Abstract
High value fruits namely, apple (cv. Royal Delicious), guava (cv. Baruipur) and litchi (cv. Shahi) harvested at their commercial maturity were considered for MA packaging to enhance storage life. Polymeric films namely LDPE, BOPP, PVC, PVDC of different thickness were used for MA packaging study and various film characteristics such as gas transmission rates, water vapour transmission rate, clarity, strength and durability were evaluated. Mathematical model was developed based on Arrhenius type equation to predict gas transmission rate (GTR) and the developed model was found to be very good fit with the mean relative deviation modulus value quite less than 10 %. The GTR of the films increased with the increase in storage temperature and the magnitude of the increase varied with the film type and thickness. Regression models have been suitably developed to predict the oxygen transmission rate and carbon dioxide transmission rate of selected polymeric films and combined film laminates as a function of temperatures. Since, none of the individual films could meet the gas transmission requirements of MAP for selected fruits, two different films were tailored to form laminates that sufficed the requirements for prolonged storage with maintaining original quality.
Keywords: Polymeric films, lamination, Modeling of GTR, Film properties, MA packaging
Introduction
Modified atmosphere packaging (MAP) is one of the food preservation methods to maintain the natural quality of commodity and extend the shelf life (Mangaraj et al. 2009). It consists of modification of the atmosphere inside the package, by the natural interplay between two processes i.e. the respiration of the fruits and the transfer of the gases through the packaging that leads to an atmosphere richer in CO2 and poorer in O2 (Montanez et al. 2010). This atmosphere can potentially reduce respiration rates, ethylene sensitivity and production, decay and physiological changes, namely oxidation (Kader et al. 1989) thereby allowing the preservation of the fresh state of the commodity without the thermo-chemical treatments generally employed in competitive preservation techniques, such as canning, freezing, dehydration etc. The MAP technique is usually suitable for short-term storage, transportation / distribution and retailing of fresh produce.
MAP technology has a great advantage in developing countries because it is economical and useful where there is dearth of refrigerated storage. MAP utilizes only the natural components of air, has achieved public acceptance due to (i) no toxic residue as synthetic chemicals are not used, (ii) little environmental impact, particularly if the plastic films are recycled (Mangaraj and Goswami 2009a, 2009b). In MAP, changes due to respiration start immediately after packing the fresh produce. The gases of the contained atmosphere and the external ambient atmosphere try to equilibrate by permeation through the package walls at a rate dependent upon the differential pressures between the gases of the headspace and ambient atmosphere. Thus, the barrier to gases and water vapour provided by the packaging material must be considered (Mangaraj et al. 2014a). The success of MA pack therefore, depends upon the polymeric film and its transmission properties e.g. gas permeability, ratio of CO2/O2 permeability, water vapor permeability, resistance to puncture, sealing reliability, antifogging properties, printability, clarity, strength and durability etc. (Kader et al. 1989; Exama et al. 1993; Abdel-Bary 2003). For most produce, a suitable film must be much more permeable to CO2 than to O2 (Kader et al. 1989; Mangaraj et al. 2009).
The polymeric films commonly available for MA packaging of fruits are low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), high-density polyethylene (HDPE), polypropylene (PP), polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyvinylidene chloride (PVDC), polyamide (Nylon) and other suitable films (Exama et al. 1993; Mangaraj et al. 2009). Although increasing choices of packaging materials are available to the MAP industry, most packs are still constructed from six basic polymers: polyvinyl chloride (PVC), polyproylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polysterene (PS) and polyvinyledene chloride (PVDC) (Kader et al. 1989; Exama et al. 1993; Abdel-Bary 2003; Ahvenainen 2003). It is reported that LDPE and PVC tends to have high ratio CO2/O2 permeability and this is of importance in MA packaging system for selecting packaging films. This allows O2 concentration to decrease without an associated excessive build of CO2 inside the package (Kader et al. 1989). The technology of manufacturing the polymeric films has permitted tailoring films for gas permeability, needed for some fruits and vegetables (Exama et al. 1993). As a result, successful MA packaging systems have been developed for a number of commodities. Most workers used polyethylene, especially LDPE, PVC and PET as the packaging materials and studied the effects of package surface area, temperature, film permeability to O2 and CO2, state of equilibrium conditions achieved and their effects on quality and shelf-life of apple (Mangaraj et al. 2014a, 2014b) and reported low mass loss, presented better colour and preserved better firmness than fruits stored in air. Apples packed in optimal MA package, had good quality after storage at 0 and 10 °C for 7 months with reduction of scald rate. The multilayer co-extruded polyolephinic film with selective permeability prolonged the storage of guava up to 3 weeks as against 14 days in LDPE and PVC film package at 10 °C with 85–90 % RH. LDPE package provided an atmosphere of 3 % O2 and 4.5 % CO2 inside the packages, which kept the fruit with good sensorial characteristics (Jacomino et al. 2001; Mangaraj et al. 2005). MA packaging of fresh guava in PET film had a strong influence on color preservation and mass loss of the guava (Jacomino et al. 2001; Mohamed et al. 1994). MA packaging of litchi using sealed polyethylene and PVC films with or without chemical treatment have been proven beneficial in maintaining high RH, essential for prevention of dehydration that leads to rapid skin browning of litchi (Chaiprasart 2003). MA packaging of treated litchi fruits using BOPP film minimized the rate of transpiration, mass loss and deterioration of fruit quality (Mangaraj et al. 2012).
No single polymers offers all the properties required for MAP to have an ability to retain the desired atmosphere for long. It can be achieved by choosing films with required gas and moisture vapour permeability characteristics and ensuring seal integrity of the packs. To achieve the desired film characteristics, different plastic films can either be laminated or co-extruded. The GTR of films vary with temperature and relative humidity. Most of the data on film permeability are determined at a single temperature and RH. Considering the above conditions an investigation was undertaken with objectives of finding out the film permeability data at realistic temperature and RH conditions employing equal pressure method and model the GTR of the films and to design of suitable package for the commodities by appropriate incorporation of the input variables for gaseous exchange in MAP system. .
Theoretical considerations
Barrier and permeation of polymeric films
The mechanism by which substances travel through an intact plastic film is known as permeation. It involves dissolution of the penetrating substance, the permeate in the plastic, followed by diffusion of the permeate through the film, and finally by evaporation of the permeate on the other side of the film, all driven by a partial pressure differential for the permeate between the two sides of the film (Pino et al. 2005; Mangaraj et al. 2009).
The barrier performance of the film is generally expressed in terms of its permeability coefficient. For one-dimensional steady-state mass transfer, the permeability coefficient (P) is related to the quantity of permeate transferred through the film as (Mangaraj et al. 2009):
| 1 |
Where, Q is the amount of permeate passing through the material, x is thickness of the plastic film, A is surface area available for mass transfer, t is time, and Δp is the change in permeate partial pressure across the film.
In general, the gases transport properties through polymers is described by three parameters viz. diffusivity, solubility and permeability and the precise nature of the correlation is dependent on the type of diffusion mechanism. Generally the Fickian diffusion process is considered for gas transport in polymer.
Diffusivity
In the diffusion process, the dissolved reentrant equilibrates with the film surface and then diffuses in the direction of lower chemical potential. It requires activation energy for generating an opening large enough to allow the penetrating molecule to perform a unit diffusion jump from one sorption site to another. The gas transmission in one direction from the atmosphere into the package, is given as:
| 2 |
Where, D is the diffusivity or diffusion coefficient of gases across the film at STP per sec (cm2 s−1), c is concentration of the gas in the membrane (mole cm−3 or cm3 cm−3), J is the flux of gas (mole sec−1 or cm3 s−1), A is Area (m2), and x is the film thickness.
