Abstract
Functional properties of the soy protein need to improve to have better applications in food industry. Alkali extracted and acid precipitated soy protein isolate (SPI) was glycosylated using D-glucose (G) and Xanthan gum (X) via Maillard reaction to improve solubility. The effects of SPI to G and SPI to X ratios (SPI:G = 2:1, 1:1, and 1:2; SPI:X = 100:1 and 10:1) and incubation time (0, 6, 12, and 24 h) on the solubility and functional properties of glycosylated SPI were evaluated. The SPI:G ratio of 1:2 yielded a maximum degree of glycosylation of 71.1 %. The solubility of SPI after glycosylation significantly increased (P < 0.05) at pH 4.0–8.0 compared to SPI alone. Although the emulsion stability of glycosylated SPIs has not significantly increased (P > 0.05), the emulsifying activity improved significantly (P < 0.05). Glycosylation with SPI-X at a ratio of 10: 1 showed maximum emulsifying activity of 191.6 m2/g (SPI alone: 66.3 m2/g). Moreover, the SPI:X (ratio of 100:1) showed the maximum foaming activity (205 mL) compared to SPI alone (155 mL). The foaming stability of SPI (2.6 %) increased to 5.5 and 8.2 % when using xanthan gum at the ratio of 100:1 and 10:1, respectively. Glycosylated SPI with enhanced emulsifying and foaming properties has potential to improve the functional quality of the food products.
Keywords: Soybean protein isolate, Glycosylation, D-glucose, Xanthan gum, Functional properties
Introduction
Soybean (Gycine max L.) is a legume crop cultivated for edible oil and is a protein source. In the food industry, soybean protein has wide range of applications especially in the processed foods (meat, milk, beverage, and bakery products), due to its nutritional and functional benefits (Liu 1999). The functional properties of proteins such as solubility, gelation, emulsification, foaming, and viscosity play a major role in the food systems. It is a common practice to modify those functional properties for effective application to improve food flavor, texture, color, and storage stability (Zhang et al. 2007). The improved functionalities of soy protein isolate will expand its usage in food processing since most commercially available soy protein isolates have undesirable functionalities due to poor solubility (Zhang et al. 2007). Chemical and enzymatic methods are commonly used for protein modifications because of its cost effective procedures. The chemical modifications (glycosylation, acetylation, alkylation, esterification, amidination, and deamination) have been extensively studied for protein functionality improvements (Aminlari et al. 2005).
Among the various chemical modifications, glycosylation describes the covalent coupling reactions between the ε- or N-terminal amino group of proteins and the carbonyl group of polysaccharide chains (Aminlari et al. 2005). Various proteins such as ovalbumin, soy protein isolate, whey protein isolate, and peanut protein isolate have been evaluated for glycosylation process and shown to improve emulsifying activity (Yu and Yu 2009; Tian et al. 2011; Liu et al. 2011). The studies conducted by Paraman et al. (2007) on the effects of glycosylation and alcalase hydrolysis on rice endosperm protein suggested that the glycosylation is more effective process than alcalase treatment to enhance the solubility and emulsifying activity of rice endosperm protein. The degrees of improvement on functional properties depend on the type of glycan and the type of carbohydrates, specifically with their size and charge bound on the protein. Mahran et al. (2011) showed improved foaming stability of buffalo casein due to Maillard reaction with glucose, galactose, lactose, and ribose, and displayed the highest emulsion stability after glucose treatment, compared to other types of reducing sugars.
Glucose is most frequently used to conjugate with proteins to improve solubility and emulsifying properties (Mahran et al. 2011). Xanthan gum is a natural polysaccharide secreted by bacteria that can also improve the solubility, emulsifying, and foaming properties of soybean protein isolate and rice endosperm protein (Xie and Hettiarachchy 1997 and 1998). However, there are no studies that reported the effects of glucose and xanthan gum, protein to glucose or xanthan gum ratios, with various incubation times of Maillard reaction on the functional properties of soy protein isolate.
Therefore, the objective of this study was to investigate the influences of glucose and xanthan gum and their ratios with protein during glycosylation, and Maillard reaction incubation time on the modification of functional properties of soy protein isolate. It is expected that the glycosylated soy protein isolate prepared from soy meal may have potential for improving quality of the food products with enhanced emulsifying and forming properties.
