Abstract
Previously, we have reported the composition, molecular mass distribution and in vivo immunomodulatory effects of common carp roe protein hydrolysates. In the current study, antioxidative activity and functional properties of common carp (Cyprinus carpio) roe (egg) protein hydrolysates, prepared by pepsin, trypsin and Alcalase, were evaluated. The three hydrolysates showed excellent antioxidant activities in a dose dependent manner in various in vitro models such as 2,2 diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, 2,2′–azino-bis(3-ethylbenzthiazoline-6)-sulfonic acid (ABTS+) radical scavenging activity, ferric reducing antioxidant power (FRAP) and ferrous ion (Fe2+) chelating ability. Enzymatic hydrolysis significantly increased protein solubility of the hydrolysates to above 62 % over a wide pH range (2–12). Carp roe hydrolysates exhibited good foaming and emulsification properties. The results suggest that bioactive carp roe protein hydrolysates (CRPHs) with good functional properties could be useful in health food/nutraceutical/pharmaceutical industry for various applications.
Keywords: Enzymatic hydrolysis, Fish egg (roe) protein hydrolysates, Antioxidant activities, Functional properties, Fish processing by-products
Introduction
Large quantities of protein rich fish processing by-products or underutilized fish species are discarded as waste, without any attempt to recover the essential nutrients and bioactive peptides. In vitro enzymatic hydrolysis is widely used method to extract bioactive peptides/protein hydrolysates from variety of food proteins. Enzymatic hydrolysis of fish proteins is an efficient way to recover the essential nutrients and potential bioactive peptides with well-defined functional properties. Application of proteolytic enzymes is often an attractive means for improving functional properties of food proteins, without losing their nutritional and biological value. Hydrolysates produced by enzymatic hydrolysis contain well defined peptide profiles. Pepsin, trypsin, and Alcalase have been tested successfully for the production of bioactive protein hydrolysates from variety of fish proteins (Chalamaiah et al. 2012). Fish protein hydrolysates have been reported to have good potential for nutritional, pharmaceutical, cosmetic and nutraceutical application as functional ingredient due to their health promoting effects (Chalamaiah et al. 2012).
Enzymatic hydrolysis has been applied to many food proteins to release bioactive peptides with desired functional properties. Several recent studies have shown that protein hydrolysates with antioxidant activity can be produced from various fish proteins by enzymatic hydrolysis (Bougatef et al. 2009; Cheung et al. 2012; Slizyte et al. 2009). During enzymatic hydrolysis, peptide bond cleavage allows the release of active peptides capable of sequestering oxygen radicals, chelating pro-oxidant metal ions and inhibiting lipid peroxidation in food systems (You et al. 2010). In addition, enzymatic hydrolysis generates low molecular weight peptides with excellent functional characteristics. The functionalities of fish protein hydrolysates are affected by the type of protein, enzyme used, and degree of hydrolysis (DH) (Klompong et al. 2007). Antioxidant activity has been reported for fish protein hydrolysates prepared from several fish protein sources, which include pink perch muscle (Naqash and Nazeer 2013), tuna liver (Je et al. 2009), cod backbone (Slizyte et al. 2009), silver carp processing by-products (Zhong et al. 2011) and Labeo rohita egg (Chalamaiah et al. 2013).
India is one of the major producers of common carp. The spawning of common carp occurs throughout the year in tropical India with fecundity ranged from 100 to 230 g eggs/kg body weight (~10 to 23 % of total fish weight) (FAO 2006). In India, large quantities of common carp roes are discarded as waste, without any attempt to recover the essential nutrients and bioactive molecules. Therefore, the production of protein hydrolysates with antioxidant activities as well as good functional properties can pave the way for better utilization of this species. In our previous study, the chemical composition, molecular mass distribution and immunomodulatory effects of common carp egg protein hydrolysates have been reported (Chalamaiah et al. 2014). Studies related to antioxidant activity and functional characteristics of common carp roe hydrolysates are scanty. Hence, in the present study enzymatic hydrolysis of common carp roe was performed using pepsin, trypsin and Alcalase to produce antioxidant protein hydrolysates with good functional characteristics. The objectives of this investigation were to study the antioxidant activity and functional properties of protein hydrolysates prepared from common carp roe (egg) using pepsin, trypsin and Alcalase enzymes.
Materials and methods
Enzymes and chemicals
Enzymes, pepsin (from HOG stomach, 1:3000) and trypsin (from bovine pancreas, 1:250) were obtained from Loba Chemie Pvt. Ltd. (Mumbai, India). Alcalase (2.4 L-AU-A/g), DPPH (2,2 diphenyl-1-picrylhydrazyl), ABTS (2,2′–azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), ferrozine and ferrous chloride were purchased from Sigma (St. Louis, MO, USA). Potassium ferricyanide, ferric chloride, ammonium thiocyanate and trichloroacetic acid (TCA) were procured from Hi Media Laboratories Pvt. Ltd. (Mumbai, India).
Roe sample preparation
Common carp (Cyprinus carpio) roes (eggs) were obtained from a local fish market (Andhra Pradesh Fisheries Department, Hyderabad) immediately after processing and brought to the laboratory, stored at 4 °C for < 2 h before experimental work. Fresh roes were separated from blood vessels, skeins, and homogenized using high speed mixer (Sumeet, India) to get fish roe homogenate. The roe homogenate was dried at 48 ± 2 °C for 8 h in a cabinet tray dryer (Chemida, Mumbai), ground to powder using a high speed mixer and passed through 180 μ mesh to get fine powder. The roe powder was stored in Schott duran screw cap bottle (Schott Duran, Germany) at −20 °C until used for experimental work.
