Abstract
The influence of Pulsed Electric Field (PEF) pre-treatment of blueberry fruits (Vaccinium myrtillus L.), both on the extraction yield and antioxidant properties of juice obtained by pressing and on the on the recovery of bioactive compounds from berry by-products (press cake) by extraction with solvent, was investigated. PEF treatments carried out at field strengths of 1, 3, and 5 kV/cm and an energy input of 10 kJ/kg achieved a cell disintegration index (Zp) of 0.70, 0.80, and 0.87, respectively. Mechanical pressing (1.32 bar for 8 min) of PEF-treated berries (1, 3, and 5 kV/cm at 10 kJ/kg) significantly increased the juice yield (+28 %) compared with the untreated sample. The juice obtained from PEF pre-treated berries also had a significantly higher total phenolic content (+43 %), total anthocyanin content (+60 %) and antioxidant activity (+31 %). However, PEF treatment intensity higher than 1 kV/cm did not significantly improve the quantitative or qualitative characteristics of the juice. Compared to the untreated sample, higher amounts of total phenolics (+63 %), total athocyanins (+78 %) and antioxidant activity (+65 %) were detected in the press cake extracts. PEF treatment of higher intensity resulted in better extractability of bioactive compounds from blueberry press cake. The results obtained from this study demonstrate the potential of PEF as a mild pre-treatment method to improve the efficiency of the industrial processing of berry fruits.
Keywords: PEF, Pressing, Extraction, Blueberry, By-products, Bioactive compounds
Introduction
The dietary intake of berry fruits has been demonstrated to positively impact human health (Paredes-Lopez et al. 2010). Various biological properties of berry fruits have been largely related to the fruits’ high levels and wide diversity of phenolic-type phytochemicals (Paredes-Lopez et al. 2010). The blueberry is an important source of many traditional nutrients such as vitamins and minerals (Barba et al. 2012). Additionally, the blueberry contains a variety of phenolic compounds with high antioxidant capacity (Može et al. 2011). Anthocyanins are believed to be the key bioactive compounds responsible for the many reported health benefits of blueberries and also other fruits (Karlsen et al. 2010; He and Giusti 2010).
In recent years, the number of food products that use blueberries has increased. In particular, tens of new juices obtained from blueberries have reached the market and found a positive consumer response due to their reported nutritional and health benefits (Mellentin and Crowford 2008). Likewise, it is expected that the amount of blueberry by-products, derived from the industrial processing of this fruit, will increase in the near future. Although these by-products, which mainly consist of skins and seeds, can create greater environmental problems, they also represents a low-cost source of anthocyanins and other phenolics with great potential industrial applications as natural colorants or nutraceuticals (Khanal et al. 2010; Puértolas et al. 2013). Blueberry anthocyanin extracts are already used in several clinical applications, mainly in the areas of ophthalmology and vasoprotection (Kalt and Dufour 1997; Ulbricht et al. 2009), and there are hundreds of pharmaceutical products on the market containing extracts of Vaccinium myrtillus berries (Yamamoto et al. 2013).
Therefore, juice expression processes and bioactive compound extraction from berries and berry by-products, which strongly depend on mass transfer phenomena through the cell membrane of the fruit tissue, are of the utmost importance for delivering natural and high quality products containing high levels of the desired bioactive compounds.
Mechanical pressing and extraction with solvents are widely used in the food industry for juice, wine, sugar and vegetable oil production. These methods are also frequently applied for the extraction of different target compounds (colorants, antioxidants, essential oils, aromas, etc.) from solid foods (sugar beets, apples, grapes, etc.). Unfortunately, the quality of solutions and extracted products (e.g., purity, turbidity, colour, flavour and nutrients) may be degraded by conventional raw material pre-treatments (grinding, heating, or the addition of chemicals/enzymes) needed to increase yield (Vorobiev and Lebovka 2010).
Pulsed Electric Fields (PEF) treatment has been shown to be promising as a milder and more efficient alternative to conventional cell disintegration techniques (Donsi et al. 2010a). The exposure of plant tissue to an electric field of moderate intensity (0.5–10 kV/cm) and relatively low energy (1–10 kJ/kg), applied in the form of repetitive very short voltage pulses (typically from few μs up to 1 ms), induces a permeability of cell membranes that facilitates the release of juice and valuable compounds from the inner parts of the cells. Due to its non-thermal impact on foodstuffs, PEF treatment may exert a selective permeability of the membranes (tonoplast and plasma membrane) whereas the cell wall remains intact, which improves the purity and the yield of the extracts. It has been shown that in combination with mechanical pressing, PEF treatment is likely to increase the yield and quality of the juice extracted from fruits and vegetables such as apples, grapes, and carrots (Grimi et al. 2011; Jaeger et al. 2012; Praporscic et al. 2007). Furthermore, PEF also helps increase the extraction rates of colorants (anthocyainins, carotenoids, betanines, etc.) and bioactive compounds (polyphenols) from foods and food by-products and helps shorten extraction time and reduce solvent consumption and/or lower extraction temperatures (Boussetta et al. 2012; Puértolas et al. 2013).
As per the literature survey, no studies have yet been published on the effect of PEF-assisted pressing and the extraction of juice and bioactive compounds from blueberries and their by-products. Therefore, the objective of this work was to investigate the use of PEF pre-treatment to permeabilize cell membranes of fresh blueberry tissue before pressing with the aim of enhancing the yield and quality of the expressed juice as well as the subsequent extraction yield of bioactive compounds from blueberry by-products (press cake left after PEF-assisted pressing). The effect of different combinations of electric field strengths and total specific energy input on the cell disintegration index of berry tissue and juice yield as well as total phenolics, total anthocyanins and antioxidant activity of both expressed juice and extracts from press cake were investigated.
Materials and methods
Raw material
Wild blueberries (Vaccinium myrtillus L.) were purchased at a local market in Lithuania and stored at 4 °C until needed.