Experimental procedure
Materials
Dried soybean seeds were provided by Dr. Cheng Penying (Department of Crop, Soil, & Environmental Sciences, University of Arkansas, AR, USA). D-glucose and Xanthan gum were obtained from TIC Gums, Inc. (MD, USA). Analytical chemicals and reagents were purchased from Sigma Chemical Corp. (MO, USA).
Preparation of SPI
SPI was prepared from soybean meal following the method of Xie and Hettiarachchy (1998) with some modifications. Dried soybean seeds were ground into homogenous flour using a Universal mill. The soybean flour was suspended in hexane (1: 4 ratio w/v) and stirred for 6 h at ambient temperature. This procedure was repeated using fresh hexane to remove the residual lipids. The defatted flour was dried under the hood for 12 h and passed through an 80-mesh sieve. The flour was suspended in deionized (DI) water at a flour to solvent ratio of 1:10. The dispersion was adjusted to a pH of 9.0 using 2.0 N NaOH, then stirred for 2 h at ambient temperature. The supernatant was collected after centrifugation at 10,000×g for 20 min and the pH was adjusted to 4.5 (soy protein isoelectric point) to precipitate proteins. The proteins were obtained by collecting the residue after centrifugation at 5000×g for 20 min, washed three times using DI water, and adjusted the pH to 7.0. The dried SPI was obtained by freeze drying and stored in air-tight plastic bags at 4 °C for further studies.
Glycosylation conditions for SPI
D-Glucose (G) and Xanthan Gum (X) were selected for SPI glycosylation. The SPI (2.0 g on protein basis) was dissolved in 40 mL of DI water to make a 5 % (w/v) solution. Different amounts of G (1.0, 2.0, and 4.0 g) were added into the protein solutions to make the SPI:G ratios of 2:1, 1:1, and 1:2 (w/w), respectively. The SPI-G mixtures were adjusted to a pH of 8.0 and stirred at ambient temperature for 2 h. The mixtures were freeze-dried, placed on aluminum plates and incubated (50 °C, 65 % relative humidity). Each glycosylated protein mixture was taken out at 6 h intervals for 24 h and stored separately in air-tight plastic bags at 4 °C for further studies. Glycosylated SPI using Xanthan Gum (X) was conducted in a manner essentially similar to the SPI-G described above, with the following modification: the SPI: Xanthan gum ratios were 100:1 and 10:1.
Degree of glycosylation
The degree of glycosylation of modified SPI was determined using the method of Yaylayan et al. (1992). Dried protein or glycosylated protein samples (25 mg, protein basis) were dispersed in 10 mL of DI water and vortexed. The dispersions were stirred for 30 min and filtered through a 0.45 um syringe filter. The filtrate was diluted 20 times with DI-water. A 200 μL sample of the diluted filtrate, 4.0 mL of borate buffer (0.02 M potassium tetraborate, pH 8.5), and 1.0 mL of fluorescamine reagent were combined and vortexed. Blank solution was prepared using buffer without protein. After 5 min of reaction, the fluorescence intensity was recorded at excitation and emission wavelengths of 390 and 475 nm, respectively.
The degree of glycosylation was calculated as follows: Degree of glycosylation (%) = (Ac–Aa)/Ac × 100 (Ac: fluorescence of unmodified protein, Aa: fluorescence of the glycosylated protein).
Solubility
The solubility profiles of glycosylated proteins were determined by the method described by Bera and Mukherjee (1989) with modifications. One hundred milligram samples were suspended in 10 mL of DI water. The pH values of protein solutions were adjusted from 2.0 to 10.0, using 1.0 N HCl and 1.0 NaOH, and readjust for specific pH point every 10 for 30 min of continuous stirring. The protein contents of sample solutions and the supernatant after centrifugation (10,000 g, 30 min) were determined by Coomassie protein assay (Bradford 1976).
The protein solubility was calculated using the following formula: Protein solubility (PS, %) = amount of protein in the supernatant × 100 / total amount of nitrogen in a 100 mg sample.