Preparation of protein hydrolysates from common carp roe (egg)
Protein hydrolysates were prepared using optimum enzyme concentration as reported in earlier study (Chalamaiah et al. 2014). Carp roe powder (5 g, protein basis) was suspended in 150 ml of distilled water. The mixture was adjusted to the optimum pH (2.0 for pepsin or 8.0 for trypsin and Alcalase) for enzyme activity. The mixtures were pre-incubated at 37 °C for pepsin and trypsin or 55 °C for Alcalase for 10 min prior to enzymatic hydrolysis. The protein hydrolysis reaction was initiated by the addition of the enzyme at a level of 1.5 % (w/w for pepsin and trypsin or v/w for Alcalase). The enzymatic reaction was performed for 180 min with continuous stirring by maintaining optimum temperature (37 ± 1 °C for pepsin and trypsin, or 55 ± 1 °C for Alcalase). The enzyme activity was terminated by keeping the mixture in boiling water bath at 85–95 °C for 20 min. After enzyme inactivation, pepsin reaction mixture was neutralized to pH 7.0 with 1N NaOH. The slurry was then centrifuged at 13,000g using Eppendorf centrifuge (Model 5810 R, Germany) for 30 min at 4 °C and the soluble aqueous fraction was decanted, vacuum dried, and stored in Schott Duran screw cap bottles (Schott Duran, Germany) at −20 °C until further experiments.
Determination of antioxidant activities
DPPH radical scavenging activity
DPPH (2,2 diphenyl-1-picrylhydrazyl) radical scavenging activity was determined according to the method of Klompong et al. (2007), with slight modification. Various concentrations (0.5, 1.0, 1.5, 2.0 and 2.5 mg/ml) of common carp roe protein hydrolysates were taken in 4 ml distilled water and l ml of 0.15 mM DPPH was added, and incubated for 30 min at room temperature in dark and absorbance was read at 517 nm using UV-Visible spectrophotometer (Perkin-Elmer Lambda 1, USA). Distilled water was used for control sample. Radical scavenging activity was calculated using the following formula.
| 1 |
C, CB, S and SB are the absorbance of the control, blank control, sample and blank samples.
ABTS+ radical scavenging activity
ABTS+ (2,2′-azinobis(3- ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity was measured by following the method of Khantaphant and Benjakul (2008). ABTS+ radical was produced by allowing the ABTS stock solution (7.4 mM in distilled water) to react with 2.6 mM potassium persulfate at ratio of 1:1 (v/v) in dark (at room temperature, 25 ± 2 °C) for 14 h. Then ABTS+ solution was diluted with methanol to obtain the absorbance of 1.1 at 734 nm. Different concentrations (0.1, 0.2, 0.3, 0.4 and 0.5 mg/ml) of the hydrolysates were taken in 1 ml of distilled water and mixed with 3.0 ml of ABTS+ solution and incubated at room temperature for 30 min in dark. Control sample was performed using distilled water. The absorbance was read at 734 nm and percent ABTS+ radical scavenging activity was calculated using Eq. (1).
Ferric reducing antioxidant power (FRAP)
Ferric reducing antioxidant power was determined by following the method of Wu et al. (2003), with minor modification. Two ml sample (1.0, 2.0, 3.0, 4.0, and 5.0 mg/ml) and 2 ml 1 % potassium ferricyanide were added to 2 ml of 0.2 M phosphate buffer (pH 6.6), and the mixture was incubated at 50 °C for 20 min. Then 2 ml of 10 % TCA was added and centrifuged for 10 min at 3000 rpm. To the upper layer (2 ml) of each sample, 2 ml of distilled water and 0.4 ml of 0.1 % ferric chloride were added. The reaction was allowed for 10 min, and absorbance of the solution was measured at 700 nm. The control was prepared using distilled water instead of the sample. Increasing absorbance at 700 nm indicates increasing reducing power of protein hydrolysates.
Ferrous (Fe 2+) ion chelating ability
Ferrous ion chelating activity of the hydrolysates was determined according to the method of Klompong et al. (2007). Various concentrations of roe hydrolysates (0.5, 1.0, 1.5, 2.0 and 2.5 mg/ml) were mixed with 4.7 ml of distilled water. 0.1 ml of 2 mM FeCl2 and 0.2 ml of 5 mM 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine) were added to the samples and incubated for 20 min at room temperature. The absorbance was measured at 562 nm. The control was prepared using distilled water. Ferrous ion chelating ability (%) was calculated using following formula.
| 2 |
C, CB, S and SB are the absorbance of the control, blank control, sample and blank samples.
Evaluation of functional properties of CRPHs
Protein solubility
Protein solubility of the hydrolysates was determined by following the method of Klompong et al. (2007). Two hundred milligrams of hydrolysates were taken in 20 ml of distilled water, and the mixture was adjusted to desired pH value (from 2 to 12) with 0.5 N hydrochloric acid (HCl) or 0.5 N sodium hydroxide (NaOH). The mixture was stirred at room temperature (25 ± 2 °C) for 30 min, and then centrifuged at 4500g for 30 min at 4 °C. Protein content in the supernatant was determined using the Biuret method (Weichselbaum 1946). Protein solubility was calculated using the following formula.
Foaming properties
Foaming properties (foam capacity and foam stability) were measured according to the method of Klompong et al. (2007), with minor modification. Roe protein hydrolysates (0.5 %) were dissolved in 20 ml of distilled water, pH was adjusted to 2, 4, 6, 8 and 10 using 0.5 N HCl or 0.5 N NaOH, and then the contents were transferred into 100 ml measuring cylinder and whipped for 30 s. Total volume was recorded immediately at 0, 30 and 60 min. Foam capacity and foam stability were calculated according to the following equations.
- A
is the volume after whipping at ‘0’ min (ml)
- B
is the volume before whipping (ml)
- A
is the volume at 30 and 60 min (ml)
- B
is the volume before whipping (ml)
Emulsifying properties
Emulsifying activity index (EAI) and emulsion stability index (ESI) were determined using the method of Pearce and Kinsella (1978). Fifteen ml of 1 % protein hydrolysate was mixed with 5 ml of sunflower oil and pH was adjusted to 2, 4, 6, 8 and 10 using 0.5 N HCl or 0.5 N NaOH. The mixture was homogenized for 1 min at a speed of 18,000 rpm, using IKA T25 digital Ultr-Turrax homogenizer. An aliquot of the emulsion (50 μl) was taken from bottom of the tube at 0 and 10 min after homogenization and mixed with 5 ml of 0.1 % sodium dodecylsulphate (SDS) solution (1:100 dilution). The absorbance of the diluted solution was measured at 500 nm using UV-Visible spectrophotometer (Perkin-Elmer Lambda 1, USA). The absorbances measured immediately (A0) and 10 min (A10) after emulsion formation were used to calculate emulsifying activity index (EAI) and emulsion stability index (ESI). EAI and ESI were calculated using the following formula.