PEF treatment
Investigations on PEF-assisted pressing of blueberries were carried out in a laboratory scale cylindrical treatment chamber that was connected to an electric pulses generator. The treatment chamber consisted of a vertical spacer of polycarbonate material, closed at both ends by two cylindrical stainless steel electrodes, with the upper electrode being able to slide within the spacer under compression exerted by loading a weight on it. The lateral surface of the polycarbonate spacer was uniformly pierced (d = 0.5 mm) to allow the flow of the juice when compression was applied. The electrode area was 9.1 cm2, and the distance between the two electrodes could be adjusted (up to 5 cm) depending on the volume of the treated sample. The two electrodes of the PEF chamber were electrically connected to a high voltage pulse generator (Modulator PG, ScandiNova, Uppsala, Sweden) designed to provide monopolar square wave pulses with an independent setting for the applied voltage (0–25 kV/cm), pulse width (1–23 μs), and pulse repetition rate (1–450 Hz), limited only by the average power of 20 kW.
The actual voltage and current signals in the treatment chamber were measured using a high voltage probe (Tektronix, P6015A, Wilsonwille, OR, USA) and a Rogowsky coil (2–0.1 Stangenes, Inc., USA) connected to a 300 MHz digital oscilloscope (Tektronix, TDS 3034B, Wilsonwille, OR, USA). The maximum electric field intensity (E, kV/cm) was evaluated as the peak voltage divided by the inter-electrode gap. The specific energy input per pulse (W, kJ/kg/pulse) was calculated according to Eq. (1):
| 1 |
where U(t) and I(t) represent the voltage across the electrodes and the current intensity through the product at time t, respectively, and m is the mass of the treated product. The total specific energy (WT, kJ/kg) was calculated by multiplying W by the number of pulses applied.
During each experiment, 10 g of crushed blueberry fruits were loaded into the treatment chamber and subjected to PEF pre-treatments of different field strengths (1, 3, and 5 kV/cm) and total specific energy inputs (1, 5, and 10 kJ/kg) at a constant frequency (10 Hz) and pulse width (20 μs). The sample was then pressed in the same chamber for 8 min at a costant pressure of 1.32 bar by loading a weight on the upper electrode. The juice expressed was collected in a plastic tube and subjected to further refinement before analysis. The corresponding press cake was subjected to solid-liquid extraction as described in the following subsection.
Control samples were collected after the application of the same protocol without PEF treatment. In all experiments, the initial temperature of the samples was 20 ± 1 °C and the final temperature never exceeded 23 ± 1 °C.
Cell disintegration index
Cell disintegration index (Zp) was used to quantify the degree of cell membrane permeabilization of blueberry tissues attained via each PEF treatment before pressing.
The determination of Zp is based on a given chamber geometry and on the frequency dependence of the absolute value (|Z|) of the electrical complex impedance of intact and permeabilized plant tissue (Donsi et al. 2010a; Pataro et al. 2011). Therefore, for the Zp determinations, measurements of electrical complex impedance in frequency sweep were carried out by loading 10 g of untreated or PEF-treated blueberries into a test vessel, which consisted of two parallel plate cylindrical electrodes (3 cm in diameter) separated by a polycarbonate tube. The electrodes were connected to an impedance analyzer (Solartron 1260, UK), which was working in the frequency range of 103–107 Hz. For each PEF treatment condition investigated, a Zp value was calculated on the basis of the measurement of the absolute value of complex impedance of untreated (Zuntr) and PEF-treated tissue (Ztr) in the low (1 kHz) and high (10 MHz) frequency ranges (Donsi et al. 2010a; Pataro et al. 2011).
| 2 |
The value of Zp varies between 0 for intact tissue and 1 for fully permeabilized tissue.
Collection of juice and yield calculation
The liquid collected during the pressing phase was centrifuged at 5000 rpm for 10 min at 5 °C (ALC PK 130R, DJB Labcare Ltd, Newport, UK) to separate the pulp from the juice. The latter was weighed to evaluate the extraction yield, which was expressed as the grams of juice per 100 g of fresh weight (fw) blueberries. The extracted juice was stored at 4 ± 1 °C until analyzed.
Extraction procedure of phenolic compounds from blueberry press cake
The press cake obtained after PEF-assisted pressing of blueberries was weighed and immediately immersed in the extraction solvent (50 % Ethanol; 0.5 % HCl, v/v). The solvent to press cake ratio was 6:1 (mL/g). The extraction process was carried out for 24 h at ambient temperature with constant shaking at 150 rpm. The final extracts, obtained after filtration with a filter paper (Watman no.1) in a Buchner funnel, were stored at 4 °C until analyzed.
Determination of total phenolic (TP) content
The total phenolic (TP) content of blueberry juice and press cake extract were determined using the Folin-Ciocalteu method previously described by Bobinaitė et al. (2012). The absorbance was measured at 765 nm in a V-650 UV-vis spectrophotometer (Jasco Inc., Easton, USA) after incubation at ambient temperature for 1 h. Gallic acid (Sigma-Aldrich, Steinheim, Germany) was used as the standard for the calibration curve, and results were expressed in mg of gallic acid equivalents (GAE) per 100 mL of juice or per 100 g of fw berry press cake.
Determination of total anthocyanin content (TAC)
Total anthocyanin content (TAC) of juice and press cake extract was determined by using the pH differential method described by Lee et al. (2005). Blueberry juice or press-cake extracts were added to buffer solutions (sodium chloride pH 1.0, and sodium acetate pH 4.5). The absorbance of the solutions were measured using V-650 UV-vis spectrophotometer (Jasco Inc., Easton, USA) at 520 and 700 nm.
The concentration of anthocyanins was expressed in mg of cyanidin-3-glucoside equivalents per 100 mL of juice or per 100 g of fw berry press cake.
Evaluation of ferric reducing antioxidant power (FRAP)
FRAP assay of control and PEF treated samples of both juice and press cake extract was carried out according to the method described by Benzie and Strain (1996) with some modification. For FRAP measurement of juice, 1 mL of blueberry juice was diluted with 2 mL of aqueous methanol (50 %), whereas for the blank sample measurements, 1 mL of water was diluted with 2 mL of aqueous methanol (50 %). For FRAP measurement of blueberry press cake extract, 1 mL of extract was diluted with 3 mL of aqueous methanol (50 %), whereas for the blank sample measurements, 1 mL of extraction solvent was diluted with 3 mL of aqueous methanol (50 %).