Emulsifying properties
The emulsifying activity and emulsifying stability were determined using the method described by Pearce and Kinsella (Pearce and Kinsella 1978). Protein solution (0.1 % w/v) was prepared using 0.01 M phosphate buffer (pH 7.0). Then, 6 mL of protein solution and 2 mL corn oil were mixed and homogenized for 1 min using a homogenizer, at a setting 6, to form a homogenous emulsion. The emulsion (50 μL) was added into a test tube containing 5 mL of 0.1 % SDS (w/v) at 0 and 10 min after homogenizing. The absorbance of the above solutions was recorded using a spectrophotometer at a wave length of 500 nm. The EAI (emulsifying activity index) was calculated using the following formula: EAI (m2/g) = (2 × 2.303 × A0 × DF) / (C × ϕ × θ × 10,000), where DF is the dilution factor (100), C is the initial concentration of protein (g/mL), ϕ is the optical path (1 cm), θ is the fraction of oil used to form the emulsion (0.25), and A0 is the absorbance of the diluted emulsions at 0 min. ES (emulsion stability) was calculated using the following equation: ES = T0 × ∆t/∆T; where ∆T is a decrease in turbidity (absorbance) of the initial absorbance (T0) which occurred after 10 min.
Foaming properties
The foaming capacity (FC) and foaming stability (FS) of the protein isolate and glycosylated protein samples were determined following the method of Kato et al. (1983). Five milliliters of 1.0 % sample solution was prepared using 0.05 M phosphate buffer (pH 7.4). Then, air was introduced into the solution at a rate of 200 cm3/min for 15 s. The volumes of the foam (V0) after 0 and 1 min air introduction were recorded. The foaming capacity (FC) of the protein was expressed by the volume (mL) of the foam measured immediately after the air was induced for 15 s. The foaming stability (FS) was calculated from the following equation: FS (min) = V0 × ∆t/∆V; where ∆V is a decrease in the volume of the foam at the time interval of ∆t, and V0 is the volume of the foam at 0 min.
Statistical analysis
All of the experiments were conducted in triplicate and the values were reported as the means of three determinations. The data were analyzed by analysis of variance (ANOVA) using the Statistical Analysis System (SAS 9.22008, SAS Institute Inc., Cary, N.C., U.S.A.). The Fisher’s protected least significant difference (LSD) test was conducted to separate the means at P ≤ 0.05.
Results and discussion
Degree of glycosylation
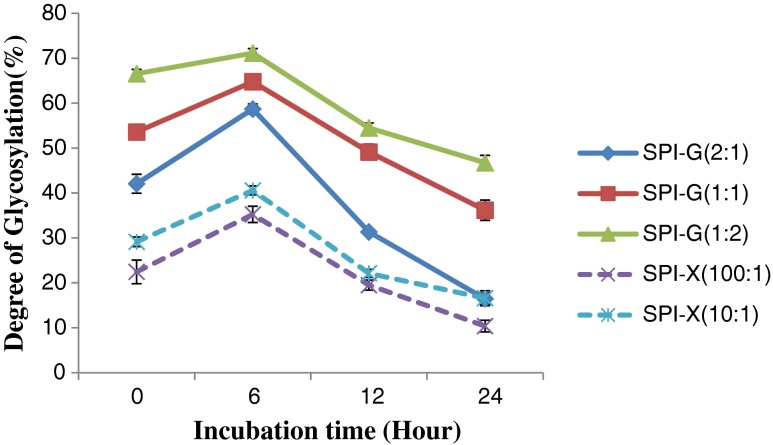
The glycosylation of SIP could be triggered by a reaction between the ε-amino group or the N-terminal amino group in proteins and the carbonyl group of glycans (Nakamura et al. 1992). All glycosylated SPI derivatives through Maillard reaction using xanthan gum and glucose showed significantly (p < 0.05) different levels of degree of glycosylation (Fig. 1). The degrees of glycosylation increased significantly (p < 0.05) after incubation for 6 h and then decreased as the incubation time increased from 6 to 24 h. A similar trend was observed in all other glycosylated proteins. In accordance with the studies conducted by Aminlari et al. (2005) the increased incubation time may be the reason for the disassociation of glycans from the protein molecules. There is another possibility that the interaction between proteins will increase and compete with the conjugation between proteins and glucose or xanthan gum molecules (Ramezani et al. 2008). The highest degree of glycosylation (71.1 %) was obtained with SPI:G of 1:2 after 6 h incubation (50 °C, 65 % relative humidity). The glycosylated SPI using D-glucose resulted in higher degrees of glycosylation compared to modified proteins using xanthan gum (P < 0.05). Since the degree of glycosylation is related to the molar ratios of protein to glycans, higher amount of glucose or xanthan gum added to the mixtures can result in higher degree of glycosylation (Fig. 1).