Where dil is the dilution factor (100);
- A
is the absorbance at 500 nm
- c
is the protein concentration (g/ml), 0.01
- θ
is the disperse phase volume fraction (0.25).
Where ΔA = A0 – A10 and Δt = 10 min
Statistical analysis
All the experiments were performed in triplicates and the mean values were reported. The statistical analysis was done using one-way analysis of variance (ANOVA) with post hoc multiple comparisons (least significant difference). Statistical significant was evaluated at P < 0.05. Statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL).
Results and discussion
The percentage of degree of hydrolysis (DH) of common carp roe (egg) after 180 min was found to be 30, 19.5, and 12.7 % for pepsin, trypsin, and Alcalase enzymes, respectively (Chalamaiah et al. 2014).
Antioxidant activity
Measurement of antioxidant capacity using single method cannot provide the compound’s ability to act as antioxidant. Hence, this study involved four in vitro methods such as 2,2 diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, 2,2′–azino-bis(3 ethylbenzthiazoline-6)-sulfonic acid (ABTS+) radical scavenging activity, ferric (Fe3+) reducing antioxidant power (FRAP) and metal chelating activity.
DPPH and ABTS+ radical scavenging activities
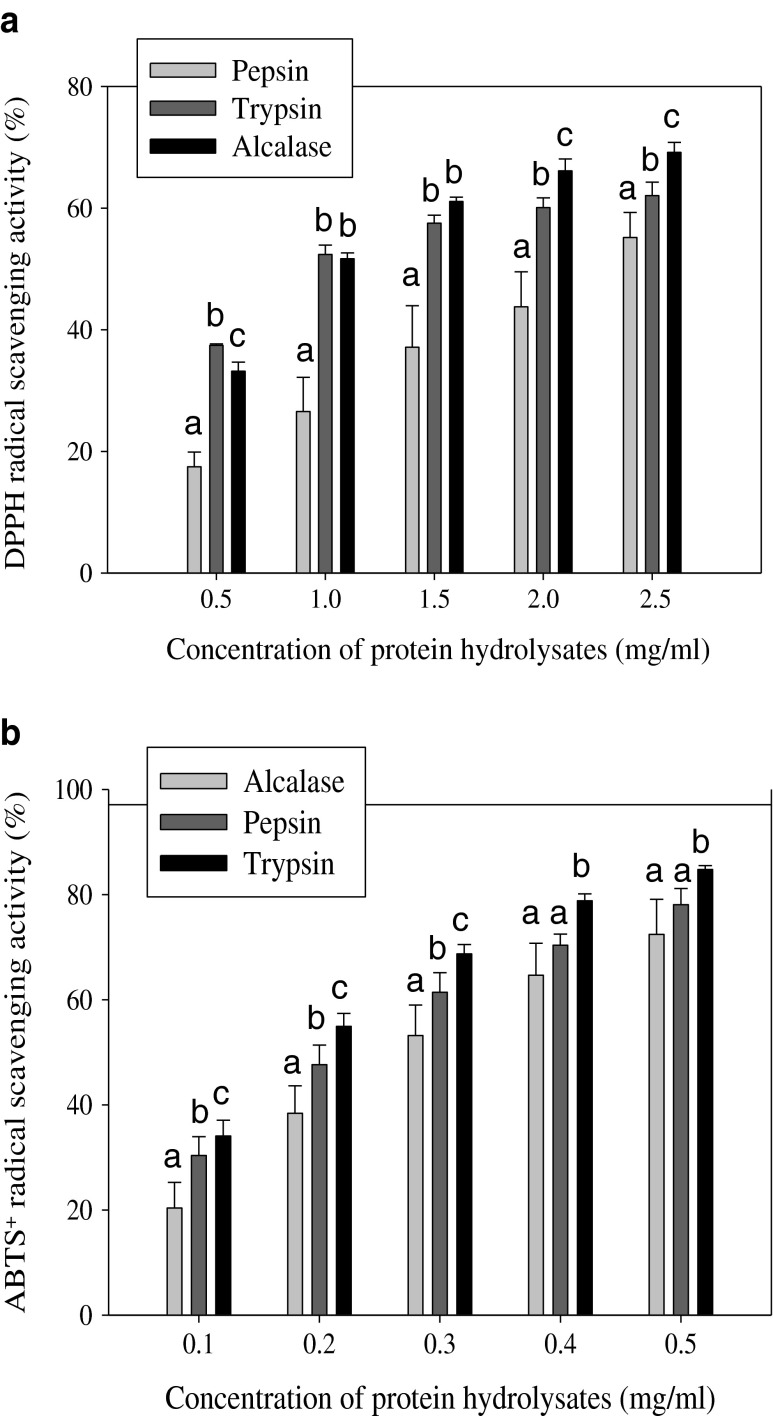
The radical scavenging activity of carp roe protein hydrolysates (CRPHs) was assessed by using two in vitro free radical models, namely, DPPH and ABTS+. When DPPH and ABTS radicals encounter a proton-donating substance such as an antioxidant, the radical is scavenged, and the absorbance is reduced by changing the color (Liu et al. 2010). As depicted in Fig. 1, carp roe protein hydrolysates showed good DPPH and ABTS+ radical-inhibiting activity in a dose-dependent manner. Our findings are in line with previous results reported by Intarasirisawat et al. (2012) and Chalamaiah et al. (2013) who reported that the DPPH and ABTS scavenging activity increased with increasing concentrations.
Fig. 1.