For the analysis, 2 mL of freshly prepared FRAP working solution and 20 μL of diluted juice or extract were mixed and incubated for 30 min at ambient temperature. The change observed in absorbance was due to the reduction of ferric-tripyridyltriazine (Fe III-TPTZ) complex by the antioxidants present in the samples, which was monitored at 593 nm using a Genesys-10 UV/Vis (Thermo Spectronic, Rochester, USA) spectrophotometer. The absorptions of blank samples (applying the same analysis conditions) were tested each time before the analysis. Trolox (Acros Oganics, Geel, Belgium) was used as the standard for the calibration curve, and the FRAP values were expressed as μmol of trolox equivalents (μmol TE) per mL of juice or g of fw berry press cake.
Statistical analysis
All experiments were carried out in triplicate and each collected sample was analyzed in duplicate. The mean values and standard deviations of the experimental data were calculated using SPSS 20 Software (SPSS Inc., Chicago, USA). Mean values were further compared using Turkey’s test, and differences were considered to be statistically significant at p ≤ 0.05.
Results and discussion
Characterization of PEF-induced damage in the cells of blueberry tissue
Electroporation of biological tissues and the consequent mass transfer process is a complex function of the interaction between the electric field applied and the material properties, which, in turn, are also spatially dependent and highly inhomogeneous.
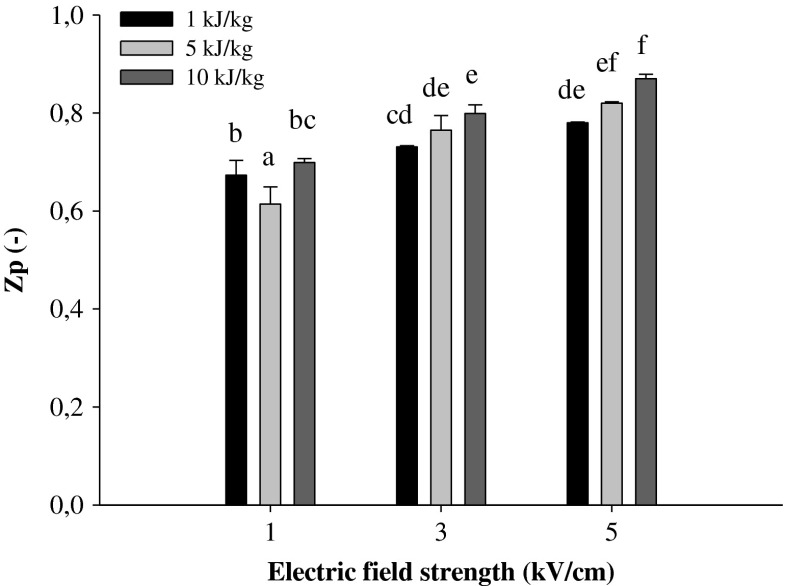
Among the different methods proposed, the assessment of the cell disintegration index (Zp) via impedance measurements has been successfully used as a reliable macroscopic indicator of the cell membrane disintegration in diverse fruits and vegetables (Donsi et al. 2010a, b; Luengo et al. 2013; Puértolas et al. 2013).
Figure 1 shows the influence of the electric field strength on the Zp of blueberry tissue at different energy inputs. The lowest value of Zp (0.61) was observed when a PEF treatment intensity of 1 kV/cm and 5 kJ/kg was applied. Increasing the electric field strength and energy input also increased the Zp up to 0.87, which was the highest value recorded when the most intense treatment was applied (5 kV/cm and 10 kJ/kg). However, it was observed that in the range of the investigated PEF treatment intensities, the permeabilization degree appears to depend mainly on the electric field strength, whereas the effect of energy input seems to be more pronounced when higher field strengths are applied. The results obtained during this study show that at each energy input, the Zp value increased significantly (p ≤ 0.05) when the field strength increased. At fixed field strength, the Zp value also increased when the energy input was increasing, but the difference was significant (p ≤ 0.05) only for field strengths of 3 and 5 kV/cm, and when energy was changed from 1 to 10 kJ/kg.
Fig. 1.
Cell disintegration index (Z p) of blueberries as a function of electric field strength and total specific energy input. Different letters above the bars indicate significant differences between the mean values (p ≤ 0.05)
The increment of Zp values with increasing intensity of PEF treatment observed in our research is in agreement with previously reported data for different plant tissues (Asavasanti et al. 2010; Luengo et al. 2013; Puértolas et al. 2013). However, it is worth noting that, in the study of Luengo et al. (2013) it was observed that, for each value of the electric field applied, the value of Zp increased as the specific energy input was increased until a threshold saturation value was reached (Luengo et al. 2013). This trend was not observed in our study; for each applied field strength, the Zp value still significantly rose when energy input increased from 1 to 10 kJ/kg.
Further investigations of PEF pre-treatment were carried out at 1, 3, and 5 kV/cm with a constant energy input of 10 kJ/kg.