Fig. 1.
Effect of glucose or xanthan gum, their ratios to soy protein isolate (SPI), and incubation time on the degree of glycosylation (%) of SPI after glycosylation. Values are means (n = 3) of three determinations. Standard derivation was shown by error bars
Solubility
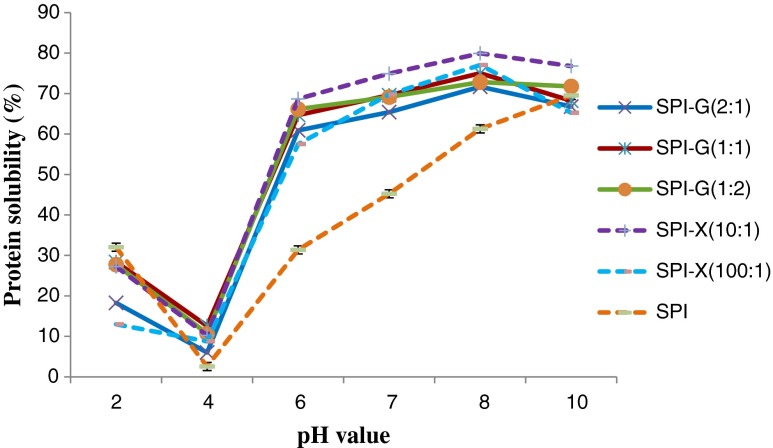
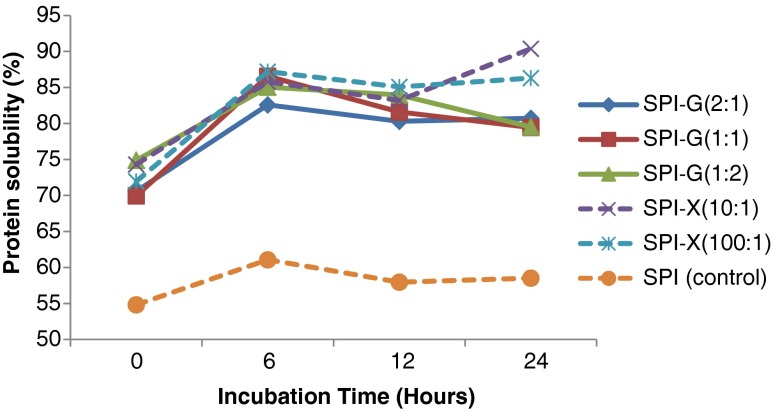
The solubility of unmodified SPI and glycosylated SPI samples at various pH values (2, 4, 6, 7, 8, and 10) were shown in Fig. 2. The solubility of modified SPI prepared under various incubation time (0, 6, 12, 24 h) were also evaluated (Fig. 3). The glycosylated SPIs displayed significant (p < 0.05) increase in solubility at pH values ranging from 4 to 8, compared to native SPI. The maximum solubility of 79.9 % was found in SPI: X ratio of 10: 1 conjugate after 6 h of Maillard reaction at a pH of eight (Fig. 2). The improvement of glycosylated SPI sample solubility is directly correlated with increasing degree of glycosylation. In general, high protein solubility is preferred for further optimization of protein functionalities (Kinsella 1979). The literature information showed that protein solubility can be increased by Maillard reaction (Aminlari et al. 2005; Liu et al. 2011; Paraman et al. 2007; Xie and Hettiarachchy 1997; Ramezani et al. 2008). All glycosylated SPIs (at pH of 7) showed significantly (p < 0.05) increasing solubility after glycosylation incubation from 0 to 24 h (Fig. 3). In addition, the glycosylated protein samples using xanthan gum after longer incubation time (12 to 24 h) exhibited relatively higher solubility compared to glucose modified SPI (Fig. 3). This indicates that the glycan-proteins in combination can be an ideal approach for improving food quality.