If D is a constant and steady state condition exists, then
| 3 |
However, c1 and c2 are difficult to measure within the membrane. Applying Henry’s law:
| 4 |
Where, S is the solubility (moles cm−3 atm−1 or cm3 cm−3 atm−1) and p is the partial pressure of gas (atm). Then combining Eqs. (3) and (4), we have:
| 5 |
Solubility
The solubility coefficient is the ratio of equilibrium concentration of the dissolved penetrates to its partial pressures in the gas phase. The equilibrium concentration depends on polymer penetrate interaction and the availability of free volume for hole filling. Differences in the solubility of specific gases in a particular film determine which gas diffuses more readily across that film (Pino et al. 2005).
Permeability
Permeability is a measure of the ease with which gas can penetrate through an intact film. Permeability coefficient (P) is the proportionality constant between the flow of the gas per unit film area per unit time and the driving force (partial pressure difference) per unit film thickness. The amount of gas penetrating through the film is expressed in terms of either, moles per unit time (flux) or mass or volume of the gas at STP. Commonly, it is expressed in terms of volume. The quantity (DS) in the Eq. (5) is known as permeability coefficient (P), which is the product of diffusivity and solubility (Ashley 1985). Then, we have:
| 6 |
Measurement of gas transmission rates
Equal pressure principle
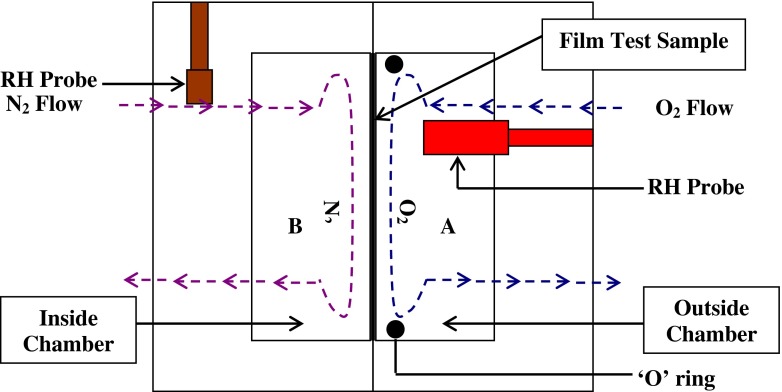
This test method employed a coulometric oxygen sensor and associated equipment in an arrangement similar to that described in Test Method ASTM D3985. The testing principle was schematically represented through Fig. 1. The system was first purged to identify the value of ‘system zero point’. The specimen (film) divided the testing chamber into chamber A and chamber B. Chamber A was purged with pure oxygen gas at 0.1 MPa pressure at certain flow rate and chamber B was purged with 0.1 MPa nitrogen gas at certain flow rate. When oxygen transmitted through the specimen from chamber A into chamber B, output value of the sensor gradually increased as corresponding electrical signals, which was an indication that there was oxygen transmitting through the specimen into chamber B. When the oxygen transmission rate (OTR) maintained at a constant value, it was considered as equilibrium of transmission and that OTR was the test result (Mangaraj et al. 2009). The appropriate flow rate of N2 during testing directly influenced the result.
Fig. 1.
Operating principle OTR measurement using equal pressure method
Water vapor permeability of films
The partial pressure difference for water vapor between the inside and the outside of the package influences the moisture gain or loss in the product. The saturated water vapor transmits through the test specimen (film) in a unit time under specified condition of temperature and humidity. The transmitted mass is determined by testing the decreasing mass of distilled water with time.
In a desiccant system of measurement, silica gel desiccant was directly placed inside films pouch whose Pwv was to be measured under controlled conditions of 38 °C temperature and 90 % RH. Water vapor permeability was computed from the measured values of the change in mass of the packages with time employing the following equations (Goswami and Mangaraj 2011; Mangaraj and Goswami 2009d).
| 7 |
Where, Pwv is the water vapor permeability of packaging film (g-mm/m2.day.pa), dw/
dt is the mass gain by desiccant with time and is obtained from the slope of the increments of mass vs. time plot,
t is the time in days, w is the mass gain by desiccant in g, x is the thickness of the film in mm, A is the area of the package in m2 and p is the water vapor pressure at 38 °C in Pa.
Factors affecting gas transmission rates of polymeric films
Gas transmission through a polymeric film depends upon the solubility of gas molecules in the polymer and their diffusivity through the film. The factors influencing the gas transmission of films are broadly classified as internal or film factors and external or penetrant factors. Internal factor relates to morphology and structure of film and vary with the polymer and its processing conditions such as functional groups on the polymer backbone, packing density, crystalline and amorphous volume fractions, degree of cross-linking, polymer chain segmental motion within the film matrix, orientation, draw temperature (DT), annealing time, etc. External factors include: penetrant’s molecular weight, size and shape, cohesive energy density and polarity of the penetrating molecules, temperature and relative humidity of the penetrant gas etc. (Kader et al. 1989; Exama et al. 1993; Goswami and Mangaraj 2011). Some important aspects are discussed here as follows.
Temperature
The permeability of O2 and CO2 in polymeric films is temperature dependent and this dependence is commonly described by an Arrhenius-type equation (Exama et al. 1993; Yam and Lee 1995) as:
| 8 |
Where, P is the permeability of gas at absolute temperature T, PP is permeability pre-exponential factor for gas, EaP is the activation energy of permeation for gas, and R is the universal gas constant.
When permeability coefficients are not available at the temperature of interest, the following equation can be used to determine the required value, from the permeability coefficient at a nearby temperature and the activation energy.
| 9 |
Where, T1 is the temperature at which P1 is known, T2 is the temperature at which P2 is to be calculated.
As thumb rule, gas transmission rate increases by 30 to 50 % and WVTR 10–100 % for every 5 °C rise in temperature (Kader et al. 1989; Exama et al. 1993; Mangaraj et al. 2009).
Temperature quotient for permeability
The influence of temperature on permeability of polymeric films was quantified with the Q10P value which is the permeability increase for a 10 °C rise in temperature and given as:
| 10 |
Where, Q10P is temperature quotient for permeability, P1 and P2 are the permeability at temperature T1 and T2, respectively.
Film thickness
Permeability of films thicker than 25 μm tends to become independent of thickness. For all polymers, P is proportional to x−a, where ‘a’ varies from 0.8 to 1.2 for most of the polymers. At higher thickness, the proportion of increase of gas transmission rates was not high with the increase in temperature (Mangaraj et al. 2009).
Relative humidity
The permeability of hydrophilic polymer films to water vapor usually increases rapidly at high RH because of sorption of water, concomitant swelling of the film and increased mobility of the polymer chains. Water has the potential to enhance flexibility of polymer chains. The increase in permeability is particularly dramatic for transport of gases through materials, which are excellent gas barriers when dry. In hydrophilic polymer films, permeability increases rapidly above 70 % RH. Permeability does not vary with RH in hydrophobic polymer films. However, WVTR of polymeric film vary considerably with temperature (Mangaraj et al. 2009, 2014b).
Permeability coefficient of multilayer films
Permeability coefficients for multiplayer plastic film or sheet, layered through either laminations or co-extrusions, can be calculated from the thickness and permeability coefficients of the individual layers (Abdel-Bary 2003; Mangaraj et al. 2009) as.
| 11 |
Where, the subscript ‘t’ indicates the value for the total structure, ‘i’ indicates the value for an individual layer, and there are ‘n’ layers in the structure.
Mathematical modeling of Gas transport through polymeric films
Various models simulating transport of gas molecules in polymeric films have been developed to predict diffusion coefficients and gas transmission:
Empirical models
Goswami and Mangaraj (2011) have developed an empirical model for prediction of GTR of polymeric films. This model incorporates an important parameter i.e. temperature, which influences the GTR of films significantly. Employing experimental values, the following polynomial Eq. (12) was used to link the relationship of GTR with temperature.
| 12 |
Where, GTR is the gas transmission rates of film to O2 and CO2 at absolute temperature T, α0, α1, and α2 are the constants of the gas transmission model.