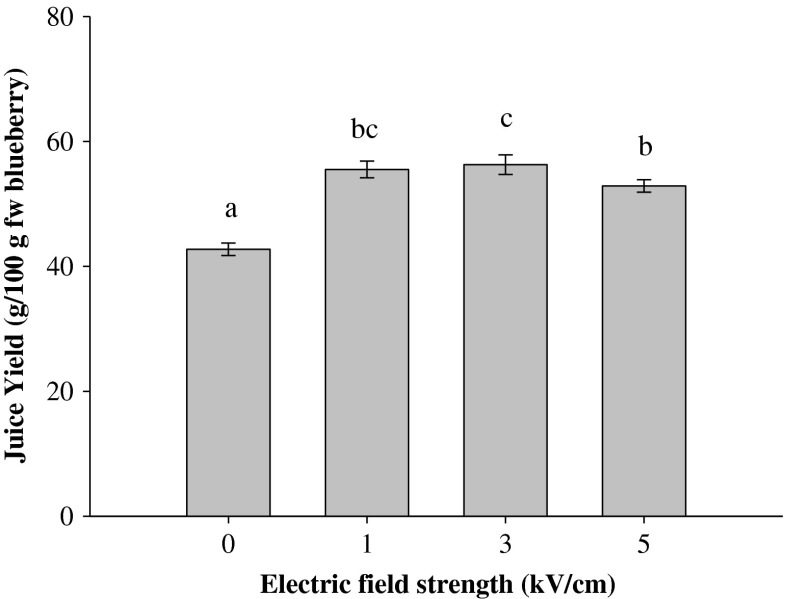
Effects of PEF pre-treatment on juice yield
Juice yield is one of the most important aspects determining the profitability of juice production. Figure 2 shows the juice yield from blueberry fruits pre-treated using PEF at different electric field intensities (0–5 kV/cm) with fixed energy input (10 kJ/kg). The average juice yield for the untreated (0 kV/cm) sample was 42.7 %. The pre-treatment of blueberries with PEF before pressing significantly (p≤0.05) increased the juice yield compared with the untreated sample. However, no significant difference was detected between the juice yield of the PEF-treated samples at 1 and 3 kV/cm, whereas a significant (p ≤ 0.05) difference was observed when the field strength was increased from 3 to 5 kV/cm. A significant increase in juice yield (up to 55.5 %) was obtained for the PEF pre-treatment at the lowest field intensity of 1 kV/cm. A further increase of field strength up to 3 kV/cm led to only a slight increase of the yield, which reached a maximum value of 56.3 %. It is interesting to note that the most intense PEF treatment condition (5 kV/cm) resulted in a juice yield of 52.9 %, which was the lowest increase in juice yield with respect to the untreated sample. This indicates that the cell disintegration level (Zp = 0.70) achieved with the lower PEF treatment intensity resulted in the most favourable de-juicing properties of blueberries. According to Jaeger et al. (2012), high intensity treatment may cause softening of the plant tissues to an extent that is unfavourable for de-juicing i.e., causes the compaction and closing of the capillaries in the press cake.
Fig. 2.
Juice yield obtained after pressing untreated (0 kV/cm) and PEF-treated blueberries as a function of the electric field strength (WT = 10 kJ/kg). Different letters above the bars indicate significant differences between the mean values (p ≤ 0.05)
The application of PEF in combination with mechanical juice expression by pressing was demonstrated to be effective for improving juice yield from different plant-based materials (Donsi et al. 2010a). The reported improvement of the juice yields varies widely, most likely due to different intrinsic characteristics of the tissues and process conditions employed in different studies (degree of particles fragmentation, PEF parameters and compression pressure) (Jaeger et al. 2012). It has been reported that PEF pre-treatment of grapes accelerated the juice expression and increased juice yield by approximately 24 % for Muscadelle, Sauvignon and Semillon varieties (Praporscic et al. 2007) and by 8 % for the Chardonnay variety (Grimi et al. 2009). PEF treatment of apple mash increased juice yield by 4.1 % (Turk et al. 2012) or from 1.7 to 7.7 % (Schilling et al. 2007). Compared to the above-mentioned studies, our results showed a higher increase in juice yield of PEF-treated berries by a 30 % increase compared with the untreated samples.
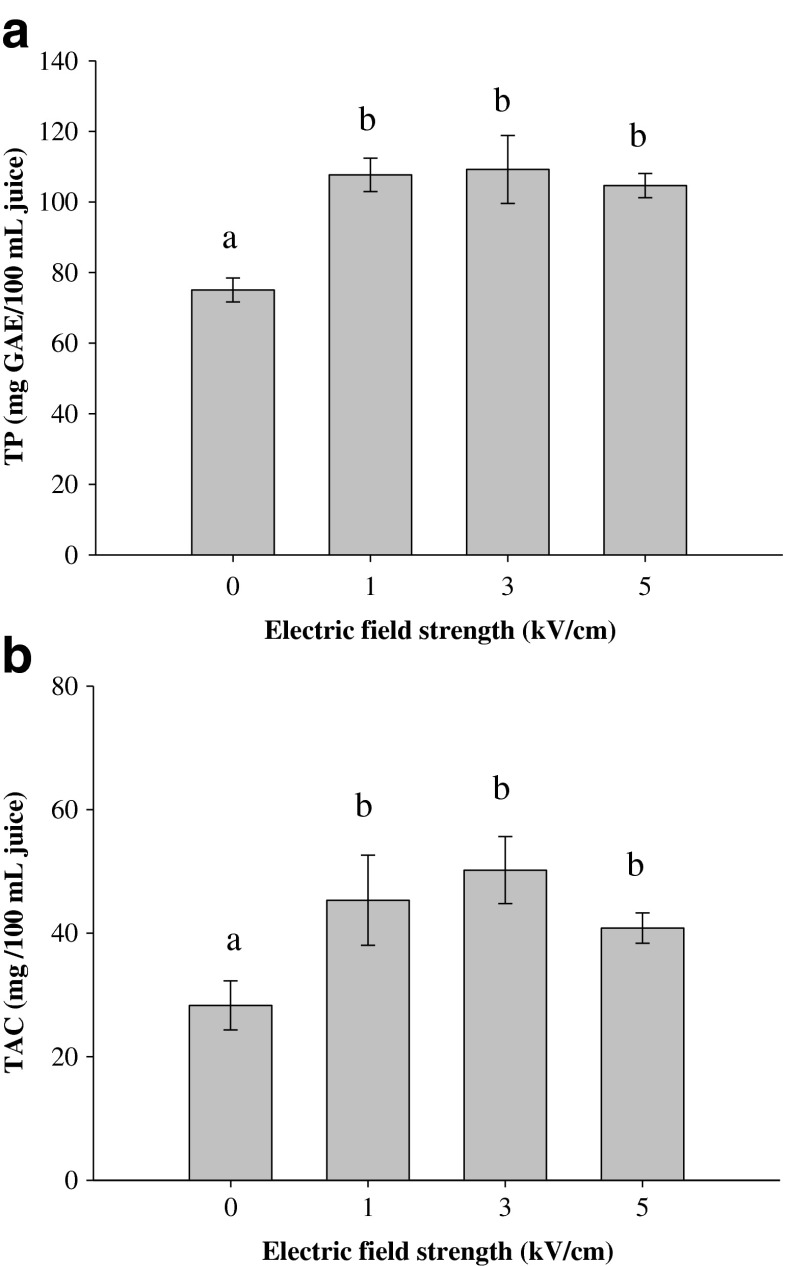
Effects of PEF pre-treatment on the TP and TAC of blueberry juice
Figure 3 shows the total content of both phenolic compounds (Fig. 3a) and anthocyanins (Fig. 3b) in the juice obtained after pressing untreated (0 kV/cm) and PEF-treated blueberries. The results show that the influence of PEF treatment intensity on TP and TAC exhibited a similar trend. The content of total phenolics and anthocyanins in the control juice (without PEF pre-treatment) was 75.0 mgGAE/100 mL and 28.3 mg/100 mL, respectively (Fig. 3). PEF pre-treatment significantly (p ≤ 0.05) increased both TP and TAC values, which rose approximately 43.5, 45.5, and 39.4 % for TP, and 60.2, 77.5, and 44.3 % for TAC, when the berry fruits were PEF-treated at 1, 3, and 5 kV/cm, respectively. These results reflect a positive effect of PEF application for the improvement of the nutritional quality of the juice.
