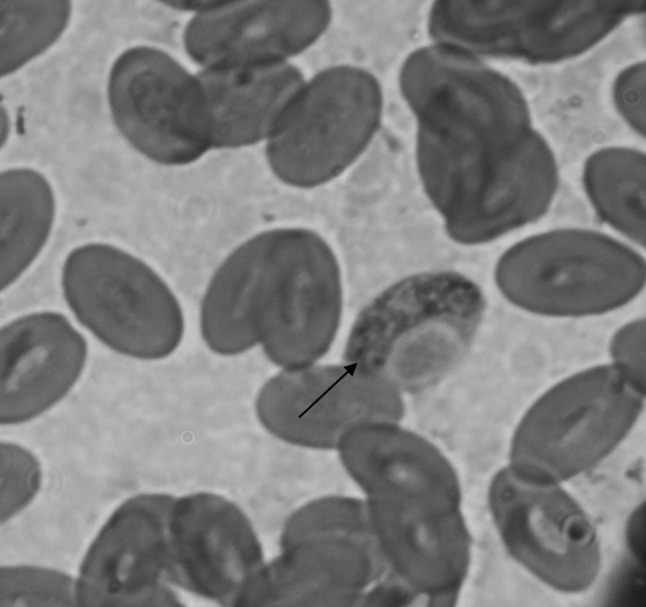
Gamont stage of Haemoproteus columbae (arrow) inside the RBC (100x, Zoom, Giemsa stain)
Fig. 2.

Dorsal view of Pseudolynchia canariensis (10x)
Discussion
Throughout the world, the prevalence of H. columbae in feral pigeons in different geographical locations varies from 14 to 100 %. It has been reported to be 65.8 % infection in San Juan, Puerto Rico (Mclaughlin 1968), 70.4 % in Izmir, Turkey (Tolgay and Cesitli 1972), 76.5 % in Kampala, Ugenda (Dranzoa et al. 1999), 80 % from Sebele (Mushi et al. 2000), 57 % in wild pigeons of Ankara (Gicik and Arslan 2001), 37 % in domestic pigeons of Morogoro municipality of Tanzania (Msoffe et al. 2010) and 17.47 % from Ganbad, Golestan Province of North Iran (Youssefi et al. 2010). In the present study, the overall incidence of H. columbae in pigeons (wild as well as domestic) was recorded to be 61.33 %. Similar finding (58.33 %) has been reported by Shinde et al. (2008) from urban localities of Mumbai, India. In tropical India, a year round abundance of parasitaemia in feral pigeons has been reported by Mandal (2002). Vector abundance was claimed to be the major factor influencing the spatial variation in prevalence of H. columbae in pigeons. Host-parasite relationship was suggested to differ in tropical from non-tropical areas (Moller 1998). In the present study, domestic pigeons showed higher incidence than wild pigeons. The reason might be the free access of vector P. canariensis to the housing system of domestic pigeon and larvae are mostly laid in dark crevices of the pigeon houses in dry dust or in the nests, while the wild pigeon may protect themselves to some extent by their wild habitat in nature.
Examination of the infected erythrocytes in this study revealed only one gametocyte so the infections were mild in the pigeons. More numbers of gametocyte per erythrocyte led severe infection in pigeon. The infected erythrocyte counts within microscopic field in the positive cases determined higher affection of 1–2 erythrocytes also indicative of milder infection in the birds which supported the findings of Gicik and Arslan (2001). The present investigation opined that Jammu—a subtropical region of Western India is harboring the H. columbae and vector P. canariensis in pigeons. The present findings open a serious investigation to study about the haemosporidian protozoa in sub-tropical birds, domesticated one in particular.
Acknowledgments
The authors are highly thankful to Dean, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-Jammu for providing necessary facilities for carrying out the study.
References
- Allander K. Reproductive investment and parasite susceptibility in the great fit. Funct Ecol. 1997;11:348–364. doi: 10.1046/j.1365-2435.1997.00095.x. [DOI] [Google Scholar]
- Ashford RW. Blood parasites and migratory fat at Lake Chand. Ibis. 1971;113:100–101. doi: 10.1111/j.1474-919X.1971.tb05127.x. [DOI] [Google Scholar]
- Benett GF, Caines JR, Bishop MA. The influenza of blood parasites on the body mass of passeriform birds. J Wildlife Dis. 1988;24:339–343. doi: 10.7589/0090-3558-24.2.339. [DOI] [PubMed] [Google Scholar]
- Dranzoa C, Ocaido M, Katete P. The ecto-, gastro-intestinal and haemo-parasites of live pigeons (Columbia) in Kampala. Uganda Avian Pathol. 1999;28(2):119–124. doi: 10.1080/03079459994830. [DOI] [PubMed] [Google Scholar]
- Gicik Y, Arslan M. Blood parasites of wild pigeons in Ankara District. Turk J Vet Anim Sci. 2001;25:169–172. [Google Scholar]
- Mandal PI. Haemoproteus columbae infection of feral pigeons in Singapore and Israel. Raf Bull Zool. 2002;50:281–286. [Google Scholar]
- Mclaughlin ET. Pigeon malaria in San Juan, Puerto Rico. Cari J Sci. 1968;8:101–102. [Google Scholar]
- Merino SJ, Moreno J, Sanz J, Arriero E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits. Proc Roy Soc London. 2000;267:2507–2510. doi: 10.1098/rspb.2000.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AP. Evidence of larger impact of parasites on hosts in tropics: investment in immune function within and outside tropics. Oikos. 1998;82:265–270. doi: 10.2307/3546966. [DOI] [Google Scholar]
- Msoffe PLM, Muhairwa AP, Chiwanga GH. A study of ecto- and endoparasites of domestic pigeon in Morogoro Municipality . Tanz Afr J Agri Res. 2010;5:264–267. [Google Scholar]
- Mushi EZ, Binta MG, Chaba Ndebele RG, Panzirah RR. Parasites of domestic pigeon (Columbia livia domestica) in Sebele, Gaborone, Botswana. J South Afr Vet Assoc. 2000;7:249–250. [PubMed] [Google Scholar]
- Shinde GN, Gantne ML, Singh A. Prevalence of parasites in pigeons (Columbia livia domestica) of Mumbai. J Vet Parasitol. 2008;22:65–66. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: ELBS and Bellaire Tindall and Casael Ltd.; 1982. pp. 256–259. [Google Scholar]
- Tolgay NK, Cesitli Plasmodium, Haemoproteus ve Leucocytozoon enfeksiyonlari uzerinde arastirmalar. Ank Uni Vet Fak Derg. 1972;19:271–286. [Google Scholar]
- Youssefi RM, Sadeghian AG, Esfandiari B. Prevalence of H. columbae infection in Columbia livia in North of Iran. World J Zoo. 2010;5:275–277. [Google Scholar]