Exponentiel model
Exama et al. (1993) developed the following Arrhenius - type equations for predicting the gas permeability of polymeric films as a function of temperature.
| 13 |
Where, Pgas is the permeability of O2 and CO2 at absolute temperature T, PgasP is the permeability pre-exponential factor for gases, and EagasP is the activation energy of permeation for O2 and CO2.
Materials and methods
Collection of fruits from orchard
The fruits apple (cv. Royal Delicious), guava (cv. Baruipur) and Litchi (cv. Shahi) were harvested from the orchard at their commercial maturity. The color values, firmness, TSS, acidity and other quality attributes were evaluated objectively to assess the maturity indices of fruits (Mangaraj and Goswami 2009c). It was ensured to maintain uniformity in terms of size and weight of individual fruits in the whole lot of samples for the MA packaging study.
Modeling of respiration rates
The respiration rates of fruits were measured for 0–30 °C in the step of 5 °C using airtight respirometer chamber of size 0.125 m × 0.175 m × 0.23 m. Headspace gas sample of respiration chamber was analyzed quantitatively for O2 and CO2 concentrations using gas chromatograph (100 Knaur, Germany) (Mangaraj and Goswami 2008). The Michaelis-Menten type equation based on principle of enzyme kinetics with uncompetitive type of inhibition was fitted to the experimental respiration data (Lee et al. 1991; Mangaraj and Goswami 2011a, b).
Selection of polymeric films for MA packaging of fruits
The selection of packaging films with suitable gas transmission properties is of crucial importance to achieve desirable gas composition within the package capable of maintaining quality and extending shelf life to the produce (Exama et al. 1993; Costa et al. 2011; Mangaraj et al. 2011). With the objective of meeting MAP requirements of fruit selected for study the polymeric high, medium and low barrier hydrophilic films namely LDPE, BOPP, PVC, PVDC were procured from Reliance Food Industry, Kolkata considering various film characteristics (Exama et al. 1993; Mangaraj et al. 2009, 2012, 2013). The concise technical details of the films are as follows:
Low-Density Polyethylene (LDPE)
LDPE has short and long-chain branching of the molecules and good barriers to water vapour, low barriers to oxygen and carbon dioxide gas. It sealed at lower and over a wider temperature range. It is relatively transparent and low cost plastic film, on a per-unit-mass basis (Abdel-Bary 2003).
Polyvinyl Chloride (PVC)
The films were soft and flexible, easy to heat-seal, have excellent self-cling, toughness, good strength, excellent resistance to chemical, high gas permeability and clarity (Berins 1991).
Polypropylene (BOPP)
BOPP has the lowest density of the commodity plastics, 0.89–0.91 g/cm3. The film has good clarity and strength. Water vapor barrier properties were better with medium gas barrier properties (Abdel-Bary 2003).
Polyvinylidene Chloride (PVDC)
The PVDC has excellent barrier to oxygen, water vapour, odours and flavours (Mangaraj et al. 2009).
Measurement of physico-mechanical and optical properties of films
The film properties namely thickness, density, haze, tensile strength, elongation strength, seal strength, tear strength, clarity, water vapor transmission rates were determined employing the standard methods and techniques in the quality control division of Reliance Industry, Kolkata .
Gas transmission through polymeric films
The gas transmission through polymeric films is considered as solution diffusion mechanism. In diffusion process, the dissolved penetrant equilibrates with the film surface and then diffuses in the direction of lower chemical potential. The gas transmission rates of the film vary considerably with the temperature (Exama et al. 1993). The MA packaging study was proposed to carry out at 10, 15, 20, and 25 °C, therefore the O2 and CO2 gas transmission rates of selected films and film laminates were determined at these temperatures levels. Then the ratio of CTR to OTR of selected films was also calculated. The GTR of films was determined employing equal pressure method as discussed in the previous section, since it facilitated similar condition under which gas transmission takes place in MAP (Yasuda et al. 1969). Mathematical models were developed to predict the GTR of films at various temperatures employing Arrhenius-type equation.
Modeling of GTR of selected films
The gas transmission rates of polymeric film are temperature dependent and hence Arrhenius type equation was fitted to the experimental data to depict the relationship of GTR with temperature as follows:
| 14 |
Where, GTR is the gas transmissions rates of films (cm3/m2 h ΔC) at absolute temperature Tabs (K), GTRp is the gas transmission pre-exponential factor for O2 and CO2 (cm3/m2 h ΔC), Eap is the activation energy of gas transmission for O2 and CO2 (kJ kg-mole−1), R is the universal gas constant (8.314 kJ kg-mole−1 K−1).
The above Eq. (14) can be expressed in a linearised form as:
| 15 |
Film laminates for MA packaging
The gas transmission rates of the selected films were compared with the gas transmission requirement of MAP for apple, guava and litchi. Not a single film could meet the gas permeability requirement for MAP of the above mentioned fruits, satisfactorily. Thus two different films were combined through the tailoring of film laminates to bring the gas transmission requirement of the laminates close to the required values (Ahvenainen 2003). The areas of the two individual films were optimized and the films were laminated employing adhesive (Mangaraj et al. 2014a, 2014b). The different combination of PVC and BOPP as well as that of PVC and LDPE was considered for MA packaging of selected fruits. Considering these combinations, five types of film laminates i.e. LFR-1 (BOPP-30 μ + PVC-50 μ), LFR-2 (BOPP-45 μ + PVC-35 μ) for apple; LFR-3 (BOPP-45 μ + PVC-25 μ), LFR-4 (LDPE-40 μ + PVC-25 μ) for guava; and LFR-5 (BOPP-30 μ + PVC-25 μ) for litchi were developed to meet the GTR requirements of the MAP. Using different coded film laminates i.e. LFR-1, LFR-2, LFR-3, LFR-4 and LFR-5, five types of packages (PCG-LFR1, PCG-LFR2, PCG-LFR3, PCG-LFR4 and PCG-LFR5) of required size based on the physical dimension of the fruits, i.e. 24 cm × 19 cm for apple; 19 cm × 19 cm for guava and 28 cm × 22 cm for litchi were developed. Six apples, four guavas and 52–55 litchis weighing 1.00 ± 0.10 kg were placed in each package and the packages were Rsealed by heat-sealing machine. Silicon rubber septums were glued to the packages to facilitate gas sampling. The MA packages were labeled, marked and subsequently kept for storage study (Mangaraj et al. 2014a, 2014b).
Circular disc cutting unit
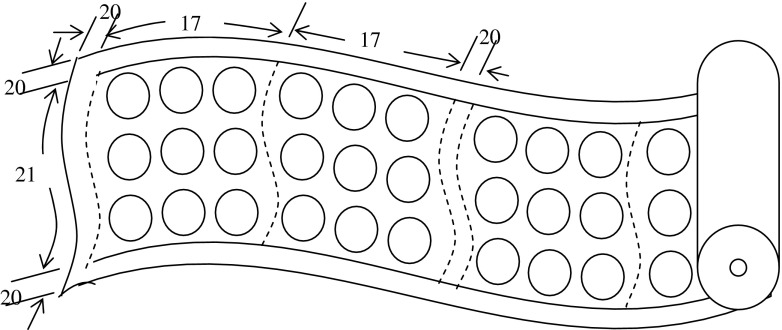
A circular disc-cutting unit was developed (Fig. 2) for the removal of required area from the polymeric film in the form of circular discs of different diameters, A-grade mild steel pipe of 27 mm inner diameter was used for the fabrication of the cutting unit. Heating coil of 100v / 83 watt was wound around the pipe. The whole cutting assembly was insulated by asbestos rope. A transformer was used to step-down the voltage from 220 to 100 V. A temperature controller was used to control the temperature of the cutting unit. Thermocouple was connected between the temperature controller and the cutting unit to measure the temperature of the film at the time of cutting. The required area of film was cut in the form of circular discs (Fig. 2). The diameter and numbers of the discs to be removed were determined from mathematical calculations of optimization of package parameters (Mangaraj et al. 2014a).
Fig. 2.