Fig. 3.
Total phenolic content (a) and total anthocyanin content (b) of blueberry juice obtained after pressing untreated (0 kV/cm) and PEF-treated berries as a function of the electric field strength (WT = 10 kJ/kg). Different letters above the bars indicate significant differences between the mean values (p ≤ 0.05)
However, it is worth noting that the increase of the treatment intensity from 1 to 5 kV/cm did not contribute to a significant increase in the extraction yield of both TP and TAC, despite the fact that an approximately 24 % increase in the cell disintegration index was observed. Furthermore, a slight decrease in both TP and TAC was detected when the most intense PEF treatment was applied (5 kV/cm). These findings are somewhat similar to those previously observed for the juice yield (Fig. 2), indicating that more intense treatment conditions (>1 kV/cm) for fresh blueberries might not be necessary to intensify the release of phenolic compounds and colorants into juice.
Partially in contrast with our results, Luengo et al. (2013) found that pressing (5 bar for 30 min) PEF-treated orange peels at 1, 3, 5 and 7 kV/cm significantly increased the total phenolic extraction yield by 20, 129, 153 and 159 %, respectively. On the other hand, a positive impact of PEF on extraction of polyphenols was also observed in juices obtained after PEF treatment of grapes (Donsi et al. 2010b; Grimi et al. 2009) and apple mash (Grimi et al. 2011; Jaeger et al. 2012; Schilling et al. 2008). For example, the PEF pre-treatment of crushed grapes during wine-making induced a significantly higher release of polyphenols (+20 %) and anthocyanins (+75 %) than in the untreated samples (Donsi et al. 2010b). TP content was also found to increase with the application of PEF treatment before the pressing of apple mash in the range from 198 to 500 mg/L depending on the mash size, PEF treatment intensity and de-juicing system (Jaeger et al. 2012).
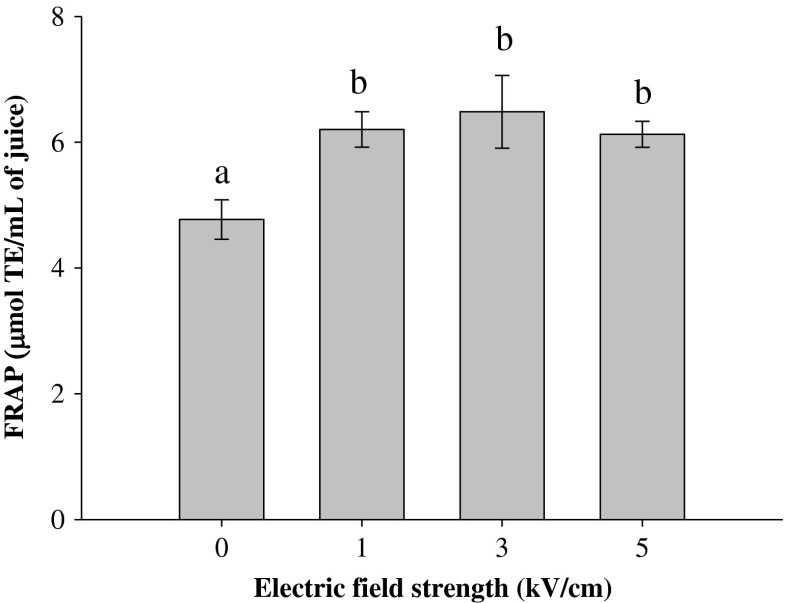
Effects of PEF pre-treatment on the antioxidant activity of blueberry juice
The effect of PEF pre-treatment on the antioxidant potential of blueberry juice obtained after pressing was evaluated using the FRAP method. Compared to the juice obtained from the untreated berries, PEF pre-treatments significantly (p≤0.05) increased the antioxidant activity of the juices by 30.0, 35.9, and 28.4 %, when berries were PEF-treated at 1, 3, and 5 kV/cm, respectively (Fig. 4).
Fig. 4.
Ferric reducing antioxidant power (FRAP) of blueberry juice obtained after pressing untreated (0 kV/cm) and PEF-treated berries as a function of the electric field strength (WT = 10 kJ/kg). Different letters above the bars indicate significant differences between the mean values (p ≤ 0.05)
It has previously been reported that PEF treatment also improves the antioxidant activity of the wine obtained after pressing PEF pre-treated grapes by 20 % (Donsi et al. 2010a). In addition, an investigation of apple juice extracts obtained after pressing PEF pre-treated cut or whole apples revealed an increase in antioxidant activity in the range 30–50 % compared to the control extraction (Grimi et al. 2011).