Fig. 2.
Effect of glucose or xanthan gum and their ratios to soy protein isolate (SPI) on solubility of glycosylated SPI under different pH values. SPI was tested for comparison. The incubation time for all the samples were 6 h. Values are means (n = 3) of three determinations. Standard derivation was shown by error bars
Fig. 3.
Effect of glucose or xanthan gum, their ratios to soy protein isolate (SPI), and incubation time on solubility of glycosylated SPI. SPI was tested for comparison. The solubility of all the samples were determined at pH value of 7.0. Values are means (n = 3) of three determinations. Standard derivation was shown by error bars
Emulsifying activity and stability
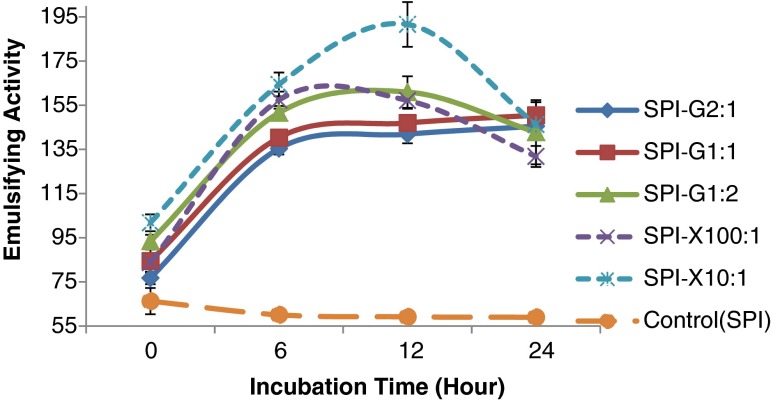
In order to determine the effect of glucose/xanthan gum on the emulsifying activity of SPI, the glycosylated proteins prepared with different SPI to glucose/xanthan gum ratio and varying incubation times were evaluated. The emulsifying activity index (EAI) of glycosylated-SPI samples using different ratios of glucose and xanthan gum as function of Maillard reaction time (0, 6, 12, and 24 h) were shown in Fig. 4. All the glycosylated SPI samples showed significant (p < 0.05) improvement in EAI (ranged from 76.8 to 191.6 m2/g) compared to native SPI (ranged from 59.0 to 66.3 m2/g). The EAI of glycosylated SPI depended on the type of glycans (glucose and xanthan gum) and their ratios to native SPI. For the xanthan gum modified SPI samples, a gradual improvement in EAI was observed which then decreased when the incubation time was extended. For the glucose glycosylated proteins, at 0 to 6 h incubation time the EAI significantly (p < 0.05) increased as the increment was insignificant from 6 to 24 h incubation. The glucose modified SPI (SPI: Glucose = 1:2) showed a decrease in EAI from 12 to 24 h. The highest values of EAI of glycosylated-SPI samples were 191.6 m2/g when using xanthan (SPI: Xanthan gum = 1: 10) and 161.0 m2/g with glucose (SPI: Glucose = 1:2) at the optimum Maillard reaction incubation time (12 h).
Fig. 4.