Disc Cutting Unit (Mangaraj et al. 2012)
Lamination of films
The film lamination (Fig. 3) was done at the Polyprint Packaging Products Pvt. Ltd in collaboration with Reliance Industry, Kolkata. The main components of the lamination plant were: (i) Nipping roller (ii) Heat chamber (iii) Air chamber (iv) Balancing roller (v) Pressure roller and (vi) Transmission system. The film roll without perforation was passed through the rubber roller where adhesive was applied. Subsequently, it was passed through the drying tunnel consisting of cooling chamber, heating chamber and normal chamber. Both heating and cooling treatment was given for the bonding of the adhesive with the film. Then it was passed through the laminating nip through the idle roller. The secondary substrate (film with perforation) coming from another idle roller was also passed through the laminating nip (Fig. 3). Both the films were pressed through the laminating nip along with the application of heat. Finally the desired laminated film roll came out through the idle roller (Fig. 4). The speed of the roller was adjusted to 35 m min−1. The temperature and pressure of the nipping roller was maintained as 70 °C and 78–98 N. The temperature of the drying heater was kept at 65 °C. The power was supplied from the main drive operating with 10 hp motor. The adhesive applied was prepared from HB 6680 (original solid content of 80 %) + dilute ethyl acetate with hardener HB 375, having final solid content of 30 %). The laminated film roll (Fig. 5) was used for the MA packaging of apple, guava and litchi fruits.
Fig. 3.

View of Lamination Process (source: Reliance Inustries, Mangaraj 2012
Fig. 4.
Schematic diagram of Laminated film rolls using un-perforated and perforated films for MA packaging of fruits
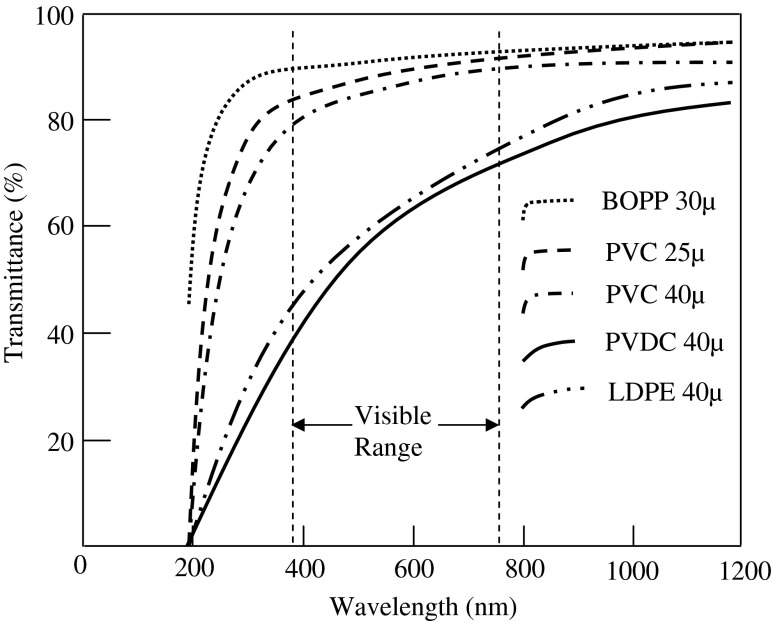
Fig. 5.
Transmittance of light through different polymeric films
Statistical methodology
Two-factor analysis of variance has been performed by using SAS 9.2 to find the effect of different levels of temperatures, film thickness and their interaction on OTR and CTR of selected polymeric films (Das and Giri 1986).
Regression analysis
Regression analysis has been performed to predict the OTR and CTR of selected polymeric films and combined film laminates using second order \polynomial equation (Mangaraj and Singh 2011). It was assumed that a continuous mathematical function ‘f’ exists for each response variable OTR and CTR of films in terms of one factor: temperatures (T), then,
| 16 |
To approximate the function ‘f’, second order polynomial equation of the following form was assumed
| 17 |
| 18 |
Where, OTR and CTR, are the predicted response (dependent variables); a0, a1, and a11; b0, b1, and b11 are the regression coefficients for the gas transmission model OTR and CTR, respectively; and T is the storage temperature.
Mean relative deviation modulus was used to find the goodness of fit of model developed with experimental data using following expression
Results and discussions
The ascertained properties of the selected polymeric films as measured by different standard procedures were presented in Table 1. It was evident that as the thickness of all the films increased the tensile strength, elongation, dart impact, tear strength increased and water WVTR decreased. This implies with thickness mechanical strength improved and the water barrier properties also improved for the produce requiring less water loss while storage. While comparing the properties of different films, it could be seen that PVC films have better mechanical strength and highest water barrier properties compared with other films and would be suitable for highly respiring fruits. Films with high WVTR have the capability to remove the condensation, which would impede microbial growth. On the other hand PVDC films have the lowest WVTR that may be useful for low respiring produce; in case of condensation it might not prove ideal, however. Therefore, films should be chosen according to the metabolic rate of the produce to be packaged and the environment they are to be handled, stored, transported and distributed.
Table 1.
Properties of the selected polymeric films
| Film properties | Units | Procedure | Types of films with code | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BOPP-I (PFR-1) | BOPP-II (PFR-2) | PVC-I (PFR-3) | PVC-II (PFR-4) | PVC-III (PFR-5) | LDPE-I (PFR-6) | LDPE-II (PFR-7) | PVDC-I (PFR-8) | |||
| Thickness | μ | ASTM D37 | 30 | 45 | 25 | 35 | 50 | 40 | 60 | 40 |
| Tensile strength at yield | Mpa | MD/TD | 14.0/16.0 | 14.3/16.4 | 52.0/58.0 | 60.0/66.0 | 70.0/75.0 | 9.2/9.9 | 9.7/10.5 | 81.0/76.5 |
| Tensile at break | Mpa | MD/TD | 14.5/16.1 | 14.9/16.3 | 52.0/53.0 | 57.0/62.5 | 61.0/68.0 | 18.5/20.1 | 19.4/20.9 | 66.5/62.0 |
| Elongation at yield | % | MD/TD | 4.5/2.7 | 4.7/3.0 | 2.2/2.0 | 3.0/2.9 | 4.5/3.8 | 7.5/5.4 | 8.0/5.7 | 8.5/6.7 |
| Elongation at break | % | MD/TD | 160/400 | 177/428 | 307/486 | 346/532 | 389/571 | 593/817 | 645/835 | 721/680 |
| Tear strength | Gms/microns | MD/TD | 0.77/3.53 | 0.81/3.79 | 0.47/0.60 | 0.83/0.97 | 1.42/1.53 | 1.93/18.6 | 2.56/19.75 | 2.4/3.1 |
| Haze | % | ASTM D1003 | 1.28 | 1.35 | 1.38 | 1.62 | 1.84 | 23.57 | 28.10 | 13.65 |
| Dart impact | Gms/microns | ASTM D2457 | 0.7 | 0.78 | 0.76 | 0.85 | 0.96 | 2.6 | 2.9 | 1.8 |
| Seal temperature at 2 kg/cm2 | °C | ASTM F88 | 170–200 | 180–200 | 125–180 | 130–180 | 137–180 | 142–180 | 150–190 | 140–190 |
| WVTR at 38 °C and 90 % RH | g/m2.day | ASTM E96 | 5.2 | 4.91 | 34.8 | 29.0 | 22.6 | 11.67 | 9.54 | 4.79 |
MD Machine direction, TD Transverse direction
Transparency and surface gloss are the principal intrinsic requirement of a fruit packaging. They facilitate better product display and influence customer appeal. The percentage transmission of light through various film samples were measured (Fig. 5) in the visible range (i.e. 390–760 nm). The percentage transmission of the selected films were considered to be fairly high with 84 % at 390 nm to 90 % at 760 nm for BOPP, 79 to 88 % for PVC film and 49 % at 390 nm to 70 % at 760 nm of PVDC film. Hence, they were categorized as clear films, which could suffice the product display requirement of MAP adequately.