Scavenging effect of carp roe protein hydrolysates on 2,2-diphenyl-1-picrylhydrazyl (DPPH) (a) and 2,2′–azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS+) radicals (b). (n = 3, mean ± SD). Error bars represent standard deviations from triplicate determinations. Different superscript letters (a, b, c) on same concentration of the three hydrolysates indicate significant difference at P < 0.05
The low molecular mass peptides (<10 kDa) present in the CRPHs might be responsible for the exhibited radical inhibiting activity (Chalamaiah et al. 2014). Presence of amino acids such as histidine, methionine, cysteine, phenylalanine and tyrosine in the CRPHs might have contributed to better DPPH and ABTS+ radicals scavenging activities (Chalamaiah et al. 2014). In the current study, IC50 value (mg/ml) is used to evaluate the scavenging activity of CRPHs. IC50 is defined as concentration of sample required to attain a 50 % reduction of the initial DPPH or ABTS+ radical concentration. For DPPH radical, the IC50 values were found to be 1.151, 1.158 and 2.255 mg/ml for Alcalase, trypsin and pepsin hydrolysates, respectively. In case of ABTS+ radical, the scavenging activity of the hydrolysates was in order of trypsin (IC50 = 0.186 mg/ml) > pepsin (IC50 = 0.235 mg/ml) > Alcalase (IC50 = 0.301 mg/ml). The three hydrolysates exhibited significant difference (P < 0.05) in DPPH and ABTS+ radicals inhibiting activity. The significant difference (P < 0.05) in radical inhibiting activity of CRPHs might be due to the difference in the sequence, amino acid constituents and size of peptides, resulted from different DH (Chalamaiah et al. 2014). The results obtained suggest that the three hydrolysates contained some peptides that were electron donors and could react with free radicals to convert them to more stable products and terminate the radical chain reaction (Jemil et al. 2014). The radical scavenging activities of fish protein hydrolysates are usually associated with the amino acid composition, sequence and hydrophobicity (Bougatef et al. 2010; Chalamaiah et al. 2012; Gimenez et al. 2009). DPPH and ABTS scavenging activity was also reported in protein hydrolysate prepared from Pangasius sutchi (Najafian and Babji 2015), Nemipterus hexodon (Nalinanon et al. 2011) and Mustelus griseus (Wang et al. 2014). The free radical antioxidant activity shown by carp roe hydrolysates could be useful in the preparation of health foods/nutraceuticals.
Ferric (Fe3+) reducing antioxidant power (FRAP)
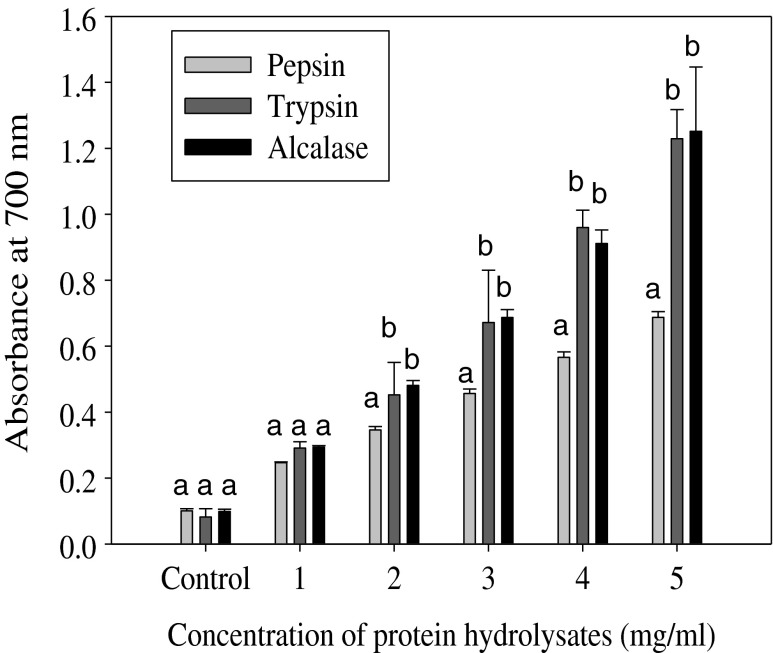
The reducing power assay is often used to evaluate the ability of an antioxidant to donate an electron or hydrogen (Klompong et al. 2007). Antioxidative peptides in protein hydrolysate were able to reduce the Fe3+/ferric cyanide complex to the ferrous form. Fe2+ complex can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm (Intarasirisawat et al. 2012). As shown in Fig. 2, carp roe protein hydrolysates (CRPHs) exhibited strong reducing power (absorbance at 700 nm) in a dose dependent manner (1–5 mg/ml). The reducing power of the three hydrolysates increased with increasing concentrations. Compounds with higher reducing power have better abilities to donate electron or hydrogen and serve as a significant indicator of its potential for use as an antioxidant (Je et al. 2009). The strong reducing power of CRPHs may be attributed to the increased availability of hydrogen ions (protons and electrons) due to release of the peptides by enzymatic hydrolysis (Liu et al. 2010). Strong ferric ion reducing power was reported for tuna liver protein hydrolysates by Je et al. (2009). At concentrations 2, 3, 4 and 5 mg/ml pepsin hydrolysate showed significantly lower (P < 0.05) reducing power compared to trypsin and Alcalase hydrolysates. In the present study, ferric reducing antioxidant power was mainly influenced by type of the protease used for hydrolysis (Klompong et al. 2007; Wiriyaphan et al. 2012). Various proteases generate peptides of varying sequences, compositions and sizes depending on the enzyme specificity. Present study results clearly showed that reducing power ability of roe protein hydrolysates mainly depend on the type of protease, and further support the other findings reported by Wiriyaphan et al. (2012), Bougatef et al. (2009) and Zhao et al. (2012). The reducing power of CRPHs is comparable to the reducing power of skipjack (Katsuwonous pelamis) roe protein hydrolysates (Intarasirisawat et al. 2012).
Fig. 2.