Moreover, it should be noted that, the antioxidant activities of the blueberry juice obtained from the berries treated at different electric field strengths (1, 3 and 5 kV/cm) were not significantly different (p > 0.05). This result is not surprising, considering the fact that the antioxidant activity of berries is mainly attributed to their polyphenolic compounds (anthocyanins in particular) (Bobinaitė et al. 2012; Giovanelli and Buratti 2009), and in our study, significant changes of polyphenolic compounds content in the juice obtained after PEF pre-treatments of different intensities were not observed (Fig. 3).
TP and TAC of extracts obtained from the press cake of PEF pre-treated blueberries
In fresh berries, the anthocyanins and other phenolic compounds are found mainly in the cell vacuoles of the fruit skins rather than in the fruit flesh (Paredes-Lopez et al. 2010). As blueberry press cake is mainly composed of berry skins, it is likely that it still retains high amounts of anthocyanins and other phenolics (Khanal et al. 2010). Therefore, the extraction process of these compounds from blueberry press cake could represent a crucial step in obtaining natural pigments and nutraceuticals to be further used in the food and pharmaceutical industries.
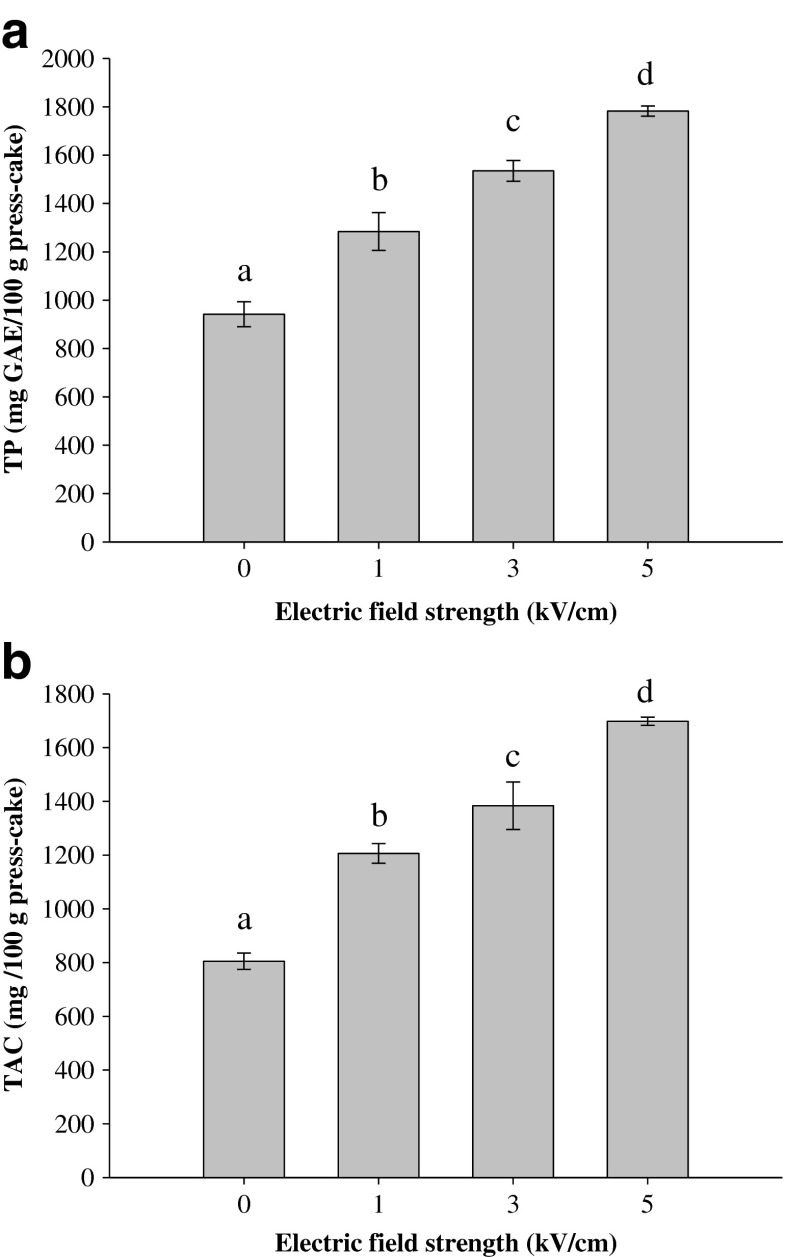
Figure 5 shows TP and TAC detected in the extracts obtained from the press cakes of untreated and PEF-treated berries. The results showed that despite the application of a PEF pre-treatment, a substantial amount of polyphenolic compounds seem to be retained inside the plant cells (Fig. 5). Compared to the control sample, significantly (p ≤ 0.05) higher amounts of TP and TAC were extracted from the press cakes of PEF pre-treated berries. In contrast to the results previously observed for blueberry juice, significant (p ≤ 0.05) differences were also detected between the extracts when different field strengths were applied. The higher the field strength applied, the greater the amount of phenolic compounds in the extracts. Therefore, it is likely that pores formed during the PEF treatment of fresh fruit were still open after the pressing. The amount of TP extracted from the control press cake was 941.7 ± 52.1 mg GAE/100 g fw, whereas the amount of these compounds increased by 36.3, 63.0 and 89.3 % when they were extracted from press cakes obtained after PEF-assisted pressing at 1, 3 and 5 kV/cm, respectively. Similarly, the lowest amount of anthocyanins (805.0 ± 30.2 mg/100 g press cake) was extracted from the press cake of untreated berries. The application of a PEF pre-treatment at 1, 3 and 5 kV/cm yielded an increase in TAC by 50, 72 and 111 %, respectively, compared with the control extraction.
Fig. 5.
Total phenolic content (a) and total anthocyanin content (b) of extracts from blueberry press cake obtained after pressing untreated (0 kV/cm) and PEF-treated berries as a function of the electric field strength (WT = 10 kJ/kg). Different letters above the bars indicate significant differences between the mean values (p ≤ 0.05)
It has been shown that the cell membranes permeabilization induced by PEF treatment improved the extraction yield of phenolic compounds from grape seeds (Boussetta et al. 2012), grape by-products (Corrales et al. 2008) and from the external bracts of artichokes (Battipaglia et al. 2009). For example, Corrales et al. (2008) extracted bioactive compounds from grape by-products and found that PEF treatment increased the extraction yields of total phenolics by 50 % and anthocyanins by 17 % compared to conventional extraction.