Effect of glucose or xanthan gum, their ratios to soy protein isolate (SPI) and incubation time on emulsifying activity of glycosylated SPI samples. SPI was tested for comparison. Values are means (n = 3) of three determinations. Standard derivation was shown by error bars
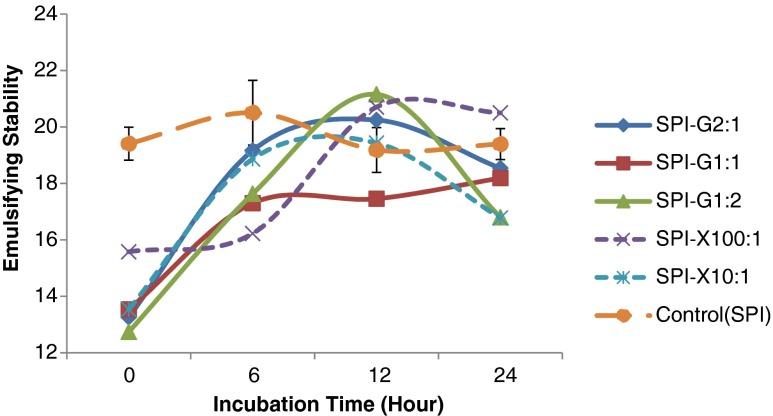
The emulsion stability (ES) of all glycosylated-SPI samples as a function of Maillard reaction time from 0 to 24 h were shown in Fig. 5. All the glycosylated proteins after 0 to 6 h of Maillard reaction showed significantly (p < 0.05) lower ES values compared to unmodified SPI. After 12 h of Maillard reaction, the glycosylated SPI samples using glucose (SPI: Glucose = 1:2 and 2:1) and xanthan gum (SPI: Xanthan gum = 100:1) demonstrated increased ES values compared to native SPI. The emulsifying activity of modified protein was affected by type of glycans (glucose or xanthan gum) along with the molar ratio of glycans and proteins. The glycosylated proteins using xanthan gum showed relatively higher emulsifying activity compared to glucose. The emulsifying activity of xanthan gum-modified proteins displayed a higher increasing rate of emulsifying activity compared to glucose utilization, and it decreased after a certain period of incubation time (from 6 to 12 h). The increase in EAI may be due to the increased capacity to form a heavily hydrated inter-facial layer, as the protein surface is able to provide several hydrophilic and/or charged groups. The SPI-Xanthan conjugate with a ratio of 10:1 showed a slower rate of reaction and higher emulsifying activity than the sample using lower amount of carbohydrate (SPI: Xanthan gum = 100:1). The amount of glycan might have influenced the degree and rate of cross-link formation of the amino group of the protein with the carbonyl group of the carbohydrate. Similar results were observed with modified proteins when using glucose. The SPI-Glucose conjugate with a ratio of 1:2 displayed a maximum value of emulsifying activity. The SPI-Glucose with a ratio of 2:1 and 1:1 showed increase in emulsifying activity, possibly due to greater amounts of glycosylated-unsaturated protein molecules. Thus, the optimum reaction time of Maillard reaction varied and depended on the type of glycans and protein source (Oliver et al. 2006).
Fig. 5.
Effect of glucose or xanthan gum, their ratios to soy protein isolate (SPI), and incubation time on emulsifying stability of glycosylated SPI samples. SPI was tested for comparison. Values are means (n = 3) of three determinations. Standard derivation was shown by error bars
Foaming capacity and foaming stability
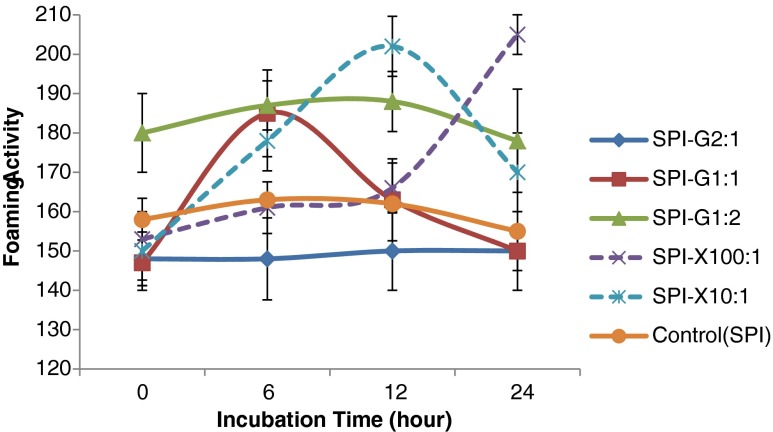
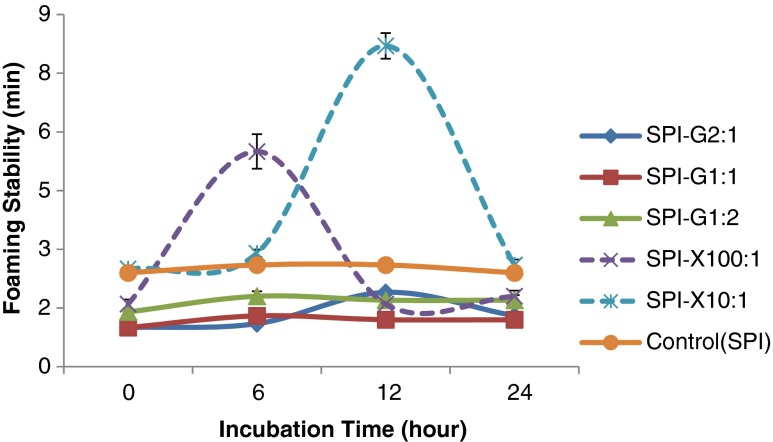