Gas transmission rates of selected polymeric films
The O2 transmission rate (OTR) and CO2 transmission rates (CTR) of the selected films as well as the combined film laminates expressed for the total film thickness and not for unit film thickness were determined at 10, 15, 20 and 25 °C (Table 2). The GTR of the films increased with the increase in temperature. However, the magnitude of the increase varied with the film type and thickness. Among the selected films, the GTR and the TR of plasticized PVC film were found to be remarkably high whereas extremely low GTR of PVDC films with comparatively small gas transmission ratio. LDPE and PVC tend to have high ratio CO2/O2 permeability and this is of importance in MA packaging system for selecting packaging films. This allows O2 concentration to decrease without an associated excessive build of CO2 inside the package.
Table 2.
GTR of selected polymeric films combined film laminates at various temperatures and RH conditions
| Films | Thickness (μ) | Gas transmission rates (cm3 (m2.h. ΔC)−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 °C, 90 % RH | 15 °C, 80 % RH | 20 °C, 75 % RH | 25 °C, 70 % RH | ||||||
| OTR | CTR | OTR | CTR | OTR | CTR | OTR | CTR | ||
| BOPP-I | 30 | 43.15 | 190.72 | 61.72 | 278.13 | 88.59 | 408.62 | 125.86 | 596.57 |
| BOPP-II | 45 | 25.76 | 87.19 | 38.54 | 171.42 | 57.93 | 263.57 | 79.13 | 368.72 |
| PVC-I | 25 | 650.91 | 3968.81 | 943.37 | 5830.16 | 1320.84 | 8263.27 | 1894.30 | 11992.64 |
| PVC-II | 35 | 417.59 | 2527.64 | 614.38 | 3784.81 | 846.17 | 5264.08 | 1289.38 | 8148.89 |
| PVC-III | 50 | 290.26 | 1712.58 | 431.29 | 2609.83 | 585.52 | 3579.78 | 896.45 | 5566.95 |
| LDPE-I | 40 | 164.73 | 912.35 | 241.49 | 1367.14 | 337.46 | 1943.73 | 502.74 | 2930.94 |
| LDPE-II | 60 | 109.82 | 589.37 | 156.73 | 863.25 | 226.70 | 1273.25 | 329.70 | 1886.86 |
| PVDC-I | 40 | 0.34 | 1.20 | 0.51 | 1.84 | 0.68 | 2.53 | 0.94 | 3.61 |
| BOPP-I + PVC-III | 80 | 92.22 | 428.967 | 132.89 | 629.79 | 188.53 | 915.48 | 271.98 | 1349.77 |
| BOPP-II + PVC-II | 80 | 43.69 | 150.95 | 65.33 | 294.37 | 97.78 | 451.01 | 134.26 | 633.21 |
| BOPP-II + PVC-I | 70 | 39.20 | 133.99 | 58.62 | 262.36 | 87.96 | 402.85 | 120.29 | 563.93 |
| LDPE-I + PVC-I | 65 | 231.12 | 1296.32 | 338.29 | 1937.62 | 472.86 | 2753.72 | 700.72 | 4131.67 |
| BOPP-I + PVC-I | 55 | 74.96 | 336.19 | 107.30 | 490.39 | 153.81 | 719.48 | 218.63 | 1050.17 |
| BOPP-I + LDPE-II | 90 | 72.48 | 347.35 | 103.58 | 507.40 | 149.18 | 746.62 | 214.11 | 1096.40 |
| BOPP-II + LDPE-I | 85 | 42.72 | 151.79 | 63.75 | 291.32 | 94.93 | 444.30 | 131.12 | 626.42 |
| PVC-II + LDPE-II | 95 | 150.75 | 821.44 | 216.01 | 1206.31 | 310.43 | 1766.68 | 454.26 | 2632.02 |
| PVC-III + LDPE-I | 90 | 216.82 | 1232.22 | 319.63 | 1858.87 | 440.70 | 2605.19 | 664.99 | 3977.18 |
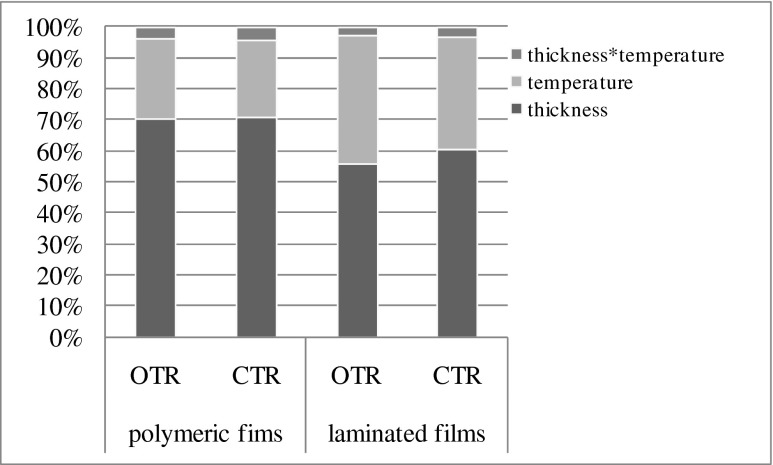
ANOVA of selected films and film laminates
From ANOVA it has been found that different levels of film thicknesses, temperatures and their interaction have significant effect on OTR (F-value more than 500) and CTR (F-value more than 8000) of films at 1 % level of significance. However, the effect of film thickness was found to have more than that of temperatures on the OTR/CTR of films. The graphical representation of F-values of OTR and CTR and the effect of various factors is represented in graphical form in Fig. 6. Film PFR-3 and temperature at 25 °C have got maximum mean on OTR/CTR, followed by PFR-4, 5, 6, 7, 1, 2 and 8 with 20, 15 and 10 °C level of temperatures. The comparison of means of OTR and CTR of selected polymeric films was presented in Table 3. The PVC-I had the highest and PFR 8 with lowest mean values of OTR at temperatures 20 and 10 °C, respectively. Similarly, for CTR the film having highest mean value was at 25 °C for PVC I and lowest value for PFR 8 at 10 °C. Similar trends were observed for analysis of variance on the combined film laminates, where the F-value for both the OTR and CTR were found to have more than the F-critical value at 1 % level of significance. The comparison of the effect of different factors on the OTR and CTR of film laminates can be seen from Fig. 6. The comparison of means of OTR and CTR of selected combined laminates was presented in Table 4, where LFR4 had the highest OTR values at 25 °C and the lowest was with LFR 3 at 10 °C. Similarly for CTR the highest mean value was for LFR 4 at 25 °C and the lowest was with LFR 3 at 10 °C.
Fig. 6.
Comparison of calculated F-values indicating the effects of different factors and their interactions on the OTR and CTR of polymeric films and laminates
Table 3.