Antioxidant activity of blueberry press cake extracts
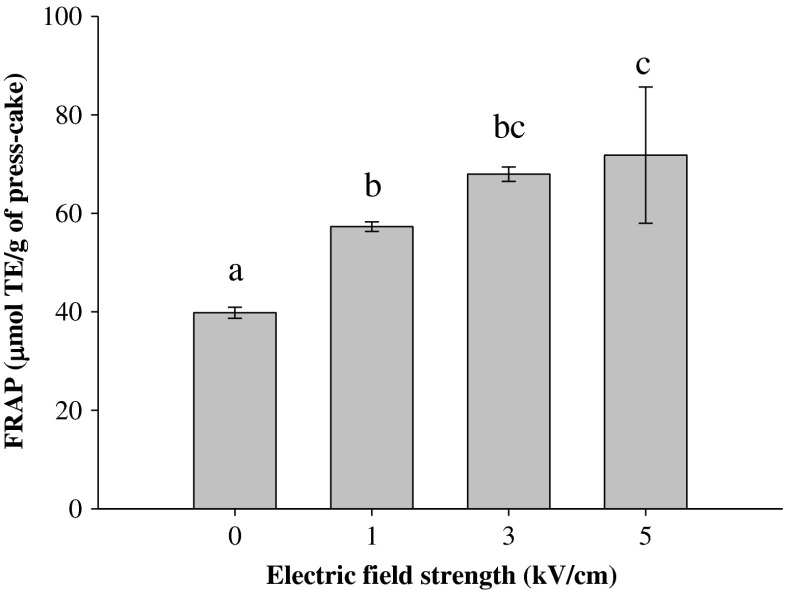
Extracts obtained from the press cake of PEF-treated blueberries possessed a significantly (p ≤ 0.05) stronger antioxidant activity than the control extracts (Fig. 6). Similar findings were reported by other scientists (Corrales et al. 2008) who observed that PEF-assisted extraction from grape by-products increased the antioxidant activity of the extract by four times.
Fig. 6.
Ferric reducing antioxidant power (FRAP) of extracts from press cake obtained after pressing untreated (0 kV/cm) and PEF-treated berries as a function of the electric field strength (WT = 10 kJ/kg). Different letters above the bars indicate significant differences between the mean values (p ≤ 0.05)
Furthermore, our results also showed that higher PEF treatment intensity resulted in significantly (p ≤ 0.05) higher antioxidant activity of the extracts. In comparison to the control extract, PEF treatments at 1, 3 and 5 kV/cm increased the FRAP values of the press-cake extracts by 44, 71 and 80 %, respectively. Increases in the antioxidant activity of the blueberry press cake extracts achieved by increasing the electric field strength intensity, correlates with the higher content of polyphenols and anthocyanins in the extracts, as previously observed for blueberry juice.
Conclusions
This work demonstrated that the PEF pre-treatment of blueberry fruits (Vaccinium myrtillus L.) has a positive impact on the yield and antioxidant properties of juice as well as on the recovery of bioactive compounds from blueberry by-products.
Experiments have shown that for the improvement of quantitative and qualitative characteristics of the expressed juice, PEF treatment at 1 kV/cm and 10 kJ/kg was fully sufficient, whereas the recovery of bioactive compounds from press cake can be further improved by increasing the electric field strength from 1 to 5 kV/cm. Therefore, optimum PEF process conditions should be selected in order to obtain high yield of high quality juice while maximizing the recovery of valuable compounds from berry by-products.
From the results obtained in this study, it is reasonable to assume that PEF technology in combination with standard operations of the food industry, such as mechanical pressing and extraction with solvent, promises to improve the efficiency of these processes and to add value to food products and by-products.
Acknowledgments
The postdoctoral fellowship is being funded by European Union Structural Funds project “Postdoctoral Fellowship Implementation in Lithuania”. The authors would also like to acknowledge networking support by the COST Action TD1104. Thanks are due to Dr. Mauro Maria Capitoli for his invaluable help with chemical analyses.
Footnotes
Highlights
>PEF induces cell membrane disintegration of blueberry tissues. > PEF pre-treatment enhances the yield and antioxidant activity of blueberry juice. >PEF pretreatment improves the extraction efficiency of bioactive compounds from berry by-products.