Ferric (Fe3+) reducing power of carp roe protein hydrolysates measured by the ability to donate electrons and reduce Fe3+to Fe2+ ions. The formation of Fe2+/ferricyanide complex was measured as the absorbance at 700 nm. The increasing absorbance at 700 nm indicates increasing reducing power. Different superscript letters (a, b, c) on same concentration of the three hydrolysates indicate significant difference at P < 0.05
Metal (Fe2+) chelating activity
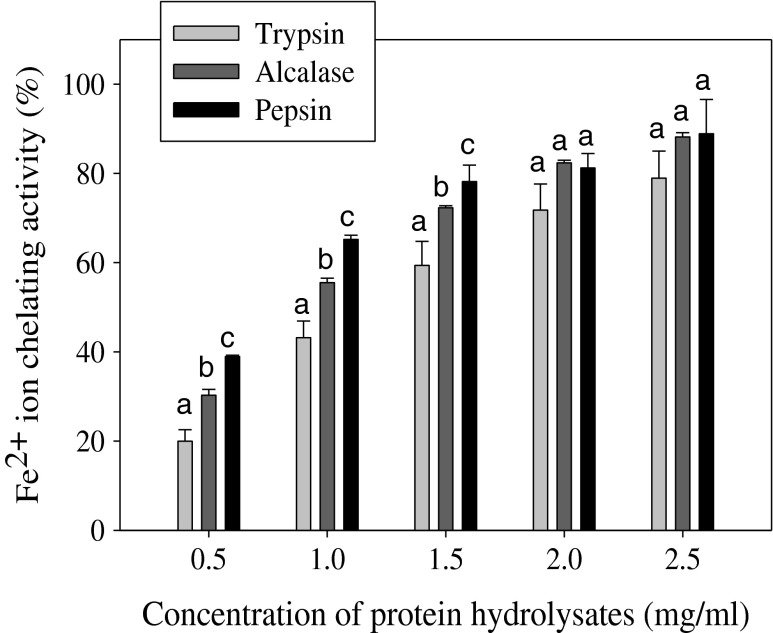
Transition metals such as iron and copper are well-known stimuli of lipid peroxidation and their chelation helps to retard the peroxidation and subsequently prevent damage to cells and tissues (Nasri et al. 2013). Metal chelating activity of carp roe protein hydrolysates (CRPHs) is presented in Fig. 3. The results showed that hydrolysates obtained by three proteases displayed excellent Fe2+ion chelating activity in a dose dependent manner. At lower concentrations (0.5, 1, and 1.5 mg/ml) the three hydrolysates showed significant difference (P < 0.05) in chelating activity, whereas at higher concentrations (2 and 2.5 mg/ml) no significant difference (P > 0.05) was observed. These results are similar to those found in protein hydrolysates prepared from cuttlefish (Sepia officinalis) muscle (Hmidet et al. 2011) and skipjack roe (Intarasirisawat et al. 2012) by enzymatic treatment. The metal chelating activity of CRPHs was in the order of pepsin (IC50 = 0.615) > Alcalase (IC50 = 0.948) > trypsin (IC50 = 1.341). Various proteases could produce peptides of varying chain lengths of different amino acids, based on their specificity. Intarasirisawat et al. (2012) reported that the peptide chain length is essential for the chelating activity of roe protein hydrolysates. Generally, histidine containing peptides are well known to act as strong metal ion chelators (Chalamaiah et al. 2012). The Fe2+ ion chelating activity obtained in the current study might be related to the presence of histidine residues (Chalamaiah et al. 2014). The low molecular mass peptides in the CRPHs could chelate the prooxidants (Fe2+, Cu2+) and thereby decrease lipid oxidation, and contribute as indirect antioxidants (Hmidet et al. 2011). These results suggest that peptides in the CRPHs could be potential antioxidants through metal chelating ability.
Fig. 3.
Metal (Fe2+) chelating ability of carp roe protein hydrolysates. Error bars represent standard deviation from triplicate experiments. Different superscript letters (a, b, c) on same concentration of the three hydrolysates indicate significant difference at P < 0.05
Functional properties
Protein solubility
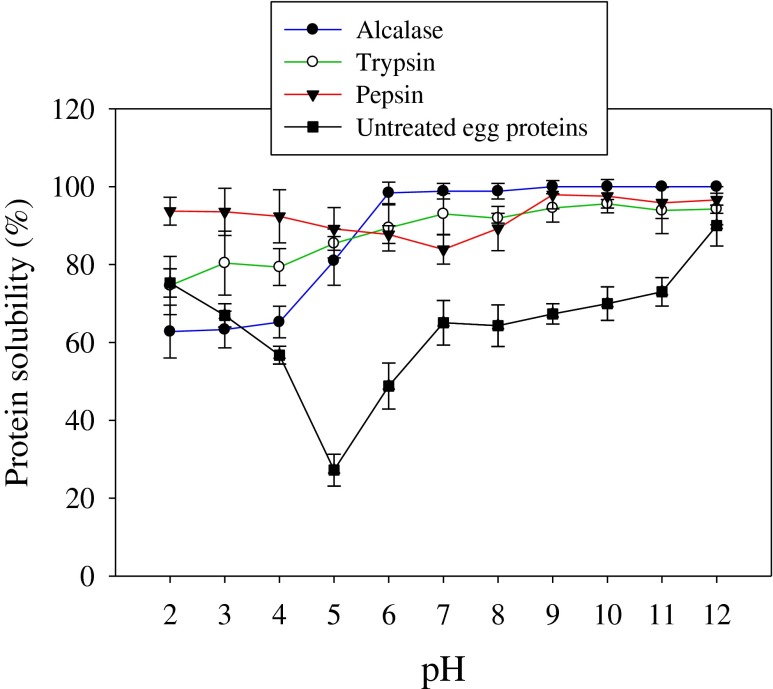
High solubility of fish protein hydrolysates is important characteristic for many food applications, and it influences the other functional properties such as foaming and emulsification properties (Kristinsson and Rasco 2000). Figure 4 shows the solubility profiles of carp roe protein hydrolysates (CRPHs). Roe hydrolysates obtained by pepsin, trypsin and Alcalase showed more than 62.8 % solubility over a wide range of pH 2 to 12. At pH 2, 3, 4 and 5, the three hydrolysates exhibited relatively lower solubility when compared to the solubility at pH values 8, 9, 10, 11 and 12. Klompong et al. (2007) reported lower solubility at pH 2, 3, 4, and 5 for yellow stripe trevally meat protein hydrolysates. Protein hydrolysates usually exhibit low solubility at their isoelectric points. The three hydrolysates showed higher solubilities (>87 %) at pH values 6 to 12. This indicates that pH influences the charge on weakly acidic and basic side chain groups of peptides. Enzymatic hydrolysis by pepsin, trypsin and Alcalase substantially improved the solubility of CRPHs when compared to solubility profile of un-hydrolyzed roe proteins. Solubility is one of the most important characteristic of proteins/protein hydrolysates which has pronounced effect on other functional properties such as emulsification and foaming capacities (Kristinsson and Rasco 2000). Higher solubility profiles (from 62.8 to 100 %) obtained in the current study might be due to reduction in the molecular size and an increase in the number of polar groups by enzymatic hydrolysis (Chalamaiah et al. 2014). Conversion of proteins into peptides leads to more soluble products (Gbogouri et al. 2004). Solubility profiles of carp roe protein hydrolysates are comparable with the solubility profiles of Cirrhinus mrigala roe protein hydrolysates (Chalamaiah et al. 2010). High solubility of CRPHs could be useful in the preparation of various food/pharmaceutical formulations.