Comparison of means of OTR and CTR of selected polymeric films
| Combined film laminates | OTR | CTR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | |||||||||
| 10 | 15 | 20 | 25 | Mean | 10 | 15 | 20 | 25 | Mean | |
| PFR-1 | 43.52 | 61.75 | 89.09 | 43.52 | 61.75 | 190.41 | 280.09 | 408.38 | 596.71 | 368.90F |
| PFR-2 | 25.63 | 38.90 | 58.08 | 25.63 | 38.90 | 88.20 | 171.60 | 263.61 | 368.81 | 223.06G |
| PFR-3 | 650.91 | 943.37 | 1320.84 | 650.91 | 943.37 | 3968.81 | 5830.16 | 8263.27 | 11992.64 | 7513.72A |
| PFR-4 | 417.98 | 614.68 | 846.60 | 417.98 | 614.68 | 2524.24 | 3781.86 | 5265.62 | 8148.26 | 4929.99B |
| PFR-5 | 291.01 | 431.76 | 584.39 | 291.01 | 431.76 | 1714.40 | 2610.74 | 3579.28 | 5566.32 | 3367.68C |
| PFR-6 | 164.37 | 240.74 | 336.66 | 164.37 | 240.74 | 910.48 | 1366.09 | 1943.98 | 2930.88 | 1787.86D |
| PFR-7 | 109.93 | 158.09 | 227.16 | 109.93 | 158.09 | 590.02 | 863.38 | 1273.18 | 1887.02 | 1153.40E |
| PFR-8 | 0.33 | 0.52 | 0.68 | 0.33 | 0.52 | 1.21 | 1.84 | 2.54 | 3.61 | 2.3H |
| Mean | 212.96D | 311.22C | 432.93B | 212.96D | 311.22C | 1248.47D | 1863.22C | 2624.98B | 3936.78A | 2418.36 |
* Means with same letter are non-significant
Table 4.
means of OTR and CTR of combined film laminates
| Combined film laminates | OTR | CTR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | |||||||||
| 10 | 15 | 20 | 25 | Mean | 10 | 15 | 20 | 25 | Mean | |
| LFR-1 | 92.218 | 132.89 | 188.53 | 271.98 | 171.40D | 428.967 | 629.79 | 915.487 | 1349.777 | 831.00D |
| LFR-2 | 43.698 | 65.328 | 97.78 | 134.266 | 85.26G | 150.95 | 294.376 | 451.005 | 633.217 | 382.38G |
| LFR-3 | 39.20 | 58.62 | 87.96 | 120.29 | 76.51I | 133.99 | 262.36 | 402.85 | 563.93 | 340.78I |
| LFR-4 | 231.12 | 338.29 | 472.86 | 700.72 | 435.74A | 1296.32 | 1937.62 | 2753.72 | 4131.67 | 2529.83A |
| LFR-5 | 74.96 | 107.30 | 153.81 | 218.63 | 138.67E | 336.19 | 490.39 | 719.48 | 1050.17 | 649.05F |
| LFR-6 | 72.487 | 103.58 | 149.178 | 214.11 | 134.83F | 347.353 | 507.408 | 746.627 | 1096.407 | 674.44E |
| LFR-7 | 42.719 | 63.75 | 94.936 | 131.122 | 83.13H | 151.797 | 291.32 | 444.30 | 626.42 | 378.45H |
| LFR-8 | 150.75 | 216.01 | 310.427 | 454.266 | 282.86C | 821.44 | 1206.31 | 1766.68 | 2632.02 | 1606.61C |
| LFR-9 | 216.825 | 319.636 | 440.70 | 664.99 | 410.53B | 1232.226 | 1858.87 | 2605.197 | 3977.184 | 2418.36B |
| Mean | 107.11D | 156.15C | 221.79B | 323.27A | 202.10 | 544.35C | 830.93C | 1200.59B | 1784.53A | 1090.10 |
*Means with same letter are non-significant
Regression analysis
A multiple regression least square analysis was carried out to fit the experimental data on OTR and CTR of individual films as well as the combined film laminates to a second order polynomial equations (Table 5).
Table 5.
Values of regression co-efficient for OTR and CTR models of selected films and combined film laminates
| Polymeric films & film laminates | OTR | CTR | ||||
|---|---|---|---|---|---|---|
| Intercept (ao) | Linear (a1) | Quadratic (a11) | Intercept (ao) | Linear (a1) | Quadratic (a11) | |
| BOPP 30 μ | 34.559 | 4.370 | 4.606 | 155.416 | 11.406 | 24.662 |
| BOPP 45 μ | 15.591 | 7.798 | 2.062 | 16.844 | 66.139 | 5.449 |
| LDPE 40 μ | 145.589 | −1.139 | 22.456 | 792.208 | −0.209 | 132.823 |
| LDPE 60 μ | 92.676 | 4.141 | 13.784 | 503.808 | 4.478 | 85.120 |
| PVC 35 μ | 389.416 | −24.490 | 61.916 | 2372.335 | −195.701 | 406.258 |
| PVC 50 μ | 272.563 | −16.932 | 42.745 | 1599.978 | −110.937 | 272.673 |
| PVDC 40 μ | 0.213 | 0.113 | 0.017 | 0.892 | 0.226 | 0.112 |
| LFR-1 | 56.279 | 8.360 | 7.724 | 291.881 | 8.117 | 48.419 |
| LFR-2 | 30.705 | 8.422 | 4.173 | 14.662 | 137.879 | 1.716 |
| LFR-3 | 74.550 | 7.805 | 10.480 | 355.346 | 17.292 | 57.714 |
| LFR-4 | 27.074 | 12.866 | 3.629 | 27.590000 | 117.936 | 8.237 |
| LFR-5 | 127.711 | 5.814 | 18.999 | 726.812500 | −15.565 | 122.822 |
| LFR-6 | 193.187 | 0.249 | 28.853 | 1104.315000 | −26.121 | 184.147 |
Modeling of GTR of selected polymeric films
The OTR and CTR of selected polymeric films and developed film laminates were measured experimentally and models were developed to predict the same at any temperature in the range of study.
Estimation of model parameters
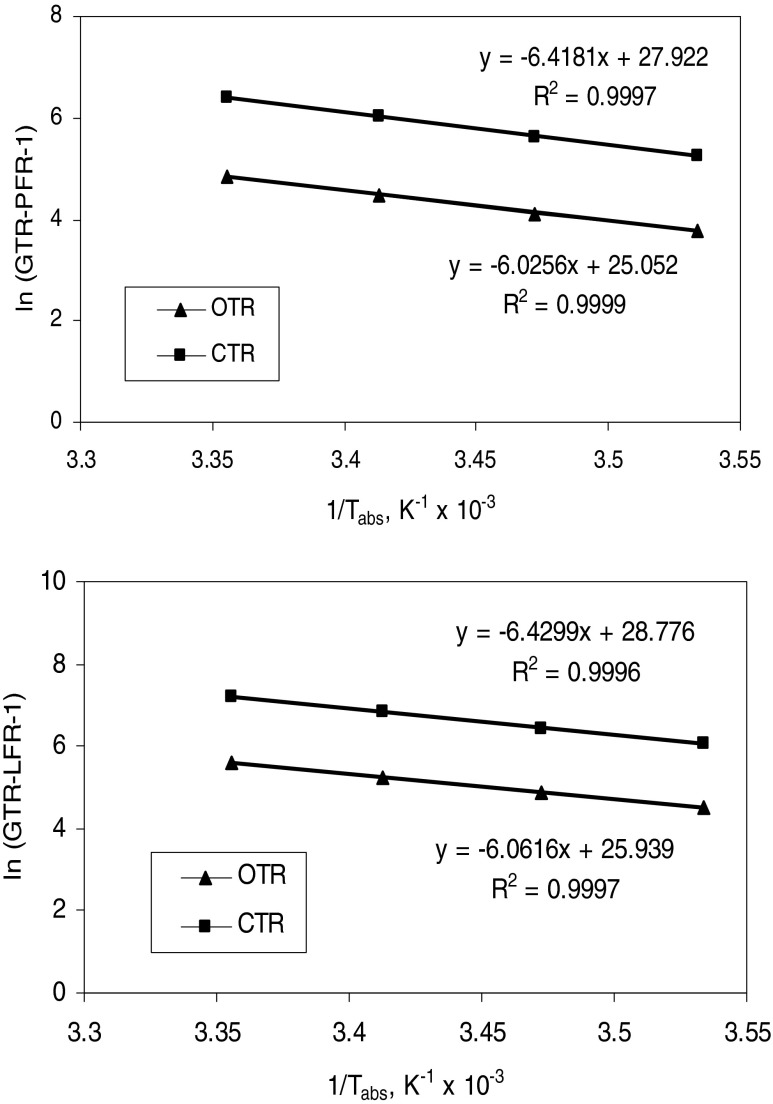
Employing Eq. (15), the temperature dependence of OTR and CTR was estimated by plotting the log values of the OTR or CTR against the inverse of corresponding temperature in absolute units (Fig. 7). The slope and the Y-axis intercept of Eq. (15) for OTR or CTR of films and film laminates were found from the linear plots (Table 6). The activation energy (Eap) and pre-exponential factor of gas transmission rates (GTRp) was calculated from the slope of the straight line and the Y-axis intercept, respectively.