The foaming capacity (FC) and foaming stability (FS) were evaluated for unmodified SPI and glycosylated SPI (Fig. 6 and Fig. 7). Foaming capacity was determined by the initial volume of foam formation followed by immediate introduction to protein solution (Kato et al. 1983). The glycosylated proteins using the highest amount of glucose (SPI: Glucose = 1:2) showed significant (p < 0.05) improvement at all incubation periods (maximum FC: 188 cm2) compared to native SPI (150 cm2). The SPI-glucose conjugate (SPI: Glucose = 1:1) displayed comparatively higher FC (185 cm2) only after at 12 h of incubation. For xanthan gum glycosylated SPI, the highest FC (202 cm2) was found after the 12 h Maillard reaction (SPI: Xanthan gum = 10:1) and significantly (p < 0.05) increased to 178 cm2 and 170 cm2after 6 and 24 h incubation, respectively. The xanthan gum glycosylated protein derivatives exhibited higher foaming stability than unmodified SPI. The SPI-Xanthan conjugate using higher amount of xanthan gum (SPI: Xanthan gum = 10:1) reached the highest value of FS (8.2 min) only after 12 h of Maillard reaction, which was longer than SPI-Xanthan conjugate using ratio of 100:1 with a maximum FS of 5.5 min after 6 h of incubation under the same condition (Fig. 7). However, the glycosylated protein samples using glucose had a negative impact on foaming stability compared to the native SPI. Protein solubility in the aqueous phase is the prerequisite for proteins to have good foaming properties. All the glycosylated proteins showed enhanced foaming activity except the SPI-glucose conjugate (SPI: Glucose = 2:1). The xanthan gum showed comparatively higher foaming capacity, which might be due the large molecular size of xanthan gum, that led to increased surface hydrophobicity and level of desired protein denaturation (Damodaran 1994). The glycosylated proteins with higher molecular weight of glycan may increase the flexibility of the native protein structure, the conformational change, and the protein surface rearrangement, that can lead to enhanced protein adsorption at air-water interface during bubbling (Were et al. 1997). The enhanced foaming activity and stability of SPI-Xanthan samples might be due to the increased incubation time that may influence the protein unfolding to a desired extent.
Fig. 6.
Effect of glucose or xanthan gum, their ratios to soy protein isolate (SPI), and incubation time on foaming activity of glycosylated SPI samples. SPI was tested for comparison. Values are means (n = 3) of three determinations. Standard derivation was shown by error bars
Fig. 7.
Effect of glucose or xanthan gum, their ratios to soy protein isolate (SPI), and incubation time on foaming stability of glycosylated SPI samples. SPI was tested as comparsion. Values are means (n = 3) of three determinations. Standard derivation was shown by error bars
Conclusion
The results of this study clearly indicate that the functional properties of the SPI improved by the Maillard reaction using glucose and xanthan gum. These observations speculate that the changes in protein functionalities such as emulsifying activity, stability, and foaming, caused by glycosylation, are not only depending on the type of glycans, it also influenced by several factors. The major factors include the number of protein glycosylable groups, protein: glycans ratio, and incubation time. The results of this study are consistent with other related studies on glycosylation of soybean protein isolate with carbohydrates and indicates the possible use of glycosylated SPI, extracted from soybean seeds as a functional components to the various food products.