Fig. 4.
Solubility profiles of carp roe protein hydrolysates as affected by pH (2–12). Results are mean ± SD from triplicate determinations
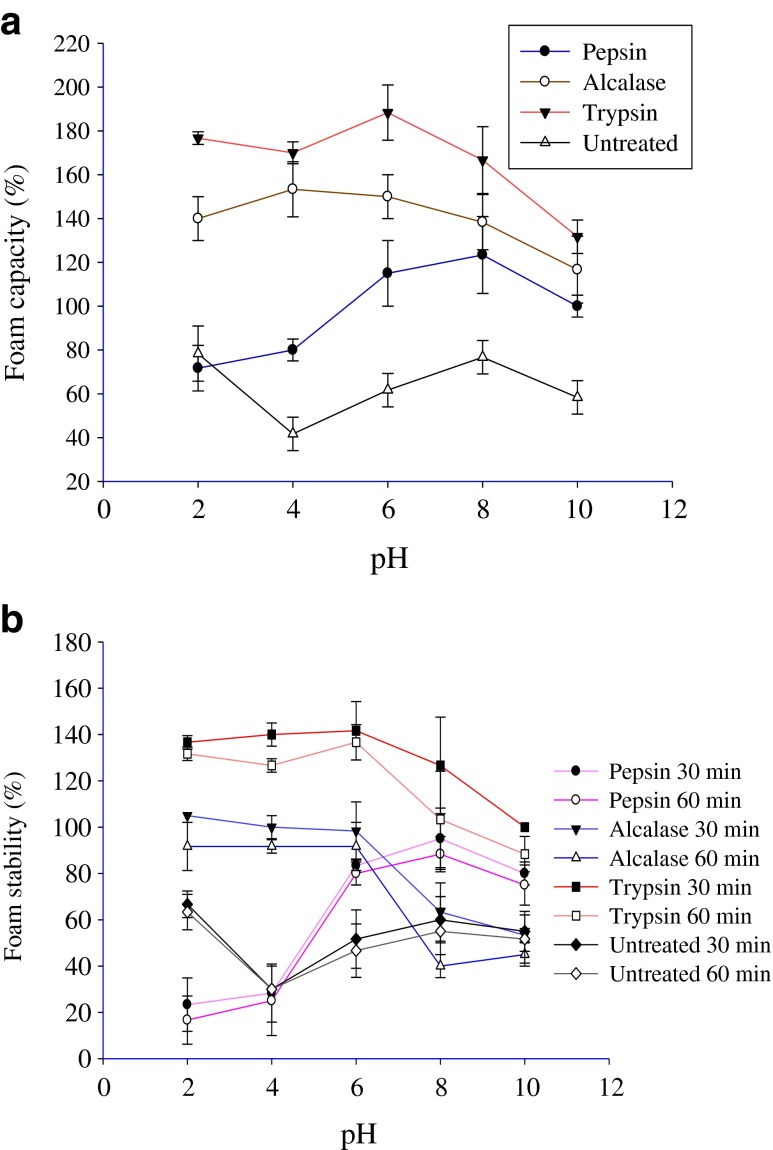
Foaming properties
Foaming capacity of carp roe protein hydrolysates (CRPHs) is presented in Fig. 5a. Foams are biphasic colloidal systems with a continuous liquid and a dispersed air phase (Liu et al. 2010). Carp roe protein hydrolysates showed superior foaming capacity (71–188 %) when compared to the untreated carp roe proteins, which exhibited lower foam capacity (41–78 %) at various pH values of 2–10. For a protein/protein hydrolysate to exhibit good foaming capacity, rapid migration to the air–water interface, unfolding and rearranging at the interface are very important characteristics. In the present study, CRPHs likely contained peptides with more hydrophobic amino acids and adsorb rapidly at the air-water interface. pH markedly affected the foaming capacity of CRPHs. The hydrolysates produced by the three enzymes showed different pattern of foaming capacity at all the pH levels (2–10). This could be attributable to the fact that peptides in the hydrolysates might have different compositions, sizes, and variations in the net charge of peptides (Pacheco-Aguilar et al. 2008). Klompong et al. (2007) reported foaming capacity for yellow stripe trevally protein hydrolysates at different pH values (2 to10), which are comparable to the present study results. Previous study by Chalamaiah et al. (2010) reported lower foam capacity values for Cirrhinus mrigala roe protein hydrolysates than the present study values.
Fig. 5.
Foaming capacity (a) and foam stability (b) of common carp roe protein hydrolysates as influenced by pH. Data is expressed as mean from three replicate experiments with standard deviations
As shown in Fig. 5b, the three hydrolysates exhibited good foam stability. Not much difference was observed between 30 and 60 min foam stability of the three hydrolysates. Foam stability of CRPHs was considerably influenced by the pH of the dispersing medium. Trypsin hydrolysate showed higher foam stability (100–141 %) after 30 min than pepsin and Alcalase hydrolysates. Generally, larger peptides in the hydrolysates could form flexible films around the air bubbles and form more stable foam. Foam stability values obtained in the present study were higher than the values reported by Pacheco-Aguilar et al. (2008) for protein hydrolysates produced from Pacific whiting (Merluccius productus) muscle. The high foaming capacity of CRPHs could be useful in food industry for various applications.