Fig. 7.
Arrhenius Relation for OTR and CTR of Film PFR-1 and Film Laminate LFR-1
Table 6.
Slope (−Ea T/R) and Y-axis intercept (ln Tp) and calculated values of Activation Energy and Pre-Exponential Factor of Arrhenius relation for different model parameters of selected polymeric films and combined film laminates
| Polymeric films and laminates | OTR | CTR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| -Ea T/R (slope) | Ea (kJ/g-mole) | Tp (intercept) | Tp (unit) | r2 | -Ea T/R (slope) | Ea (kJ/g-mole) | Tp (intercept) | Tp (unit) | r2 | |
| BOPP-I | −6025.6 | 50.09 | 25.05 | 7.58 × 1010 | 0.99 | −6418.1 | 53.36 | 27.92 | 1.34 × 1012 | 0.99 |
| BOPP-II | −6370.2 | 52.96 | 25.79 | 1.55 × 1011 | 0.98 | −8040.3 | 66.84 | 32.96 | 2.06 × 1014 | 0.97 |
| PVC-I | −5972.5 | 49.65 | 27.58 | 9.52 × 1011 | 0.99 | −6183.4 | 51.4 | 30.13 | 1.22 × 1013 | 0.99 |
| PVC-II | −6241.6 | 51.89 | 28.08 | 1.57 × 1012 | 0.99 | −6476.7 | 53.84 | 30.71 | 2.18 × 1013 | 0.99 |
| PVC-III | −6212.9 | 51.65 | 27.62 | 9.90 × 1011 | 0.99 | −6494.9 | 53.99 | 30.39 | 1.58 × 1013 | 0.99 |
| LDPE-I | −6208.2 | 51.61 | 27.04 | 5.52 × 1011 | 0.99 | −6497.4 | 54.01 | 29.77 | 8.51× 1012 | 0.99 |
| LDPE-II | −6183.1 | 51.4 | 26.53 | 3.34 × 1011 | 0.97 | −6541.6 | 54.38 | 29.48 | 6.38 × 1012 | 0.99 |
| PVDC-I | −5634.7 | 46.84 | 18.85 | 1.54 × 108 | 0.99 | −6113 | 50.82 | 21.8 | 2.94 × 109 | 0.99 |
| LFR-1 | −6061.6 | 50.39 | 25.93 | 1.82 × 1011 | 0.99 | −6429.9 | 53.45 | 28.77 | 3.12 × 1012 | 0.99 |
| LFR-2 | −6363.9 | 52.9 | 26.27 | 2.56 × 1011 | 0.99 | −7992.5 | 66.45 | 33.34 | 3.01 × 1014 | 0.97 |
| LFR-3 | −6361.3 | 52.88 | 26.15 | 2.27 × 1011 | 0.99 | −8013.4 | 66.62 | 33.29 | 2.86 × 1014 | 0.98 |
| LFR-4 | −6175.3 | 51.34 | 27.26 | 6.9 × 1011 | 0.99 | −6456.9 | 53.68 | 29.98 | 1.04 × 1013 | 0.97 |
| LFR-5 | −6022.8 | 50.07 | 25.59 | 1.29 × 1011 | 0.99 | −6409 | 53.28 | 28.45 | 2.26 × 1012 | 0.99 |
| LFR-6 | −6094.4 | 50.66 | 25.81 | 1.61 × 1011 | 0.99 | −6466.3 | 53.76 | 28.69 | 2.88 × 1012 | 0.98 |
| LFR-7 | −6349.7 | 52.79 | 26.2 | 2.57 × 1011 | 0.99 | −7900.9 | 65.68 | 33.01 | 3.01 × 1014 | 0.99 |
| LFR-8 | −6190.7 | 51.46 | 26.88 | 4.71 × 1011 | 0.99 | −6533.7 | 54.32 | 29.78 | 8.65 × 1012 | 0.97 |
| LFR-9 | −6210 | 51.62 | 27.31 | 7.31 × 1011 | 0.99 | −6496.6 | 54.01 | 30.07 | 1.14 × 1013 | 0.98 |
The activation energy and pre-exponential factor for OTR or CTR of polymeric films as well as film laminates is presented (Table 6). Using these constants, the GTR (OTR and CTR) at any temperatures were predicted by using Eq. (14) and the GTRp and Eap of O2 and CO2 of selected films found to be within the range reported (Exama et al. 1993; Mangaraj et al. 2009).
Verification of the arrhenius type model
The mean relative deviation moduli between the OTR and CTR of films as well as the film laminates predicted by Arrhenius model and that obtained through experiments varied from 5.26 to 8.73 % and 7.15–9.94 %, respectively. The results indicate that these models have good agreement for predicting the GTR of films and film laminates for MA packaging of fruits.
Storage of MA packages
MAP is essentially a packet for storage, which is kept in proper containers for transportation. Under these conditions, MAP is not likely to get exposed to such forces, which would delaminate the packaging film laminates. Thus, lamination strength is not a critical parameter. However, proper sticking of the two film surfaces was considered to be important. Two types of packages for apple (PCG-LFR-1 and PCG-LFR-2), two types of packages for guava (PCG-LFR-3 and PCG-LFR-4) and one type of package for litchi (PCG-LFR-5) were developed for MA packaging of these fruits. Package sizes of 24 cm × 19 cm (Ap = 0.0912 m2), 19 cm × 19 cm (Ap = 0.0722 m2), and 28 cm × 22 cm (Ap = 0.1232 m2) were found to be appropriate for packaging of six medium size apples, four medium size guavas, and fifty-two medium size litchi, respectively. Packages were designed to accommodate a fill weight (Wp) range of 0.90–1.10 kg. It suggests an optimal range of Wp: Ap ratio of 9.86–12.06, 12.47–15.23 and 7.30–8.92 for apple, guava and litchi, respectively. The shape and size of the apple, guava and litchi fruits were found to have affected Wp: Ap ratio as well as package free volume (Vfp). Large variations in the free volume of various packages were found. The Vfp was found to have varied between 762 and 897 ml, 490–625 ml, and 1572–1705 ml for MA package of apple, guava and litchi, respectively. In general, Vfp was found to have varied inversely with the Wp: Ap ratio.
Equilibrium conditions in MA packages
Most of the packages have established equilibrium at such levels of O2 and CO2, which were fairly close to the target levels. The experimental values of YO2eq varied between 3.10 and 3.31; 5.00–5.37; 4.95–5.28 % whereas those of ZCO2eq between 3.34 and 4.17; 3.14–3.72; 3.56–4.20 % for all types of MA packages for apple, guava and litchi, respectively at all the reference temperature levels. During steady state period, the experimental values of O2 and CO2 were found to be nearly constant for an extended period of storage. By and large, all types of MA packages have established dynamic equilibrium state without causing any unfavorable deviation from the target levels of O2 and CO2 at all the reference storage temperatures. The time taken by the MA package to establish dynamic equilibrium from the time of packaging is considered as equilibrium time and the predicted values of equilibrium time varied between 36–72 h; 12–30 h; and 26–50 h, whereas experimental values of 34–80 h; 10–34 h and 24–56 for all types of MA packages for apple, guava and litchi, respectively.
Conclusions
The application of Arrhenius equation to develop models for predicting the gas transmission rates of selected polymeric films and film laminates at different temperatures was attempted to predict the model parameters at any temperature of storage. The GTR of films predicted by models were verified with experimental data at 12 °C. The mean relative deviation moduli (E) between the OTR and CTR of film and film laminates predicted and that obtained through experiments varied from 6.48 to 9.57 % and 7.35 to 10.14 %, respectively. The results indicate that these models are capable of predicting the GTR of selected films and film laminates used for MA packaging of fruits. The GTR of none of the selected films could match the GTR requirements of MAP. Hence, judicious combinations of two different films were combined to form film laminates for MA packaging of selected fruits. Various package parameters were optimized to facilitate establishment of dynamic equilibrium at target levels of O2 and CO2 concentration for all fruits. It has been found that different levels of film thicknesses, temperatures and their interaction have significant effect on OTR and CTR of films at 1 % level of significance. However, the effect of film thickness is more than the temperatures on the gas transmission rates of films.