References
- Asavasanti S, Ersus S, Ristenpart W, Stroeve P, Barret DM. Critical electric field strengths of onion tissues treated by pulsed electric fields. J Food Sci. 2010;75:433–443. doi: 10.1111/j.1750-3841.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- Barba FJ, Jäger H, Meneses N, Esteve MJ, Frígola A, Knorr D. Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innov Food Sci Emerg Tech. 2012;14:18–24. doi: 10.1016/j.ifset.2011.12.004. [DOI] [Google Scholar]
- Battipaglia G, De Vito G, Donsì F, Ferrari G, Pataro G. Enhancement of polyphenols extraction from involucral bracts of artichokes. In: Lebovka N, Van Hecke E, Lanoiselle JL, Vorobiev E, editors. International conference on bio and food electrotechnologies. Compiègne: Universite’ de Technologie de Compiègne; 2009. pp. 40–44. [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bobinaitė R, Viškelis P, Venskutonis PR. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012;132:1495–1501. doi: 10.1016/j.foodchem.2011.11.137. [DOI] [PubMed] [Google Scholar]
- Boussetta N, Vorobiev E, Le LH, Cordin-Falcimaigne A, Lanoisselle JL. Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT-Food Sci Technol. 2012;46:127–134. doi: 10.1016/j.lwt.2011.10.016. [DOI] [Google Scholar]
- Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci EmergTech. 2008;9:85–91. doi: 10.1016/j.ifset.2007.06.002. [DOI] [Google Scholar]
- Donsi F, Ferrari G, Pataro G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng Rev. 2010;2:109–130. doi: 10.1007/s12393-010-9015-3. [DOI] [Google Scholar]
- Donsi F, Ferrari G, Fruilo M, Pataro G. Pulsed electric field-assisted vinification of Aglianico and Piedirosso grapes. J Agric Food Chem. 2010;58:11606–11615. doi: 10.1021/jf102065v. [DOI] [PubMed] [Google Scholar]
- Giovanelli G, Buratti S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009;112:903–908. doi: 10.1016/j.foodchem.2008.06.066. [DOI] [Google Scholar]
- Grimi N, Lebovka NI, Vorobiev E, Vaxelaire J. Effect of a pulsed electric field treatment on expression behavior and juice quality of Chardonnay grape. Food Biophys. 2009;4:191–198. doi: 10.1007/s11483-009-9117-8. [DOI] [Google Scholar]
- Grimi N, Mamouni F, Lebovka N, Vorobiev E, Vaxelaire J. Impact of apple processing modes on extracted juice quality: pressing assisted by pulsed electric fields. J Food Eng. 2011;103:52–61. doi: 10.1016/j.jfoodeng.2010.09.019. [DOI] [Google Scholar]
- He J, Giusti MM. Anthocyanins: natural colorants with health-promotingproperties. Annu Rev Food Sci Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- Jaeger H, Schulz M, Lu P, Knorr D. Adjustment of milling, mash electroporation and pressing for the development of a PEF assisted juice production in industrial scale. Innov Food Sci Emerg Tech. 2012;14:46–60. doi: 10.1016/j.ifset.2011.11.008. [DOI] [Google Scholar]
- Kalt W, Dufour D. Health functionality of blueberries. Hortic Technol. 1997;7:216–221. [Google Scholar]
- Karlsen A, Paur I, Bøhn SV, Sakhi AK, Borge GI, Serafini M, Erlund I, Laake P, Tonstad S, Blomhoff R. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr. 2010;49:345–355. doi: 10.1007/s00394-010-0092-0. [DOI] [PubMed] [Google Scholar]
- Khanal RC, Howard LR, Prior RL. Effect of heating on the stability of grape and blueberry pomace procyanidinsand total anthocyanins. Food Res Int. 2010;43:1464–1469. doi: 10.1016/j.foodres.2010.04.018. [DOI] [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- Luengo E, Alvarez I, Raso J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov Food Sci Emerg Tech. 2013;17:79–84. doi: 10.1016/j.ifset.2012.10.005. [DOI] [Google Scholar]
- Mellentin J, Crowford K. Marketing healthy fruit. In: Tomas-Barberan FA, Gil MI, editors. Improving the health-promoting properties of fruit and vegetable products. Boca Raton: CRC Press; 2008. pp. 55–68. [Google Scholar]
- Može Š, Polak T, Gašperlin L, Koron D, Vanzo A, Ulrih NP, Abram V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.) J Agric Food Chem. 2011;59:6998–7004. doi: 10.1021/jf200765n. [DOI] [PubMed] [Google Scholar]
- Paredes-Lopez O, Cervantes-Ceja ML, Vigna-Perez M, Hernandez-Perez T. Berries: improving human health and healthy aging, and promoting quality life—review. Plant Foods Hum Nutr. 2010;65:299–308. doi: 10.1007/s11130-010-0177-1. [DOI] [PubMed] [Google Scholar]
- Pataro G, Ferrari G, Donsì G. Mass transfer enhancement by means of electroporation. In: Markoš J, editor. Mass transfer in chimica engineering processes. Rijeka: InTech; 2011. pp. 151–176. [Google Scholar]
- Praporscic I, Lebovka N, Vorobiev E, Mietton-Peuchot M. Pulsed electric field enhanced expression and juice quality of white grapes. Sep Purif Technol. 2007;52:520–526. doi: 10.1016/j.seppur.2006.06.007. [DOI] [Google Scholar]
- Puértolas E, Cregenzán O, Luengo E, Álvarez I, Raso J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013;136:1330–1336. doi: 10.1016/j.foodchem.2012.09.080. [DOI] [PubMed] [Google Scholar]
- Schilling S, Alber T, Toepfl S, Neidhart S, Knorr D, Schieber A, Carle R. Effects of pulsed electric field treatment of apple mash on juice yield and quality attributes of apple juices. Innov Food Sci Emerg Tech. 2007;8:127–134. doi: 10.1016/j.ifset.2006.08.005. [DOI] [Google Scholar]
- Schilling S, Toepfl S, Ludwig M, Dietrich H, Knorr D, Neidhart S, Schieber A, Carle R. Comparative study of juice production by pulsed electric field treatment and enzymatic maceration of apple mash. Eur Food Res Technol. 2008;226:1389–1398. doi: 10.1007/s00217-007-0669-x. [DOI] [Google Scholar]
- Turk MF, Billaud C, Vorobiev E, Baron A. Continuous pulsed electric field treatment of French cider apple and juice expression on the pilot scale belt press. Innov Food Sci Emerg Tech. 2012;14:61–69. doi: 10.1016/j.ifset.2012.02.001. [DOI] [Google Scholar]
- Ulbricht C, Basch E, Basch S, Bent S, Boon H, Burke D, Costa D, Falkson C, Giese N, Goble M, Hashmi S, Mukarjee S, Papaliodis G, Seamon E, Tanguay-Colucci S, Weissner W, Woods J. An evidence-based systematic review of bilberry (Vaccinium myrtillus) by the natural standard research collaboration. J Diet Suppl. 2009;6:162–200. doi: 10.1080/19390210902861858. [DOI] [PubMed] [Google Scholar]
- Vorobiev E, Lebovka N. Enhanced extraction from solid foods and biosuspensions by pulsed electrical energy. Food Eng Rev. 2010;2:95–108. doi: 10.1007/s12393-010-9021-5. [DOI] [Google Scholar]
- Yamamoto M, Yamaura K, Ishiwatari M, Ueno K. Difficulty for consumers in choosing commercial bilberry supplements by relying only on product label information. Pharmacogn Res. 2013;5:212–215. doi: 10.4103/0974-8490.112432. [DOI] [PMC free article] [PubMed] [Google Scholar]