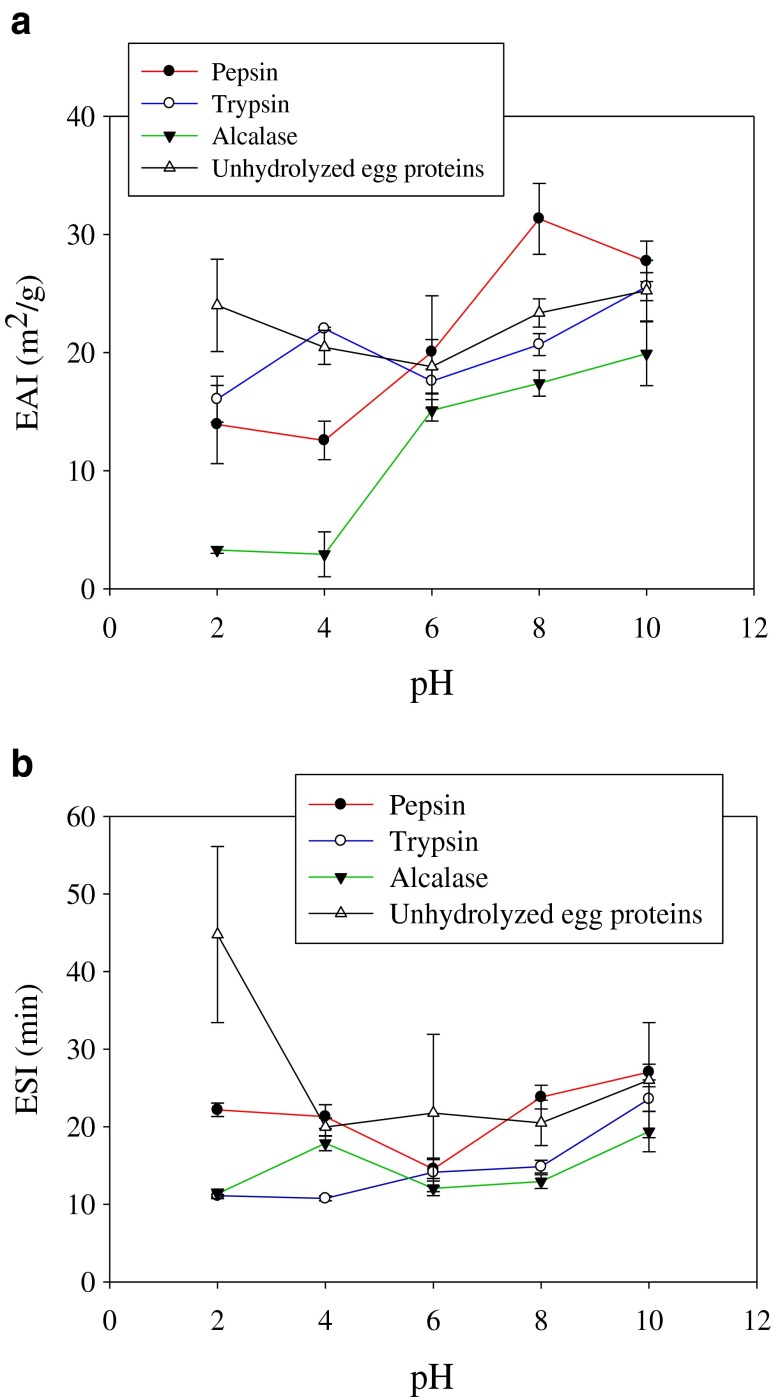
Emulsifying properties
The capacity of protein hydrolysates to form stable emulsions is important since interaction between proteins and lipids is essential for application in many food systems. Emulsifying activity index (EAI) and emulsion stability index (ESI) of carp roe hydrolysates are depicted in Fig. 6a and b. Roe hydrolysates obtained by pepsin, trypsin and Alcalase exhibited good EAI and ESI at various pH values (2, 4, 6, 8 and 10). pH markedly affected the EAI and ESI of CRPHs. This indicates that the sequences and compositions of amino acids in peptides of pepsin, trypsin and Alcalase hydrolysates might be different, leading to varying charge of the resulting peptides at a particular pH and might have resulted in varying EAI and ESI. Pacheco-Aguilar et al. (2008) reported significant effect of pH on EAI and ESI of Pacific whiting (Merluccius productus) muscle protein hydrolysates. In the present study, pepsin hydrolysate with 30 % DH showed higher EAI and ESI (at pH 6, 8 and 10) than trypsin and Alcalase hydrolysates. Generally, higher degree of hydrolysis brings about the loss of emulsifying properties (Kristinsson and Rasco 2000; Gbogouri et al. 2004). Several previous studies reported positive relationship between peptide length and emulsifying properties of protein hydrolysates (Kristinsson and Rasco 2000; Liu et al. 2010). Klompong et al. (2007) reported similar EAI at both 15 and 25 % DH for yellow stripe trevally protein hydrolysates. Pepsin hydrolysate with 30 % DH likely contained peptides with more amphiphilic characteristics that might favour the migration of peptides to oil–water interface (Chalamaiah et al. 2014). The results obtained in the present study are comparable to the results of Zhao et al. (2012), who reported higher EAI at higher %DH for rice dreg protein hydrolysates.
Fig. 6.
Emulsifying activity index (a) and emulsion stability index (b) of common carp roe protein hydrolysates as influenced by pH. Data is expressed as mean from three replicate experiments with standard deviations
Conclusions
The present study demonstrated that the three proteases (pepsin, trypsin and Alcalase) are efficient in converting underutilized carp roe proteins into bioactive protein hydrolysates with excellent functional properties. The three hydrolysates exhibited excellent antioxidant activity in a dose dependent manner in various in vitro models such as DPPH radical scavenging activity, ABTS+ radical scavenging activity, ferric reducing antioxidant power (FRAP) and metal (Fe2+) chelating activity. The results suggested that the three hydrolysates had good functional properties at various pH values (2–12). The present study results suggest that the carp roe protein hydrolysates with biological activities could be useful in functional foods/health foods/nutraceuticals/pharmaceutical formulations for various food and therapeutic applications.
Acknowledgments
The authors wish to thank Indian Council of Medical Research (ICMR), New Delhi, for providing senior research fellowship (SRF) (No. 3/1/2/19/2010-RHN) for Mr. M. Chalamaiah. The authors would like to thank Mr. V. Venubabu, NIN for his assistance during the study. The authors thank Director, NIN-ICMR and Director, CSIR-CFTRI for their keen interest and permission for publication.