Contributor Information
S. Mangaraj, Phone: 07552521064, Email: sukhdev0108@gmail.com
T. K. Goswami, Email: tkg@agfe.iitkgp.ac.in
D. K. Panda, Email: dileeppanda@rediffmail.com
References
- Abdel-Bary EM (2003) Hand book of plastic films. Rapra Technology Ltd., Shawbury, Shrewbury, Shropshri, SY4 4NR, UK pp 463
- Ahvenainen R. Novel food packaging technology. Cambridge: Published in CRC Press, Boca Raton Boston New York Washinton, DC and Published by Woodhead Publishing Ltd; 2003. p. 535. [Google Scholar]
- Ashley RJ. Permeability and plastics packaging. In: Comyn J, editor. Polymer permeability. New York: Elsevier; 1985. pp. 269–308. [Google Scholar]
- Berins ML. Plastic engineering handbooks of the society of the plastic industry. London: 5th edition; 1991. [Google Scholar]
- Chaiprasart P. Effect of modified atmosphere packaging by PE and PVC on quality changes of litchi fruits. Acta Hortic. 2003;665:43–48. [Google Scholar]
- Costa C, Lucera A, Conte A, Mastromatteo M, Speranza B, Antonacci A, Del Nobile MA. Effects of passive and active modified atmosphere packaging conditions on ready-to-eat table grape. J Food Engg. 2011;102:115–121. doi: 10.1016/j.jfoodeng.2010.08.001. [DOI] [Google Scholar]
- Das MN, Giri NC. Design and analysis of experiments. New Delhi: Wiley Eastern Limited; 1986. [Google Scholar]
- Exama A, Arul J, Lencki RW, Lee LZ, Toupin C. Suitability of plastic films for modified atmosphere packaging of fruits and vegetables. J Food Sci. 1993;58:1365–1370. doi: 10.1111/j.1365-2621.1993.tb06184.x. [DOI] [Google Scholar]
- Goswami TK, Mangaraj S (2011) Advances in polymeric materials for modified atmosphere packaging (MAP). In: Multifunctional and nanoreinforced polymers for food packaging. (Ed. JM Lagaron). Woodhead Publishing Limited, Cambridge UK pp: 163–242.
- Jacomino AP, Kluge RA, Sarantopoulos CIGL, Sigrist JMM. Evaluation of plastic packages for guava refrigerated preservation. Packag Technol Sci. 2001;14:11–19. doi: 10.1002/pts.522. [DOI] [Google Scholar]
- Kader AA, Zagory D, Kerbel EL. Modified atmosphere packaging of fruits and vegetables. CRC Crit Rev Food Sci Nutri. 1989;28:1–30. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Lee DS, Hagger PE, Lee J, Yam KL. Model for fresh produce respiration in modified atmosphere based on principles of enzyme kinetics. J Food Sci. 1991;56:1580–1585. doi: 10.1111/j.1365-2621.1991.tb08645.x. [DOI] [Google Scholar]
- Mangaraj S (2012) Modified atmosphere packaging of apple (cv. Royal Delicious), guava (cv. Baruipur) and litchi (cv. Shahi). Unpublished Ph. D. Thesis, Department of Agricultural Engineering, Indian Institute of technology, Kharagpur
- Mangaraj S, Agrawal S, Gandhi AP. Studies on physico-chemical changes in selected fruits during storage. Bev Food World. 2005;32(11):72–75. [Google Scholar]
- Mangaraj S, Goswami TK. Respiration rate modelling of royal delicious apple at different temperature. Fresh Produce. 2008;2(2):72–80. [Google Scholar]
- Mangaraj S, Goswami TK. Modified atmosphere packaging – an ideal food preservation technique. J Food Sci Technol. 2009;46(5):399–410. [Google Scholar]
- Mangaraj S, Goswami TK. Modified atmosphere packaging of fruits and vegetables for extending shelf-life-a review. Fresh Produce. 2009;3(1):1–31. [Google Scholar]
- Mangaraj S, Goswami TK. Determination of maturity indices of fruits based on physico-chemical properties. Indian Food Packers. 2009;63(1):67–79. [Google Scholar]
- Mangaraj S, Goswami TK. Measurement and modelling of respirtion rates of guava (cv. Baruipur) for modfied atmosphere packaging. Inter J Food Prop. 2011;14(3):609–628. doi: 10.1080/10942910903312403. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK. Modelling of respiration rates of litchi fruit under aerobic condition. Food Bioprocess Technol. 2011;4:272–281. doi: 10.1007/s11947-008-0145-z. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK, Mahajan PV. Application of plastic films in modified atmosphere packaging of fruits and vegetables - a review. Food Engg rev. 2009;1:133–158. doi: 10.1007/s12393-009-9007-3. [DOI] [Google Scholar]
- Mangaraj S, Sadawat IJ, Prasad S. Assessment of quality of pears stored under laminated modified atmosphere packages. Inter J Food Prop. 2011;14:1–14. doi: 10.1080/10942910903580900. [DOI] [Google Scholar]
- Mangaraj S, Singh KP. Optimisation of machine parameters for milling of pigeon pea using RSM. Food Bioprocess Technol. 2011;4:762–769. doi: 10.1007/s11947-009-0215-x. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK (2009d) Control and modified atmosphere storage of fruits and vegetables. In: Introduction to advanced Food processing Technology. (Ed. Sahu JK). Taylor and Francis group of publication, USA. ISBN10:1439880719.
- Mangaraj S, Goswami TK, Giri SK, Tripathi MK. Permselective MA packaging of litchi (cv. Shahi) for preserving quality and extension of shelf-life. Postharvest Biol Te chnol. 2012;71:1–12. doi: 10.1016/j.postharvbio.2012.04.007. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK, Giri SK, Chandra P. Development and evaluation of MA packages employing lamination technique for royal delicious apple. Emirates J Food Agri. 2013;25(5):358–375. doi: 10.9755/ejfa.v25i5.11597. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK, Giri SK, Joshy CG. Design and develoepeemnt of a modified atmosphere packaging system for guava (cv. Baruipur) J Food Sci Technol. 2014;51(11):2925–2946. doi: 10.1007/s13197-012-0860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangaraj S, Goswami TK, Mahajan PV. Development and validation of a comprehensive model for MAP of fruits based on enzyme kinetics theory and Arrhenious relation. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S, Ma K, Yusof S. Effects of various surface treatments on the storage life of guava (Psidium Guajava L.) at 10 °C. J Sci Food Agric. 1994;66:9–11. doi: 10.1002/jsfa.2740660103. [DOI] [Google Scholar]
- Montanez JC, Rodriguez FAS, Mahajan PV, Frias JM. Modelling the effect of gas composition on the gas exchange rate in perforation-mediated modified atmosphere packaging. J Food Engg. 2010;96:348–355. doi: 10.1016/j.jfoodeng.2009.08.007. [DOI] [Google Scholar]
- Pino M, Duckett RA, Ward IM. Single and mixed gas diffusion through polyethylene films. Polymer. 2005;46:4882–4890. doi: 10.1016/j.polymer.2005.02.118. [DOI] [Google Scholar]
- Yam KL, Lee DS. Design of modified atmosphere packaging for fresh produce. In: Rooney ML, editor. Active food packaging. New Zealand: Blackie Academic and Professional; 1995. pp. 55–105. [Google Scholar]
- Yasuda H, Clark HG, Stannett V. Permeability. Encyl Poly Sci Technol. 1969;9:794–80. [Google Scholar]