References
- Aminlari M, Ramezani R, Jadidi F. Effect of Maillard-based conjugation with dextran on the functional properties of lysozyme and casein. J Sci Food Agric. 2005;85:2617–24. doi: 10.1002/jsfa.2320. [DOI] [Google Scholar]
- Bera MB, Mukherjee RK. Solubility, emulsifying, and foaming properties of rice bran protein concentrates. J Food Sci. 1989;54:142–145. doi: 10.1111/j.1365-2621.1989.tb08587.x. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Damodaran S. Structure-function relationship of food proteins. In: Hettiarachchy NS, Ziegler GR, editors. Protein functionality in food system. New York: Marcel Dekker; 1994. pp. 1–37. [Google Scholar]
- Kato A, Takahashi A, Matsudomi N, Kobayashi K. Determination of foaming properties of proteins by conductivity measurement. J Food Sci. 1983;48:62–65. doi: 10.1111/j.1365-2621.1983.tb14788.x. [DOI] [Google Scholar]
- Kinsella JE. Functional properties of soy proteins. J Am Oil Chem Soc. 1979;56:242–258. doi: 10.1007/BF02671468. [DOI] [Google Scholar]
- Liu K. Soybean: chemistry, technology, and utilization. New York: Aspen Publishers Inc.; 1999. [Google Scholar]
- Liu Y, Zhao G, Zhao M, Ren J, Yang B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2011;09:074. [Google Scholar]
- Mahran GA, Haggag HF, Yousef MSH, Ali JB. Improvement of functional properties of glycated buffalo casein conjugated with different sugars through Maillard reaction. World J Dairy Food Sci. 2011;6:166–174. [Google Scholar]
- Nakamura S, Kato A, Kobayashi K. Enhanced antioxidative effect of ovalbumin due to covalent binding of polysaccharides. J Agric Food Chem. 1992;40:1734–1739. [Google Scholar]
- Oliver CM, Melton LD, Stanley RA. Creating proteins with novel functionality via the maillard reaction: a review. Crit Rev Food Sci Nutr. 2006;46:337–350. doi: 10.1080/10408690590957250. [DOI] [PubMed] [Google Scholar]
- Paraman I, Hettiarachchy NS, Schaefer C. Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chem. 2007;84:593–599. doi: 10.1094/CCHEM-84-6-0593. [DOI] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric techniques. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Ramezani R, Esmailpour M, Aminlari M. Effect of conjugation with glucosamine on the functional properties of lysozyme and casein. Society. 2008;2737:2730–2737. [Google Scholar]
- Tian S, Chen J, Small DM. Enhancement of solubility and emulsifying properties of soy protein isolates by glucose conjugation. J Food Process Preserv. 2011;35:80–95. doi: 10.1111/j.1745-4549.2009.00456.x. [DOI] [Google Scholar]
- Were L, Hettiarachchy NS, Kalapathy U. Modified soy proteins with improved foaming and water hydration properties. J Food Sci. 1997;62:821–824. doi: 10.1111/j.1365-2621.1997.tb15463.x. [DOI] [Google Scholar]
- Xie YR, Hettiarachchy NS. Xanthan gum effects on solubility and emulsification properties. J Food Sci. 1997;62:1101–1104. doi: 10.1111/j.1365-2621.1997.tb12222.x. [DOI] [Google Scholar]
- Xie YR, Hettiarachchy NS. Effect of xanthan gum on enhancing the foaming properties of soy protein isolate. J Am Oil Chem Soc. 1998;75:729–732. doi: 10.1007/s11746-998-0214-5. [DOI] [Google Scholar]
- Yaylayan VA, Despointes AH, Polydorides A. A fluorescamine-based assay for the degree of glycation in bovine serum albumin. Food Res Int. 1992;25:269–275. doi: 10.1016/0963-9969(92)90123-M. [DOI] [Google Scholar]
- Yu B, Yu JC. Effect of glycosylation on molecular characteristics and emulsifying properties of ovalbumin. Sci Agric Sin. 2009;7:15–20. [Google Scholar]
- Zhang K, Li Y, Ren Y. Research on the phosphorylation of soy protein isolate with sodium tripoly phosphate. J Food Eng. 2007;79:1233–1237. doi: 10.1016/j.jfoodeng.2006.04.009. [DOI] [Google Scholar]