References
- Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009;114:1198–1205. doi: 10.1016/j.foodchem.2008.10.075. [DOI] [Google Scholar]
- Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010;118:559–565. doi: 10.1016/j.foodchem.2009.05.021. [DOI] [Google Scholar]
- Chalamaiah M, Narsing Rao G, Rao DG, Jyothirmayi T. Protein hydrolysates from meriga (Cirrhinus mrigala) roe and evaluation of their functional properties. Food Chem. 2010;120:652–657. doi: 10.1016/j.foodchem.2009.10.057. [DOI] [Google Scholar]
- Chalamaiah M, Dinesh kumar B, Hemalatha R, Jyothirmayi T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M, Jyothirmayi T, Bhaskarachary K, Vajreswari A, Hemalatha R, Dinesh Kumar B. Chemical composition, molecular mass distribution and antioxidant capacity of rohu (Labeo rohita) roe (egg) protein hydrolysates prepared by gastrointestinal proteases. Food Res Int. 2013;52:221–229. doi: 10.1016/j.foodres.2013.03.020. [DOI] [Google Scholar]
- Chalamaiah M, Hemalatha R, Jyothirmayi T, Diwan PV, Bhaskarachary K, Vajreswari A, Ramesh Kumar R, Dinesh Kumar B. Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg (roe) Nutrition. 2014 doi: 10.1016/j.nut.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Cheung IWY, Cheung LKY, Tan NY, Li-Chan ECY. The role of molecular size in antioxidant activity of peptide fractions from Pacific hake (Merluccius productus) hydrolysates. Food Chem. 2012;134:1297–1306. doi: 10.1016/j.foodchem.2012.02.215. [DOI] [PubMed] [Google Scholar]
- FAO (2006) Cultured aquatic species information program Cyprinus carpio (Linnaeus, 1758), http://www.fao.org/fishery/culturedspecies/Cyprinus_carpio/en
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates. J Food Sci. 2004;69:615–622. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- Gimenez B, Aleman A, Montero P, Gomez-Guillen MC. Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chem. 2009;114:976–983. doi: 10.1016/j.foodchem.2008.10.050. [DOI] [Google Scholar]
- Hmidet N, Balti R, Nasri R, Sila A, Bougatef A, Nasri M. Improvement of functional properties and antioxidant activities of cuttlefish (Sepia officinalis) muscle proteins hydrolyzed by Bacillus mojavensis A21 proteases. Food Res Int. 2011;44:2703–2711. doi: 10.1016/j.foodres.2011.05.023. [DOI] [Google Scholar]
- Intarasirisawat R, Benjakul S, Visessanguan W. Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chem. 2012;135:3039–3048. doi: 10.1016/j.foodchem.2012.06.076. [DOI] [PubMed] [Google Scholar]
- Je JY, Lee KH, Lee MH, Ahn CB. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res Int. 2009;42:1266–1272. doi: 10.1016/j.foodres.2009.06.013. [DOI] [Google Scholar]
- Jemil I, Jridi M, Nasri R, Ktari N, Slama-Ben Salem RB, Mehiri M, Hajji M, Nasri M. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem. 2014;49:963–972. doi: 10.1016/j.procbio.2014.03.004. [DOI] [Google Scholar]
- Khantaphant S, Benjakul S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp Biochem Physiol B. 2008;151:410–419. doi: 10.1016/j.cbpb.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical and functional properties. Crit Rev Food Sci Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kong B, Xiong YL, Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- Najafian L, Babji AS. Isolation, purification and identification of three novel antioxidative peptides from patin (Pangasius sutchi) myofibrillar protein hydrolysates. LWT Food Sci Technol. 2015;60:452–461. doi: 10.1016/j.lwt.2014.07.046. [DOI] [Google Scholar]
- Nalinanon S, Benjakul S, Kishimura H, Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011;124:1354–1362. doi: 10.1016/j.foodchem.2010.07.089. [DOI] [Google Scholar]
- Naqash SY, Nazeer RA. Antioxidant and functional properties of protein hydrolysates from pink perch (Nemipterus japonicus) muscle. J Food Sci Technol. 2013;50:972–978. doi: 10.1007/s13197-011-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, Dhulster P, Nasri M, Karra-Châabouni M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int. 2013;54:552–561. doi: 10.1016/j.foodres.2013.07.001. [DOI] [Google Scholar]
- Pacheco-Aguilar R, Mazorra-Manzano MA, Ramirez-Suarez JC. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem. 2008;109:782–789. doi: 10.1016/j.foodchem.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Slizyte R, Mozuraityte R, Martinez-Alvarez O, Falch E, Fouchereau-Peron M, Rustad T. Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadus morhua) backbones. Process Biochem. 2009;44:668–677. doi: 10.1016/j.procbio.2009.02.010. [DOI] [Google Scholar]
- Wang B, Gong Y, Li Z, Yu D, Chi C, Ma J. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J Funct Foods. 2014;6:176–185. doi: 10.1016/j.jff.2013.10.004. [DOI] [Google Scholar]
- Weichselbaum TE. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am J Clin Pathol. 1946;16(Tech. Suppl):40. [PubMed] [Google Scholar]
- Wiriyaphan C, Chitsomboon B, Yongsawadigul J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012;132:104–111. doi: 10.1016/j.foodchem.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen H, Shiau C. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- You L, Zhao M, Regenstein JM, Ren J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res Int. 2010;43:1167–1173. doi: 10.1016/j.foodres.2010.02.009. [DOI] [Google Scholar]
- Zhao Q, Xiong H, Selomulya C, Dong Chen X, Zhong H, Wang S, Sun W, Zhou Q. Enzymatic hydrolysis of rice dreg protein: effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012;134:1360–1367. doi: 10.1016/j.foodchem.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Zhong S, Ma C, Lin YC, Luo Y. Antioxidant properties of peptide fractions from silver carp (Hypophthalmichthys molitrix) processing by-product protein hydrolysates evaluated by electron spin resonance spectrometry. Food Chem. 2011;126:1636–1642. doi: 10.1016/j.foodchem.2010.12.046. [DOI] [PubMed] [Google Scholar]